Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jose M. Mulet | -- | 2994 | 2023-12-05 11:04:46 | | | |
| 2 | Rita Xu | Meta information modification | 2994 | 2023-12-06 02:44:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Boyno, G.; Rezaee Danesh, Y.; Demir, S.; Teniz, N.; Mulet, J.M.; Porcel, R. Arbuscular Mycorrhizal Fungi and Strigolactone. Encyclopedia. Available online: https://encyclopedia.pub/entry/52363 (accessed on 07 February 2026).
Boyno G, Rezaee Danesh Y, Demir S, Teniz N, Mulet JM, Porcel R. Arbuscular Mycorrhizal Fungi and Strigolactone. Encyclopedia. Available at: https://encyclopedia.pub/entry/52363. Accessed February 07, 2026.
Boyno, Gökhan, Younes Rezaee Danesh, Semra Demir, Necmettin Teniz, José M. Mulet, Rosa Porcel. "Arbuscular Mycorrhizal Fungi and Strigolactone" Encyclopedia, https://encyclopedia.pub/entry/52363 (accessed February 07, 2026).
Boyno, G., Rezaee Danesh, Y., Demir, S., Teniz, N., Mulet, J.M., & Porcel, R. (2023, December 05). Arbuscular Mycorrhizal Fungi and Strigolactone. In Encyclopedia. https://encyclopedia.pub/entry/52363
Boyno, Gökhan, et al. "Arbuscular Mycorrhizal Fungi and Strigolactone." Encyclopedia. Web. 05 December, 2023.
Copy Citation
Plants, the cornerstone of life on Earth, are constantly struggling with a number of challenges arising from both biotic and abiotic stressors. To overcome these adverse factors, plants have evolved complex defense mechanisms involving both a number of cell signaling pathways and a complex network of interactions with microorganisms. Among these interactions, the relationship between symbiotic arbuscular mycorrhizal fungi (AMF) and strigolactones (SLs) stands as an important interplay that has a significant impact on increased resistance to environmental stresses and improved nutrient uptake and the subsequent enhanced plant growth.
arbuscular mycorrhizal fungi
strigolactone
synergistic interaction
1. Introduction
Plants are pivotal for many ecosystems and thus essential for the survival of virtually all living organisms. They are not only a source of food for humans and animals and the main point of entrance of solar energy and organic carbon in ecosystems, but they also play a critical role in regulating the Earth’s climate and sustaining the planet’s biodiversity. However, plants are constantly under threat from various biotic and abiotic stresses, such as pests, diseases and environmental factors, like drought and salinity [1][2][3]. Under the current context of anthropogenic global warming, forest and cultivated plants must adapt to the novel conditions or become extinct [4][5]. To overcome these challenges, plants have evolved complex mechanisms that involve a wide range of signaling pathways and interactions with other organisms, including microbes.
The significance of arbuscular mycorrhizal fungi (AMF) and strigolactones (SLs) in plant–microbe interactions lies in their ability to positively influence plant growth, development and overall health [6][7]. AMF establish mutualistic associations with plants, colonizing their root systems and providing various benefits [8]. These benefits include enhanced nutrient acquisition, such as an increased availability of phosphorus and micronutrients, improved water uptake and protection against biotic and abiotic stresses [2]. AMF can also induce systemic resistance in plants, making them more resistant to pathogens and pests and abiotic stresses [9][10]. On the other hand, SLs, a class of plant hormones, regulate several critical processes in plants [11]. They are involved in shaping root architecture, promoting the development of lateral roots and stimulating the establishment of beneficial associations with symbiotic microbes, such as AMF [7][11][12]. SLs also play a role in plant defense responses, including the activation of systemic defense mechanisms against pathogens and the induction of plant secondary metabolites [13].
Recent studies have shown that the synergistic interaction between AMF and SLs can have a significant impact on plant–microbe interactions and plant development. The combined effects of these two components result in improved plant growth, an increased resistance to biotic and abiotic stresses and enhanced nutrient uptake [6][7][14]. Therefore, understanding the interplay between these two components is crucial for developing sustainable agricultural practices and improving crop yields.
Mechanistically, AMF have been found to influence the production and release of SLs by plants [15]. They can stimulate the synthesis and secretion of SLs, which act as signaling molecules to attract beneficial microbes and promote symbiotic interactions [16]. In turn, SLs can modulate the colonization and establishment of AMF within the plant root system, facilitating their beneficial effects [6][7][14]. The significance of this synergistic interaction becomes evident in its potential to improve crop productivity, nutrient utilization and plant resistance in the face of environmental challenges [6][14]. By harnessing the combined effects of AMF and SLs, agricultural practices can be optimized to enhance nutrient acquisition efficiency, leading to increased crop yields.
2. Overview of Arbuscular Mycorrhizal Fungi
Arbuscular mycorrhizal fungi extend their hyphae into the soil, exploring a larger volume and accessing nutrients inaccessible to the root [17][18]. In addition, AMF spore dynamics are found at higher densities in rhizosphere soil [19]. They can solubilize nutrients from solid soil particles and organic matter, making them available for plant uptake [20]. Furthermore, AMF release enzymes that break down complex organic compounds, releasing nutrients for plant uptake [21]. They can convert inorganic forms of macronutrients, such as phosphorus, into molecules that plants can assimilate [22]. This promotes efficient nutrient utilization by plants. Furthermore, AMF can affect the synthesis, release and signaling pathways of growth-promoting phytohormones, such as SLs, auxins and cytokinins in plants, leading to enhanced plant growth and development [15][23].
AMF can induce systemic resistance in plants, preparing them for pathogen and pest attacks. For example, AMF activate plant defense mechanisms by triggering the expression of defense-related genes [24]. This leads to the production of defense compounds, such as pathogenesis-related proteins and antimicrobial peptides that protect plants against pathogens and pests [25]. Furthermore, AMF prime the plant’s immune system, enabling a more rapid and effective defense response upon pathogen or pest attacks [25]. This priming improves the plant’s ability to recognize and respond to subsequent challenges, increasing its overall disease resistance.
