Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Anesthesiology
Anaesthesia plays an important role in diagnosis procedures and the treatment and pain management of oncological patients. However, studies suggest that anaesthetic drugs may increase the risk of tumour dissemination in the perioperative period by directly and indirectly suppressing the immune system, which is primarily responsible for controlling tumour growth.
- perioperative period
- surgery
- immune system
- tumours
- intravenous anaesthetics
- volatile anaesthetics
- veterinary oncology
1. Introduction
The growing attention paid to animal health [1] and the development of novel diagnostic and therapeutic procedures have significantly impacted the lifespan of companion animals [2]. Malignant tumours tend to be more prevalent in older animals [3,4], which can be linked to various age-related factors such as alterations in cell-mediated immunity and the phenomenon of immunosenescence [5]. The diagnosis of cancer in dogs and cats has increased significantly in recent years, making it one of the most prevalent causes of death. [6,7]. This fact has led to novel therapeutic oncology approaches, which are considered based on the tumour line and the patient’s health [8]. Surgical excision is still considered the gold standard for treating solid tumours [9]; however, even in tumour resections with histologically clean margins, an undetected residual risk of the intra-surgical dissemination of tumour cells still exists [10]. For this reason, it is often required to combine surgical excision with other treatment alternatives, such as chemotherapy, radiation or biological therapies [11,12,13,14].
Considering human and animal studies, the main risk of tumour dissemination occurs in the perioperative period [15,16], during which cancer cells and the immune system are influenced by stress, pain [17,18], anaesthesia [19] and surgical procedures [20]. These factors induce a stress response that starts a cascade of physiological mechanisms beneficial to the restoration of homeostasis but also favourable to tumour growth [20], which include immunomodulatory and pro-tumour effects such as immune suppression and tumour angiogenesis [17,18]. Therefore, the perioperative period can be seen as a window of opportunity to minimise the pro-tumour and anti-immune mechanisms triggered by surgical stress and anaesthetic drugs, suggesting new clinical approaches with neurophysiological and immune preservation. Alternative surgical and anaesthetic procedures, such as minimally invasive surgery [21], regional anaesthesia [22], opioid-free anaesthesia [23], total intravenous anaesthesia [24] and multimodal analgesia [25,26], are gaining significance in clinical practice, with particular emphasis on oncological patients due to their immunoprotective effects.
2. The Immune System as the Main Player in the Tumour’s Defence
The tumour growth and spread process is influenced by the tumour’s characteristics but mainly by the host’s immune system [8]. The scientific community recognises that a high number and high activity of natural killer cells and cytotoxic T lymphocytes correlate with enhanced anti-tumour immune responses and the elimination of neoplastic cells from blood circulation [27,28]. Likewise, dendritic cells (DCs) are an essential subpopulation of leukocytes for inducing and regulating an adaptive anti-tumour immune response [29]. Cancer immunoediting is part of a dynamic concept that explains the role of the immune system and immune cells in malignant transformation, emphasising cancer immunotherapy [30].
In veterinary and human searches, a high count and ratio of circulating leukocytes (CD4+ and CD8+ T cells, dendritic cells, macrophages and regulatory T cells) [31,32], as well as their presence in the tumour microenvironment [33,34], may be associated with the prognosis of certain solid tumours. In felines with mammary carcinoma, the neutrophil-to-lymphocyte ratio has been suggested as a prognostic factor. In contrast, high numbers of total leukocyte counts, neutrophils, and the neutrophil/lymphocyte ratio were associated with an increased risk of tumour-related death [31]. Moreover, the lymphocyte/monocyte ratio can predict the prognosis of newly diagnosed canines with diffuse large B-cell lymphoma in terms of time progression and lymphoma-specific survival [32] and the histopathological grade of canine mast cell tumours [35]. In canine osteosarcoma, monocyte and lymphocyte counts were used as prognostic indicators [36]. Other studies observed that in dogs with oncological disease, the percentage of Th1 was considerably lower and Th2 was significantly higher compared to healthy dogs [37]. In addition, they determined that dogs with metastases had even lower Th1 values than ill dogs without metastases [37]. Researchers observed a correlation between tumour-infiltrating lymphocytes and histopathological characteristics in canines with mammary carcinoma, suggesting that a significant quantity of lymphocytes infiltrating mammary carcinoma is associated with lymphatic invasion and a high histopathological grade [38]. Furthermore, the tumour-infiltrating lymphocytes proved indispensable for tumour growth and their spread potential [38]. In the review “A role for T-lymphocytes in human breast cancer and in canine mammary tumours”, the authors compare tumour T-lymphocyte infiltration and the CD4+/CD8+ T-cell ratio with low survival rates, the action of Th2 cells in the acceleration of tumour progression and a poor prognosis with the presence of large numbers of Treg cells [39]. In human breast cancer, the role of macrophages in tumour progression was observed as a facilitator of the invasion of tumour cells [40] due to their plasticity and ability to adapt to the different physiological conditions that the body presents, which promote the production of cytokines type II, anti-inflammatory responses, and pro-tumour functions [41]. In pancreatic and colorectal human cancers, elevated Th2 levels and an imbalance of the Th1/Th2 ratio lead to an increase in pro-inflammatory interleukins that promote the progression of the disease [42,43].
