
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carl Philpott | -- | 1678 | 2023-12-05 10:45:02 | | | |
| 2 | Jessie Wu | Meta information modification | 1678 | 2023-12-06 06:09:24 | | |
Video Upload Options
Olfactory dysfunction affects approximately 20% of the population globally, with incidence increasing over the age of 60. The pathophysiology is complex, not yet fully understood, and depends on many factors, including the underlying cause. Despite this, the present literature on olfaction is limited due to significant heterogeneity in methodological approaches. This has resulted in limited effective treatments available for olfactory dysfunction. Medications for olfactory dysfunction can be administered locally (directly to the olfactory epithelium) or systemically (orally or intravenously). There are various methods for local drug delivery to the olfactory epithelium (nasal drops, nasal sprays, atomisers, pressured meter-dosed inhalers, rinses, and exhalation delivery systems).
1. Introduction
2. Nasal Sprays
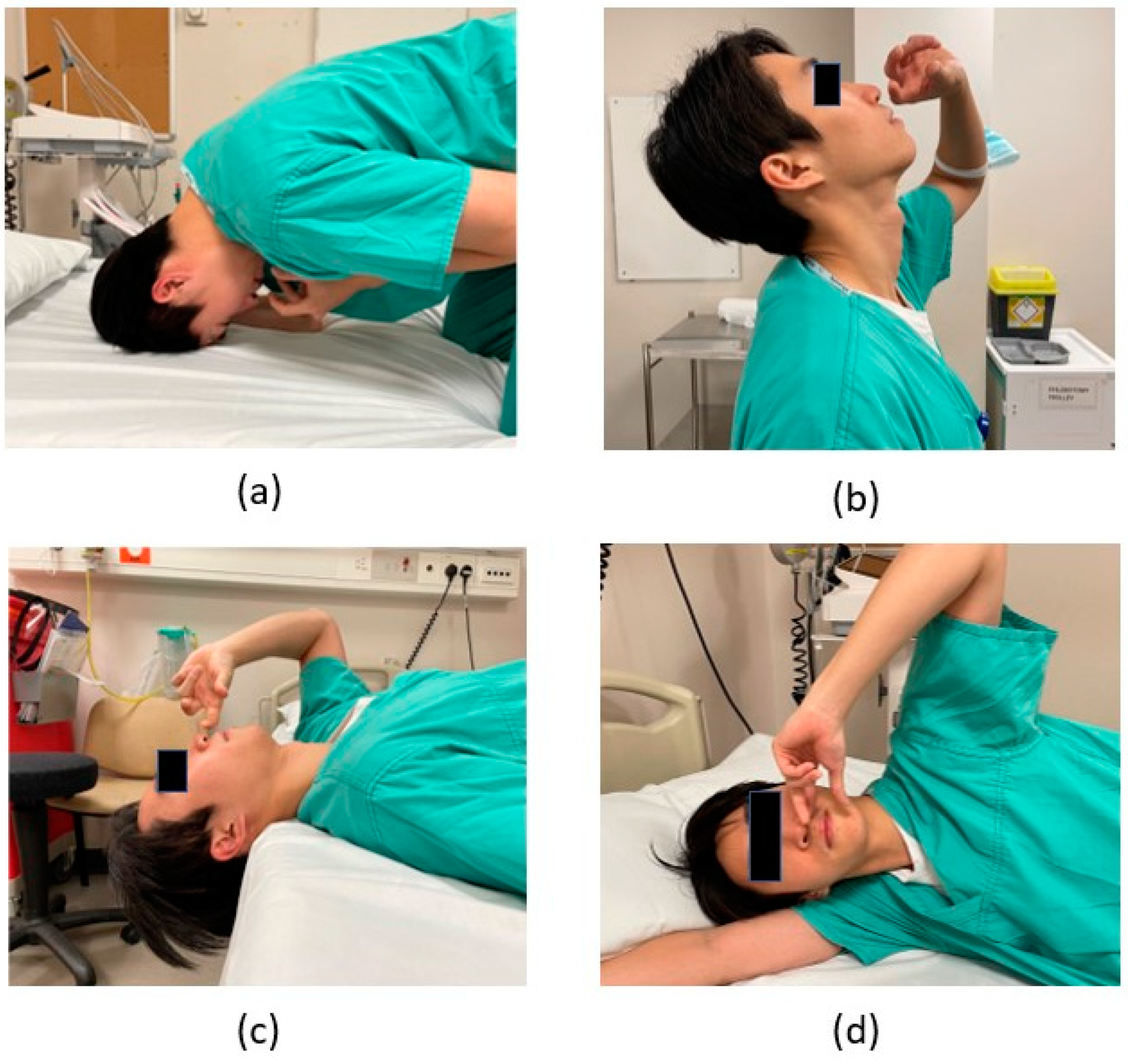
3. Nasal Drops with Various Head Positions
4. Rinses/Irrigation
5. Atomisers
6. The Exhalation Delivery System (OptiNose)
7. Direct Administration under Endoscopic Guidance
References
- Scheibe, M.; Bethge, C.; Witt, M.; Hummel, T. Intranasal Administration of Drugs. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 643–646.