2.1. Molecular Signaling
AMF are beneficial soil microorganisms that form symbiotic relationships with plants, enhancing nutrient uptake and contributing to the health and sustainability of terrestrial ecosystems [2]. In the early stages of AMF symbiosis, molecular signals are exchanged between the plant and the fungus [26][27]. The plant releases signaling molecules, such as SLs, into the soil in response to nutrient stress [28]. AMF hyphae in the soil sense these signals and initiate a molecular response [7][29]. This includes the expression of genes related to hyphal growth and colonization.
The growth and branching of AMF hyphae towards plant roots are regulated by various signaling pathways [17][18]. These pathways involve receptor proteins on the fungal hyphae that recognize specific plant signals. The interaction between plant root cells and AMF hyphae involves molecular cross-talk, allowing the hyphae to penetrate root cells [30]. This process involves the exchange of signaling molecules and the activation of genes that facilitate the establishment of arbuscules, specialized structures within root cells where nutrient exchange occurs [31].
Nutrient exchange in AMF symbiosis is highly dependent on molecular processes. Within arbuscules, specific transporters and channels facilitate the movement of nutrients between the plant and the fungus [7][28]. The plant provides sugars obtained by photosynthesis and organic compounds from the plant’s own metabolic pathways through molecular transporters, while the fungus supplies the plant with essential nutrients, like phosphorus and nitrogen, which the fungi have solubilized from the soil [32][33]. AMF also influence plant defense and stress responses at the molecular level [9]. They can enhance the plant’s ability to withstand various environmental stresses, such as biotic and abiotic [2][10]. The molecular mechanisms involved in these responses include the activation of defense-related genes and the modulation of plant hormone signaling pathways [9][34]. The molecular interactions between plants and AMF are highly intricate and involve the exchange of signaling molecules, gene regulation and the coordination of various molecular processes to establish and maintain this symbiotic relationship [34][35]. The synergy between the two organisms at the molecular level results in improved nutrient uptake and enhanced plant resilience.
2.2. Mycorrhizal Symbiosis Genes
Mycorrhizal symbiosis involves the interaction between plants and mycorrhizal fungi, and several genes in both partners are crucial for the establishment and maintenance of this symbiotic relationship (Figure 1). Some of the key genes and molecular components involved in mycorrhizal symbiosis are established below.
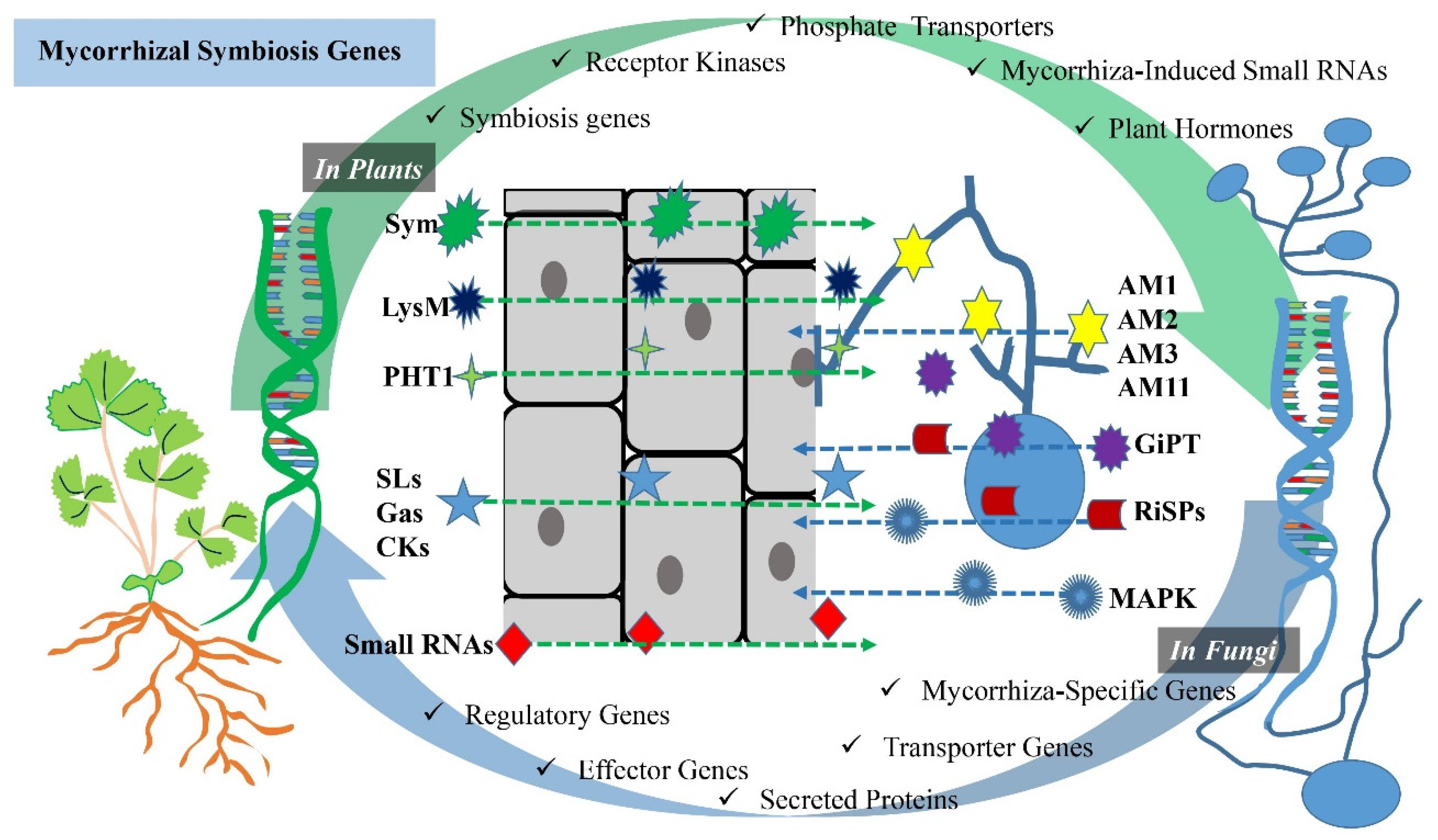
Figure 1. Schematic visualization of genes involved in AM symbiosis. The interaction between plants and AMF involves several genes and molecular components that are crucial for the establishment and maintenance of the symbiotic relationship. While Sym genes, receptor kinases, phosphate (Pi) transporters, mycorrhiza-derived small RNAs and plant hormones in plants are plant-derived genes and components, mycorrhiza-specific genes, transporter genes, secreted proteins, effector genes and regulatory genes are genes of a mycorrhizal fungi origin. These genes and molecular components in both plants and mycorrhizal fungi are essential for the successful establishment and maintenance of mycorrhizal symbiosis, which benefits both partners by improving nutrient exchange and enhancing plant growth.