Considering the immune system as a main player in the body’s defence against tumour growth, some human and animal studies suggest a new approach to minimising the impact on the immune cells of several suppressive factors such as stress, pain, anaesthesia and surgery [19,44,45]. According to studies in dogs, the administration of recombinant canine interferon (rCaIFN-γ) before, during and after anaesthesia may reduce the suppression of natural killer (NK) activity, inhibit intraoperative cancer cell dissemination and prevent postoperative cancer recurrence and metastasis [46,47]. In humans, it has been demonstrated that the administration of nonsteroidal anti-inflammatory agents to oncological patients has anti-tumour properties by inhibiting the pro-tumour activity of the enzyme COX-2 of macrophages and increasing the activity of T regulatory cells [48,49], both of which have been shown to promote tumour progression in humans. Dendritic cells also play an important role in immunotherapies, eliciting Th1 and Th17 cell differentiation [50] and infiltrating solid malignancies [51].
3. Tumour Surgery as a “Starting Point” for Tumour Progression
Excisional tumour surgery is the main approach for treating primary solid tumours [9] and the major facilitator of metastatic cell dissemination [52]. Since the eighteenth century, this condition has been researched as a “starting point” for accelerating tumour progression [53]. Since then, several studies have been published on the relationship between surgery and cancer progression to understand this biological behaviour, including 1. the dissemination of tumour cells via the manipulation of the neoplastic tissue [54]; 2. the effect of surgery as a window of opportunity for the development and spread of tumour cells [55]; 3. the change in the state of tumour dormancy via surgical tumour excision [56] and decreased antiangiogenic factor secretion [57].
First, the surgical manipulation of neoplastic tissue promotes the release of neoplastic cells into the blood and lymphatic system [54], where they seek pre-metastatic niches to initiate a new cell growth cycle [58]. Besides that, before surgery, most oncology patients already have cancer cells in their circulatory system, which does not necessarily indicate the presence of metastases or tumour recurrence [59,60]. In human research, less than 0.01% of circulating tumour cells are successful in tumour metastasis, depending on the immune response and the tumour cells’ ability to avoid the defence cells [61]. Nonetheless, human investigations have demonstrated a correlation between tumour cells in peripheral blood after surgery and poor prognosis prediction [62].
Secondly, the microenvironment of the damaged tissue in the area of the surgical procedure changes, and minutes after the incision, a proportional local and systemic stress response is generated [20]. Physiological mechanisms such as local inflammation, sympathetic nervous system activation, and the hypothalamic–pituitary–adrenal axis (HPA) are activated to restore cellular and tissue homeostasis following tissue injury [20], as demonstrated in Figure 1. In response, immunomodulatory chemicals are released, specifically catecholamines and glucocorticoids, which induce prostaglandins (PGE2), pro-inflammatory cytokines (e.g., IL-10), metalloproteinases and endothelial growth factor secretion [17]. These immunomodulatory effects can result in a suppression of the immune system, producing direct and indirect pro-tumour effects such as tumour angiogenesis, the activation of 2-adrenergic and cyclooxygenase-2 receptors, the release of coagulation factors, and the depletion of natural killer cell (lymphocytes CD3- and CD56+) activity [18], which plays a significant role in anti-tumour immunity by controlling the dissemination of cancer cells [27,28]. Platelets and coagulation factors are released to reestablish haemostasis, forming protective aggregates that shield tumour cells from natural killer cells [63]. In actuality, this biological process, which simultaneously attempts to restore homeostasis, modifies the immunological, metabolic, endocrinological, neuronal, inflammatory and immune microenvironments, thereby fostering tumour growth and dissemination [18].