2.2.1. In Plants
Sym genes (symbiosis genes): these are plant genes that are specifically involved in the establishment and regulation of mycorrhizal symbiosis [36]. They encode various proteins and transcription factors necessary for the recognition of fungal partners, the development of symbiotic structures and the regulation of nutrient exchange [37].
Receptor kinases: plant receptor kinases, such as the LysM receptor kinases, play a role in recognizing fungal signals and initiating the signaling cascade leading to mycorrhizal symbiosis [38][39]. These receptors are essential for the early recognition of mycorrhizal fungi [38].
Phosphate (Pi) transporters: plants upregulate genes encoding phosphate transporters in response to mycorrhizal colonization [40]. These transporters facilitate the uptake of phosphorus from the fungal partner [41].
Mycorrhiza-induced small RNAs: plants produce small RNAs in response to mycorrhizal colonization, which may play a role in regulating the symbiotic interaction [42].
Plant hormones: in general, plant hormones include activities directed towards the development and branching of plants. For example, strigolactones play an important role in stimulating branching activity in plants, contributing to the development of an extensive root system [43]. The gibberellin hormone promotes root elongation and influences plant growth and development [44]. Cytokinins are also involved in the regulation of plant branching. Cytokinins influence the overall architecture of the plant by promoting the growth of lateral buds [45]. Furthermore, various plant hormones, such as strigolactones, gibberellin and cytokinins, are involved in signaling and regulating mycorrhizal symbiosis [7][46][47]. Strigolactones, for example, are known to promote hyphal growth and root colonization by mycorrhizal fungi [6].
2.2.2. In Mycorrhizal Fungi
Mycorrhiza-specific genes: fungi possess genes that are specifically expressed during mycorrhizal symbiosis [48]. These genes are involved in hyphal growth, the establishment of symbiotic structures, like arbuscules, and nutrient exchange [48][49]. For example, Colard et al. [48] reported that AMF-specific AM1, AM2, AM3 and AM11 genes were activated at the pre-symbiotic stage.
Transporter genes: mycorrhizal fungi have transporter genes that code for proteins responsible for the uptake and transfer of nutrients to the plant host [50] (Table 1). For example, phosphate transporters are crucial for delivering phosphorus to the plant [51]. Maldonado-Mendoza et al. [52] revealed that this was thanks to the GiPT gene for Glomus intraradices.
Secreted proteins: fungi produce secreted proteins, some of which may be involved in facilitating the interaction with plant roots or modulating plant immune responses [53][54]. For example, Kamel et al.’s [55] study on Rhizophagus irregularis revealed that this species had a large number of putative secreted proteins (RiSPs), which could be of great importance in establishing symbiosis.
Effector genes: some mycorrhizal fungi may produce effector proteins that can manipulate plant host defenses or signaling pathways to promote symbiosis [56][57].
Regulatory genes: fungi have genes involved in the regulation of their responses to the plant host and environmental cues [58][59]. These genes help the fungi adapt to different plant partners and environmental conditions [60]. For example, Huang et al. [59] reported that using mitogen-activated protein kinase (MAPK) signaling for the interactions between AMF and apple plant hosts was shown to increase apple drought tolerance.
miRNA: there have also been some miRNAs identified as participants in this regulation, such as miR167, miR394 and miR156 [60].
Understanding the genetic and molecular basis of mycorrhizal symbiosis is an active area of research. These genes and molecular components play a critical role in the formation and maintenance of this beneficial mutualistic relationship, contributing to plant nutrient acquisition, stress tolerance and overall ecosystem health (Table 1).
Table 1. AMF-derived genes involved in mycorrhizal symbiosis.
| AMF-Induced Genes | References | ||
|---|---|---|---|
| Mycorrhiza-Specific Genes | AM1, AM2, AM3, AM11 | Genes active in the AMF-induced pre-symbiotic stage | [48] |
| AM10, AM14, AM15, AM20, AM24, AM25, AM26, AM29 | Genes active in the AMF-induced early and mature symbiotic stages | [49] | |
| Transporter Genes | GiPT | AMF-induced plant P transporter genes | [52] |
| StPT3 | [61] | ||
| OsPT11 | [62] | ||
| MtPT4 | [63] | ||
| PT11 | [49] | ||
| MtZIP5 | AMF-induced plant Zn transporter gene | [64] | |
| Secreted Proteins | LbMiSSP7 | Secreted proteins regulated by AMF | [65] |
| LjCLE19, LjCLE20 | [66] | ||
| RiSP | [55] | ||
| Effector Genes | RiSLM | AMF-induced effector genes | [67] |
| RirG175680, RirG165580, RirG263220, RirG200050, jgi.p|Gloin1|346360, RirG013260, RirG267270, jgi.p|Gloin1|154898, RirG043250, RirG045350, RirG101100, RirG043650, RirG257590, RirG187640, RirG180400, jgi.p|Gloin1|161262 | [68] | ||
| PvRxLR18, PvAVH52, PvRxLR28, PvRxLR67 | Effector genes against AMF-induced pathogen | [69] | |
| Regulatory Genes | 14-3-3 | Gene regulating AMF-induced ABA-related signaling pathway | [58] |
| MAPK | Genes regulated by AMF to enhance drought tolerance | [59] | |
| miR167, miR394, miR156 | [60] | ||
3. Strigolactones
Strigolactones (SLs), a class of plant hormones, have emerged as key regulators of plant growth, development and interactions with the environment. Striga lutea’s strigol, the first natural SL, was found as a germination stimulant; as a result, these compounds have subsequently been referred to as SLs [70]. They play a crucial role in various plant processes, such as root development, branching and responses to environmental stresses. These hormones are also known to interact with beneficial soil microorganisms, such as mycorrhizal fungi, to promote nutrient uptake and improve plant health. However, SLs secreted by plants cause the seeds of parasitic plants to germinate. This can cause problems in agricultural areas. In particular, the damage caused to agriculture by witchweed in Africa due to the parasitism on SL signaling is a major issue for farmers in developing countries [71].
3.1. Biosynthetic Pathway
According to Matusova et al. [72], SLs are derived from carotenoids, as evidenced by minimal SL accumulation after treatment with the carotenoid biosynthesis inhibitor fluridone in maize plants. The functional role of SLs can be related to their formation and production in response to the needs of the system during evolution [73]. Since it has been discovered that the gene involved in SL production has been reported for many plant species, including algae and bryophytes, it can be hypothesized that these SLs are important molecules that have long persisted in the evolutionary chain [73]. SLs are four-ring (A–D) compounds that change function by the attachment of various groups to the A and B rings [12][15][73][74]. SLs were initially thought to be sesquiterpene lactones, but were later shown to be apocarotenoid derivatives of carotenoid cleavage mediated by carotenoid cleavage dioxygenase (CCDs) enzymes [75]. A member of the CCD family is involved in the production of various apocarotenoid compounds, such as cyclohexenone and mycorradicin [76]. Initial biosynthesis occurs in plastids with the help of three plastid-specific enzymes: D (DWARF)27, CCD7 and CCD8. Carotenoid isomerase D27, carotenoid cleavage dioxygenases CCD7 and CCD8 and cytochrome P450 monooxygenases were identified as SL biosynthesis enzymes through genetic screening for shoot-branching mutants [15]. Furthermore, from mutants with excessive shoot branching, SL biosynthesis genes were found and called more axillary growth (MAX) in Arabidopsis thaliana [75], Ramosus (RMS) in Pisum sativum [77], decreased apical dominance (DAD) in petunia [78] and dwarf (D) in Oryza sativa [79][80].
D27 isomerase converts all-trans-β-carotene to 9-cys-β-carotene, and subsequent processes catalyzed by CCD7 and CCD8 convert 9-cys-β-carotene to carlactone (CL) with A- and D-ring structures [15][73]. CL is subsequently oxidized to different SL species by the cytochrome P450 monooxygenase MAX1 or other recently discovered enzymes. Briefly, researchers show the pathway of SL biosynthesis in Figure 2. Furthermore, not only enzymes downstream of CL but also enzymes upstream of CL may be structurally important for the formation of various SLs in SL biosynthesis. CCD7 and CCD8 carotenoid isomerases convert all-trans-carotene to CL as well as 3-hydroxy-carlactone (3-OH-CL) via zeaxanthin [81]. Although hydroxy-carlactone derivatives are the most common SLs in Arabidopsis [82], their significance for plant growth and development control is unknown.