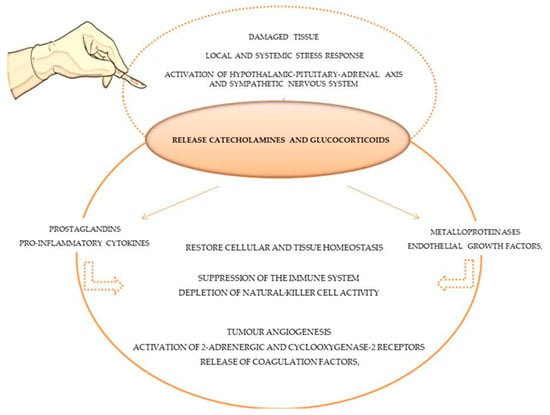
Figure 1. Stress and inflammatory response of tissue surgery damage in immune system and tumour cells.
Considering the inflammatory, immunosuppressive and tumour-spreading effects of surgery trauma, the potential advantages of minimally invasive surgery (MIS) over conventional open surgery have been discussed in human and animal studies, but with inconsistent results [21,64]. MIS seems to offer certain benefits, including reduced tissue inflammation and immune suppression, as well as less blood loss, decreased postoperative pain and reduced hospitalisation [21,64,65]. However, longer surgical and anaesthesia times and an increased risk of technical complications, such as wound dehiscence and local inflammation, could potentially be disadvantages when compared to open surgery [56,66,67].
Lastly, tumour dormancy is a condition characterised by the presence of tumour cells in the absence of evidence of oncological disease [68]. This phenomenon may explain both cancer recurrences after long periods of remission and the maintenance of reduced neoplastic cells after adjuvant oncological therapies [69], as a result of the absence of angiogenic competence, a homeostatic equilibrium between tumour cells and the immune response or the presence of an environment that does not promote tumour growth [68]. Surgical tumour excision helps cancer survive by altering the biological characteristics of neoplastic cells, such as proliferation, apoptosis, metastasis, and dormancy condition [70], and blood levels of tumour growth promoters (e.g., IL-6, TNF-α, VEGF) are higher after surgery, whereas antiangiogenic mediators such as endostatin and angiostatin are undetectable [71]. This finding supports the hypothesis that surgery and neuroendocrine mediators that control angiogenesis and inflammation can “awaken” a dormant tumour condition and dormant micrometastases [72]. As well, tumour angiogenic activity, which is the physiological process of generating new blood vessels from existing ones, is essential for the survival and development of tumour cells, including their growth, invasion and spread [73].
Even though some biological factors may paradoxically enhance the proliferation and dissemination of metastatic cells, surgical tumour removal provides an evident benefit in preventing the growth and spread of cancer [9]. Furthermore, both animal and human studies have demonstrated that the use of a less traumatic surgical technique and pharmaceutical substances, such as COX-2 selective nonsteroidal anti-inflammatory drugs [74,75], antithrombotics [63] and antiangiogenics [76,77], can make these biological effects simpler to control.
4. The Impact of the General Anaesthetics on the Immune System and Their “Anti-” and “Pro-Tumoral” Effects
Oncological diseases often require the use of anaesthetic and analgesic agents during diagnostic [78,79], therapeutic or palliative procedures to control pain [80,81] and minimise the effects of the stress response [76]. General anaesthetics include volatile agents like isoflurane and intravenous agents like propofol, which have been linked to immunomodulatory properties such as the inhibition of NK cell activity [82] and pro-tumour effects such as the inhibition of prostaglandin synthesis by tumour cells [47]. Due to their immunosuppressive effects, volatile anaesthesia may be associated with poorer outcomes than intravenous anaesthesia [83,84], as demonstrated in Figure 2. However, human studies suggest that general anaesthetics in combination with regional anaesthesia can reduce the inflammatory response and endothelial permeability, preventing the spread of cancer cells [65]. Also, studies in dogs and cats suggest that loco-regional therapy serves to reduce the quantity of intra and post-operative opioids, allowing their adverse effects to be reduced [85,86,87].
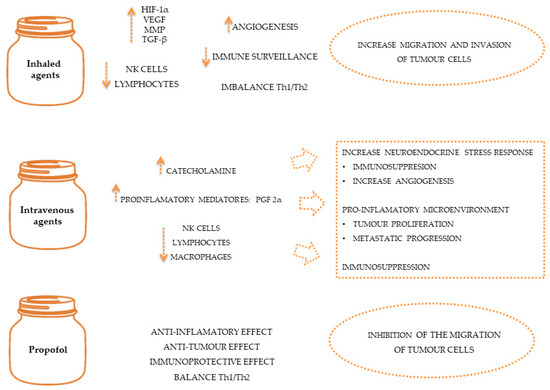
Figure 2. The effect of anaesthetics on immune system and on tumour spread.
This entry is adapted from the peer-reviewed paper 10.3390/ani13213392
This entry is offline, you can click here to edit this entry!