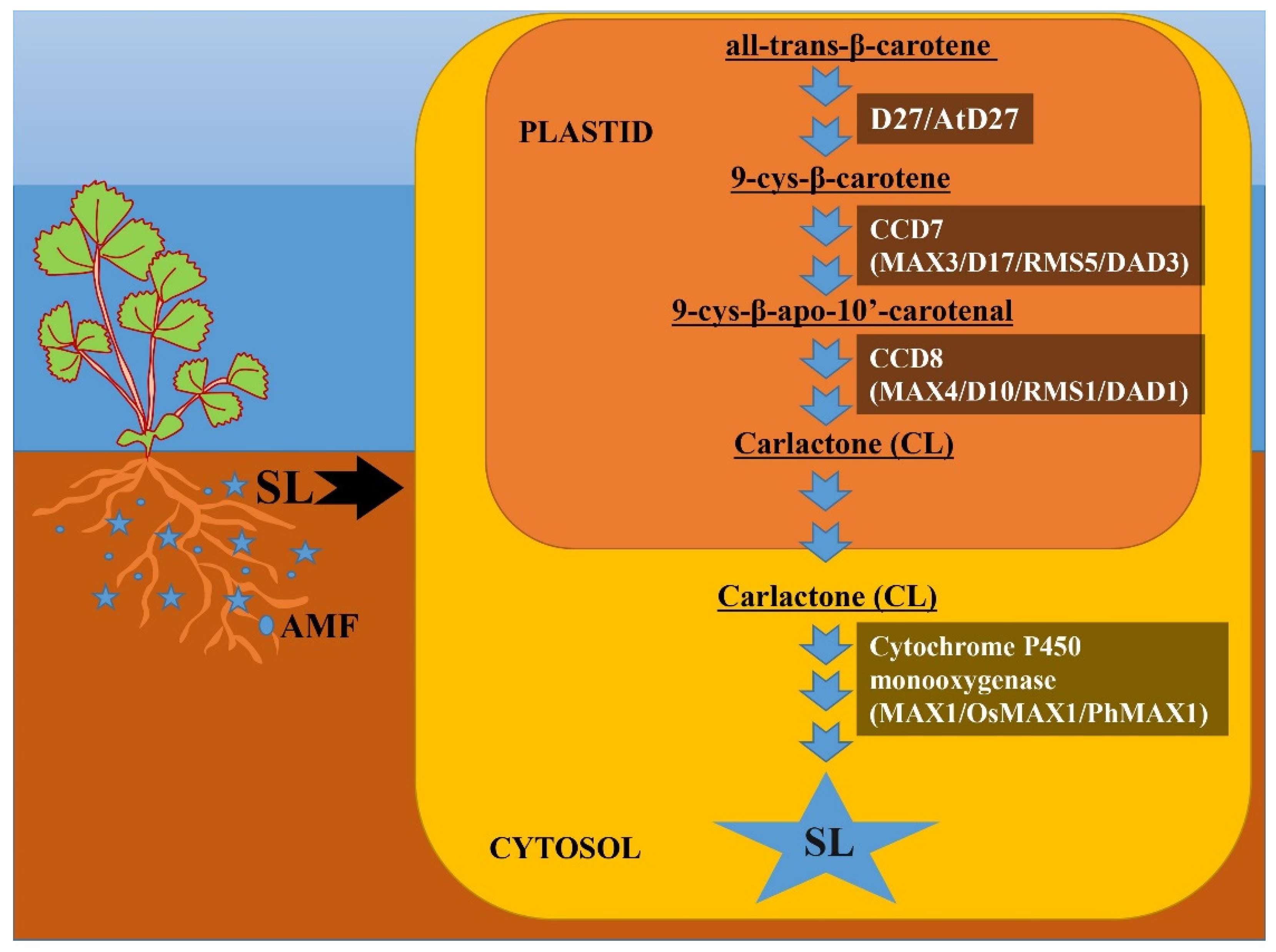
Figure 2. Biosynthetic pathway of strigolactone (SL). This figure depicts the SL biosynthetic route and important enzymes involved in biosynthesis. SL biosynthesis occurs in two distinct compartments: plastid and cytosol. All-trans-β-carotene is converted to carlactone (CL) in plastids via three intermediary stages catalyzed by D27 (At27), CCD7 (MAX3, D17, RMS5, DAD3) and CCD8 (MAX4, D10, RMS1, DAD1), respectively. Carlactone then enters the cytosol, where it is metabolized to several other SLs via cytochrome P450 monooxygenase (MAX1, OsMAX1, PhMAX1) and numerous other unidentified enzymes.
Recent studies have focused on modifying genes in the Strigolactone biosynthesis pathway using CRISPR/Cas9 gene-editing techniques [83][84][85]. Such genetic modifications can affect the biological processes of plants, such as root development, water use and nutrient uptake, and hence increase their interaction with AMF and abiotic stress tolerance. For example, the carotenoid cleavage dioxygenase 8 (CCD8) genes (SbCCD8a and SbCCD8b), which have been shown to be involved in strigolactone biosynthesis in sorghum, were manipulated by two CRISPR/Cas9-mediated genes and were found to enhance weed control and the activity of beneficial microorganisms [85].
3.2. Physiological Functions
Symbiotic interactions: SLs are involved in the establishment of symbiotic associations, particularly with arbuscular mycorrhizal fungi [6][12][14]. They act as rhizosphere signaling molecules, attracting AMF hyphae towards the plant roots and stimulating the branching of fungal hyphae in the soil. Akiyama et al. [86] demonstrated that sesquiterpenes, 5-deoxy-strigol, sorgolactone and strigol extracted from Lotus japonicus exudates promoted extended hyphal branching in AMF. A comparable finding was also seen in the synthetic counterpart of sorgolactone [87]. This association enhanced nutrient uptake, particularly phosphorus, by increasing the surface area available for nutrient absorption [6][7][14]. SLs also regulate the development of symbiotic structures, such as arbuscules, which facilitate nutrient exchange between the fungus and the plant [7].
Plant defense responses: SLs contribute to plant defense responses against pathogens and pests [88]. They can prime plants for enhanced resistance by activating defense-related genes and signaling pathways [89]. For example, Nasir et al. [90] found that SLs positively regulated defense against Magnaporthe oryzae in rice, and Xu et al. [91] positively regulated defense against root-knot nematodes in tomatoes. SLs can also influence the synthesis of secondary metabolites involved in plant defense mechanisms [92].
Shoot branching: one of the well-known roles of SLs is their influence on shoot branching. SLs act as inhibitors of bud outgrowth, promoting apical dominance and limiting the growth of lateral buds [93]. By suppressing the branching of shoots, SLs ensure the allocation of resources to the main shoot and control the overall architecture of the plant [43]. Manipulating SL levels or signaling can lead to alterations in shoot-branching patterns and can be utilized to enhance branching or promote lateral bud growth when desired [94]. For example, the involvement of SLs in regulating bud growth in Pisum sativum and Arabidopsis thaliana was demonstrated by Brewer et al. [95].
Overall plant growth and development: SLs have broader effects on plant growth and development. They contribute to various processes, such as seed germination, stomatal closure and responses to environmental stresses [96][97][98]. For example, Ha et al. [99] showed that the genetic modulation of SL content/response could provide a new approach for the development of plants with better environmental stress tolerance. SLs can affect seed dormancy and germination by inhibiting or promoting the germination process, depending on the species and environmental conditions [100]. They also regulate stomatal aperture, reducing water loss through transpiration and aiding in water-use efficiency [97]. SLs influence plant responses to abiotic stresses, such as drought and salinity, by modulating physiological and molecular responses that enhance stress tolerance [99].
References
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: Rijeka, Croatia, 2019; pp. 1–19.
- Demir, S.; Danesh, Y.R.; Boyno, G.; Najafi, S. Arbuscular mycorrhizal fungi in biotic and abiotic stress conditions: Function and management in horticulture. In Sustainable Horticulture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 157–183.
- Teniz, N.; Demirer Durak, E. Van’ın Erciş, Gevaş ve Edremit ilçelerinde Biber, Domates ve Kavundan Fusarium spp. ve Rhizoctonia spp.’nin Teşhisi ve Patojeniteleri. Yüzüncü Yıl Üniversitesi Fen Bilim. Enstitüsü Derg. 2023, 28, 704–714.
- Taïbi, K.; Del Campo, A.; Mulet, J.; Flors, J.; Aguado, A. Testing Aleppo pine seed sources response to climate change by using trial sites reflecting future conditions. New For. 2014, 45, 603–624.
- Chevilly, S.; Dolz-Edo, L.; Martínez-Sánchez, G.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Blanca, J.; Yenush, L.; Mulet, J.M. Distinctive traits for drought and salt stress tolerance in melon (Cucumis melo L.). Front. Plant Sci. 2021, 12, 2471.
- Mitra, D.; BE, G.S.; Khoshru, B.; De Los Santos Villalobos, S.; Belz, C.; Chaudhary, P.; Shahri, F.N.; Djebaili, R.; Adeyemi, N.O.; El-Ballat, E.M. Impacts of arbuscular mycorrhizal fungi on rice growth, development, and stress management with a particular emphasis on strigolactone effects on root development. Commun. Soil Sci. Plant Anal. 2021, 52, 1591–1621.
- Boyno, G.; Demir, S. Plant-mycorrhiza communication and mycorrhizae in inter-plant communication. Symbiosis 2022, 86, 155–168.
- Kalamulla, R.; Karunarathna, S.C.; Tibpromma, S.; Galappaththi, M.C.; Suwannarach, N.; Stephenson, S.L.; Asad, S.; Salem, Z.S.; Yapa, N. Arbuscular mycorrhizal fungi in sustainable agriculture. Sustainability 2022, 14, 12250.
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664.
- Boyno, G.; Demir, S.; Danesh, Y.R. Effects of some biological agents on the growth and biochemical parameters of tomato plants infected with Alternaria solani (Ellis & Martin) Sorauer. Eur. J. Plant Pathol. 2022, 162, 19–29.
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant hormones with promising features. Angew Chem. Int. Ed. 2019, 58, 12778–12786.
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010, 51, 1104–1117.
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007, 12, 1290–1306.
- Badri, D.V.; Weir, T.L.; van der Lelie, D.; Vivanco, J.M. Rhizosphere chemical dialogues: Plant–Microbe interactions. COBIOT 2009, 20, 642–650.
- Yoneyama, K.; Brewer, P.B. Strigolactones, how are they synthesized to regulate plant growth and development? Curr. Opin. Plant Biol. 2021, 63, 102072.
- Rochange, S.; Goormachtig, S.; Lopez-Raez, J.A.; Gutjahr, C. The role of strigolactones in plant–microbe interactions. In Strigolactones-Bilogy and Aplications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–142.
- Bagyaraj, D.; Sharma, M.P.; Maiti, D. Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr. Sci. 2015, 108, 1288–1293.
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881.
- Boyno, G.; Demir, S.; Rezaee Danesh, Y.; Durak, E.D.; Çevik, R.; Farda, B.; Djebaili, R.; Pellegrini, M. A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere. J. Fungi 2023, 9, 845.
- Wen, Z.; Chen, Y.; Liu, Z.; Meng, J. Biochar and arbuscular mycorrhizal fungi stimulate rice root growth strategy and soil nutrient availability. Eur. J. Soil Biol. 2022, 113, 103448.
- Qin, M.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Peng, Z.; Yang, Y.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2020, 71, 84–92.
- Reynolds, H.L.; Vogelsang, K.M.; Hartley, A.E.; Bever, J.D.; Schultz, P. Variable responses of old-field perennials to arbuscular mycorrhizal fungi and phosphorus source. Oecologia 2006, 147, 348–358.
- Selwal, N.; Wani, A.K.; Akhtar, N.; Kaur, M.; Jassal, P.S. Molecular insights of Strigolactone biosynthesis, signalling pathways, regulatory roles, and hormonal crosstalks in plant systems. S. Afr. J. Bot. 2023, 160, 9–22.
- Khan, M.H.; Meghvansi, M.; Panwar, V.; Gogoi, H.; Singh, L. Arbuscular mycorrhizal fungi-induced signalling in plant defence against phytopathogens. J. Phytol. 2010, 2, 53–69.
- Pozo, M.J.; Jung, S.C.; López-Ráez, J.A.; Azcón-Aguilar, C. Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: The role of plant defence mechanisms. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–207.
- Gianinazzi-Pearson, V.; Séjalon-Delmas, N.; Genre, A.; Jeandroz, S.; Bonfante, P. Plants and arbuscular mycorrhizal fungi: Cues and communication in the early steps of symbiotic interactions. Adv. Bot. Res. 2007, 46, 181–219.
- Schmitz, A.M.; Harrison, M.J. Signaling events during initiation of arbuscular mycorrhizal symbiosis. J. Integr. Plant Biol. 2014, 56, 250–261.
- Crosino, A.; Genre, A. Peace talks: Symbiotic signaling molecules in arbuscular mycorrhizas and their potential application. J. Plant Interact. 2022, 17, 824–839.
- Requena, N.; Serrano, E.; Ocón, A.; Breuninger, M. Plant signals and fungal perception during arbuscular mycorrhiza establishment. Phytochemistry 2007, 68, 33–40.
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 21, 1739–1760.
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142.
- Luo, X.; Liu, Y.; Li, S.; He, X. Interplant carbon and nitrogen transfers mediated by common arbuscular mycorrhizal networks: Beneficial pathways for system functionality. Front. Plant Sci. 2023, 14, 1169310.
- Zhang, Y.; Feng, H.; Druzhinina, I.S.; Xie, X.; Wang, E.; Martin, F.; Yuan, Z. Phosphorus/nitrogen sensing and signaling in diverse root–fungus symbioses. Trends Microbiol. 2023.
- Vos, C.; Schouteden, N.; Van Tuinen, D.; Chatagnier, O.; Elsen, A.; De Waele, D.; Panis, B.; Gianinazzi-Pearson, V. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 2013, 60, 45–54.
- Zhuang, X.; Gao, J.; Ma, A.; Fu, S.; Zhuang, G. Bioactive molecules in soil ecosystems: Masters of the underground. Int. J. Mol. Sci. 2013, 14, 8841–8868.
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48.
- Banba, M.; Gutjahr, C.; Miyao, A.; Hirochika, H.; Paszkowski, U.; Kouchi, H.; Imaizumi-Anraku, H. Divergence of evolutionary ways among common sym genes: CASTOR and CCaMK show functional conservation between two symbiosis systems and constitute the root of a common signaling pathway. Plant Cell Physiol. 2008, 49, 1659–1671.
- Zhang, X.; Dong, W.; Sun, J.; Feng, F.; Deng, Y.; He, Z.; Oldroyd, G.E.; Wang, E. The receptor kinase CERK 1 has dual functions in symbiosis and immunity signalling. Plant J. 2015, 81, 258–267.
- Kelly, S.; Radutoiu, S.; Stougaard, J. Legume LysM receptors mediate symbiotic and pathogenic signalling. Curr. Opin. Plant Biol. 2017, 39, 152–158.
- Walder, F.; Brulé, D.; Koegel, S.; Wiemken, A.; Boller, T.; Courty, P.E. Plant phosphorus acquisition in a common mycorrhizal network: Regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol. 2015, 205, 1632–1645.
- Christophersen, H.; Smith, F.; Smith, S. Arbuscular mycorrhizal colonization reduces arsenate uptake in barley via downregulation of transporters in the direct epidermal phosphate uptake pathway. New Phytol. 2009, 184, 962–974.
- Devers, E.A.; Branscheid, A.; May, P.; Krajinski, F. Stars and symbiosis: microRNA-and microRNA*-mediated transcript cleavage involved in arbuscular mycorrhizal symbiosis. Plant Physiol. 2011, 156, 1990–2010.
- Waldie, T.; McCulloch, H.; Leyser, O. Strigolactones and the control of plant development: Lessons from shoot branching. Plant J. 2014, 79, 607–622.
- Wang, G.-L.; Que, F.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290.
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435.
- Foo, E.; Ross, J.J.; Jones, W.T.; Reid, J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013, 111, 769–779.
- Giron, D.; Frago, E.; Glevarec, G.; Pieterse, C.M.; Dicke, M. Cytokinins as key regulators in plant–microbe–insect interactions: Connecting plant growth and defence. Funct. Ecol. 2013, 27, 599–609.
- Colard, A.; Angelard, C.; Sanders, I.R. Genetic exchange in an arbuscular mycorrhizal fungus results in increased rice growth and altered mycorrhiza-specific gene transcription. AEM 2011, 77, 6510–6515.
- Gutjahr, C.; Banba, M.; Croset, V.; An, K.; Miyao, A.; An, G.; Hirochika, H.; Imaizumi-Anraku, H.; Paszkowski, U. Arbuscular mycorrhiza–specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 2008, 20, 2989–3005.
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.B.; Xu, G.; Jakobsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250.
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057.
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. MPMI 2001, 14, 1140–1148.
- Zamioudis, C.; Pieterse, C.M. Modulation of host immunity by beneficial microbes. MPMI 2012, 25, 139–150.
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front Microbiol. 2020, 11, 1298.
- Kamel, L.; Tang, N.; Malbreil, M.; San Clemente, H.; Le Marquer, M.; Roux, C.; Frei dit Frey, N. The comparison of expressed candidate secreted proteins from two arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Front. Plant Sci. 2017, 8, 124.
- De Wit, P.J.; Mehrabi, R.; Van den Burg, H.A.; Stergiopoulos, I. Fungal effector proteins: Past, present and future. Mol. Plant Pathol. 2009, 10, 735–747.
- Sędzielewska Toro, K.; Brachmann, A. The effector candidate repertoire of the arbuscular mycorrhizal fungus Rhizophagus clarus. BMC Genom. 2016, 17, 101.
- Li, T.; Sun, Y.; Ruan, Y.; Xu, L.; Hu, Y.; Hao, Z.; Zhang, X.; Li, H.; Wang, Y.; Yang, L. Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 2016, 26, 879–893.
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255.
- Fileccia, V.; Ingraffia, R.; Amato, G.; Giambalvo, D.; Martinelli, F. Identification of microRNAS differentially regulated by water deficit in relation to mycorrhizal treatment in wheat. Mol. Biol. Rep. 2019, 46, 5163–5174.
- Rausch, C.; Daram, P.; Brunner, S.; Jansa, J.; Laloi, M.; Leggewie, G.; Amrhein, N.; Bucher, M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 2001, 414, 462–465.
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329.
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725.
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: A physiological and molecular assessment. Sci. Rep. 2019, 9, 14880.
- Plett, J.M.; Kemppainen, M.; Kale, S.D.; Kohler, A.; Legué, V.; Brun, A.; Tyler, B.M.; Pardo, A.G.; Martin, F. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 2011, 21, 1197–1203.
- Handa, Y.; Nishide, H.; Takeda, N.; Suzuki, Y.; Kawaguchi, M.; Saito, K. RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant Cell Physiol. 2015, 56, 1490–1511.
- Schmitz, A.M.; Pawlowska, T.E.; Harrison, M.J. A short LysM protein with high molecular diversity from an arbuscular mycorrhizal fungus, Rhizophagus irregularis. Mycoscience 2018, 60, 63–70.
- Zeng, T.; Holmer, R.; Hontelez, J.; te Lintel-Hekkert, B.; Marufu, L.; de Zeeuw, T.; Wu, F.; Schijlen, E.; Bisseling, T.; Limpens, E. Host-and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant J. 2018, 94, 411–425.
- Cruz-Silva, A.; Figueiredo, A.; Sebastiana, M. First insights into the effect of mycorrhizae on the expression of pathogen effectors during the infection of grapevine with Plasmopara viticola. Sustainability 2021, 13, 1226.
- Cook, C.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199.
- Li, C.; Dong, L.; Durairaj, J.; Guan, J.-C.; Yoshimura, M.; Quinodoz, P.; Horber, R.; Gaus, K.; Li, J.; Setotaw, Y.B. Maize resistance to witchweed through changes in strigolactone biosynthesis. Science 2023, 379, 94–99.
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant. Physiol. 2005, 139, 920–934.
- Mishra, S.; Upadhyay, S.; Shukla, R.K. The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front. Physiol. 2017, 7, 691.
- Boyer, F.-D.; de Saint Germain, A.; Pillot, J.-P.; Pouvreau, J.-B.; Chen, V.X.; Ramos, S.; Stévenin, A.; Simier, P.; Delavault, P.; Beau, J.-M. Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: Molecule design for shoot branching. Plant Physiol. 2012, 159, 1524–1544.
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449.
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321.
- Beveridge, C.A.; Symons, G.M.; Turnbull, C.G. Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 2000, 123, 689–698.
- Simons, J.L.; Napoli, C.A.; Janssen, B.J.; Plummer, K.M.; Snowden, K.C. Analysis of the decreased apical dominance genes of petunia in the control of axillary branching. Plant Physiol. 2007, 143, 697–706.
- Zou, J.; Zhang, S.; Zhang, W.; Li, G.; Chen, Z.; Zhai, W.; Zhao, X.; Pan, X.; Xie, Q.; Zhu, L. The rice HIGH—TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006, 48, 687–698.
- Zhang, Y.; Van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; Van Der Krol, S.; Leyser, O. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033.
- Baz, L.; Mori, N.; Mi, J.; Jamil, M.; Kountche, B.A.; Guo, X.; Balakrishna, A.; Jia, K.-P.; Vermathen, M.; Akiyama, K. 3-Hydroxycarlactone, a novel product of the strigolactone biosynthesis core pathway. Mol. Plant 2018, 11, 1312–1314.
- Yoneyama, K.; Akiyama, K.; Brewer, P.B.; Mori, N.; Kawano-Kawada, M.; Haruta, S.; Nishiwaki, H.; Yamauchi, S.; Xie, X.; Umehara, M. Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct. 2020, 4, e00219.
- Butt, H.; Jamil, M.; Wang, J.Y.; Al-Babili, S.; Mahfouz, M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018, 18, 174.
- Bari, V.K.; Nassar, J.A.; Kheredin, S.M.; Gal-On, A.; Ron, M.; Britt, A.; Steele, D.; Yoder, J.; Aly, R. CRISPR/Cas9-mediated mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in tomato provides resistance against the parasitic weed Phelipanche aegyptiaca. Sci. Rep. 2019, 9, 11438.
- Hao, J.; Yang, Y.; Futrell, S.; Kelly, E.A.; Lorts, C.M.; Nebie, B.; Runo, S.; Yang, J.; Alvarez, S.; Lasky, J.R. CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase (CCD) genes in Sorghum alters strigolactone biosynthesis and plant biotic interactions. Phytobiomes J. 2023, 7, 339–351.
- Akiyama, K.; Matsuzaki, K.-i.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827.
- Liu, W.; Kohlen, W.; Lillo, A.; Op den Camp, R.; Ivanov, S.; Hartog, M.; Limpens, E.; Jamil, M.; Smaczniak, C.; Kaufmann, K. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 2011, 23, 3853–3865.
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014, 15, 211–216.
- Kalliola, M.; Jakobson, L.; Davidsson, P.; Pennanen, V.; Waszczak, C.; Yarmolinsky, D.; Zamora, O.; Palva, E.T.; Kariola, T.; Kollist, H. Differential role of MAX2 and strigolactones in pathogen, ozone, and stomatal responses. Plant Direct. 2020, 4, e00206.
- Nasir, F.; Tian, L.; Shi, S.; Chang, C.; Ma, L.; Gao, Y.; Tian, C. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Biochem. 2019, 142, 106–116.
- Xu, X.; Fang, P.; Zhang, H.; Chi, C.; Song, L.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Strigolactones positively regulate defense against root-knot nematodes in tomato. J. Exp. Bot. 2019, 70, 1325–1337.
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992.
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944.
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28.
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493.
- Toh, S.; Kamiya, Y.; Kawakami, N.; Nambara, E.; McCourt, P.; Tsuchiya, Y. Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol. 2012, 53, 107–117.
- Zhang, Y.; Lv, S.; Wang, G. Strigolactones are common regulators in induction of stomatal closure in planta. Plant Signal. Behav. 2018, 13, e1444322.
- Tariq, A.; Ullah, I.; Sardans, J.; Graciano, C.; Mussarat, S.; Ullah, A.; Zeng, F.; Wang, W.; Al-Bakre, D.A.; Ahmed, Z. Strigolactones can be a potential tool to fight environmental stresses in arid lands. Environ. Res. 2023, 229, 115966.
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856.
- Waters, M.T.; Smith, S.M.; Nelson, D.C. Smoke signals and seed dormancy: Where next for MAX2? Plant Signal. Behav. 2011, 6, 1418–1422.
More
Information
Subjects:
Biochemistry & Molecular Biology; Agronomy; Horticulture
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
979
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