- Cheng, Y.; Holmes, T.; Gao, J.; Guilmette, R.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of Nasal Spray Pumps and Deposition Pattern in a Replica of the Human Nasal Airway. J. Aerosol Med. 2001, 14, 267–280.
- Benninger, M.S.; Hadley, J.A.; Osguthorpe, J.D.; Marple, B.F.; Leopold, D.A.; Derebery, M.J.; Hannley, M. Techniques of Intranasal Steroid Use. Otolaryngol.-Head Neck Surg. 2004, 130, 5–24.
- Hardy, J.G.; Lee, S.W.; Wilson, C.G. Intranasal drug delivery by spray and drops. J. Pharm. Pharmacol. 2011, 37, 294–297.
- Newman, S.P.; Morén, F.; Clarke, S.W. Deposition pattern of nasal sprays in man. Rhinology 1988, 26, 112–120.
- Weber, R.; Keerl, R.; Radziwill, R.; Schick, B.; Jaspersen, D.; Dshambazov, K.; Mlynski, G.; Draf, W. Videoendoscopic analysis of nasal steroid distribution. Rhinology 1999, 37, 69–73.
- Heilmann, S.; Huettenbrink, K.-B.; Hummel, T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am. J. Rhinol. 2018, 18, 29–33.
- Muenkaew, Y.; Tangbumrungtham, N.; Roongpuvapaht, B.; Tanjararak, K. Comparison of sinus distribution between nasal irrigation and nasal spray using fluorescein-labelled in patients with chronic rhinosinusitis: A randomised clinical trial. Clin. Otolaryngol. 2023, 48, 286–293.
- Kayarkar, R.; Clifton, N.; Woolford, T. An evaluation of the best head position for instillation of steroid nose drops. Clin. Otolaryngol. Allied Sci. 2002, 27, 18–21.
- Trabut, S.; Friedrich, H.; Caversaccio, M.; Negoias, S. Challenges in topical therapy of chronic rhinosinusitis: The case of nasal drops application—A systematic review. Auris Nasus Larynx 2020, 47, 536–543.
- Morén, F.; Björnek, K.; Klint, T.; Wagner, Z.G. A comparative distribution study of two procedures for administration of nose drops. Acta Oto-Laryngol. 2009, 106, 286–290.
- Kubba, H.; Spinou, E.; Robertson, A. The Effect of Head Position on the Distribution of Drops within the Nose. Am. J. Rhinol. 2018, 14, 83–86.
- Mori, E.; Merkonidis, C.; Cuevas, M.; Gudziol, V.; Matsuwaki, Y.; Hummel, T. The administration of nasal drops in the “Kaiteki” position allows for delivery of the drug to the olfactory cleft: A pilot study in healthy subjects. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 939–943.
- Milk, D.G.; Khong, G.C.; Çam, O.H.; Alfaro-Iraheta, F.; Tierney, C.; Kassem, F.; Leong, S.C. A Comparison between Mygind and Kaiteki positions in administration of drops to the olfactory cleft. Clin. Otolaryngol. 2021, 46, 406–411.
- Rawal, R.B.; Deal, A.M.; Ebert, C.S.; Dhandha, V.H.; Mitchell, C.A.; Hang, A.X.; Gore, M.R.; Senior, B.A.; Zanation, A.M. Post-operative budesonide irrigations for patients with polyposis: A blinded, randomized controlled trial. Rhinology 2015, 53, 227–234.
- Rotenberg, B.W.; Zhang, I.; Arra, I.; Payton, K.B. Postoperative care for Samter’s triad patients undergoing endoscopic sinus surgery: A double-blinded, randomized controlled trial. Laryngoscope 2011, 121, 2702–2705.
- Harvey, R.J.; Snidvongs, K.; Kalish, L.H.; Oakley, G.M.; Sacks, R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int. Forum Allergy Rhinol. 2018, 8, 461–470.
- Soudry, E.; Wang, J.; Vaezeafshar, R.; Katznelson, L.; Hwang, P.H. Safety analysis of long-term budesonide nasal irrigations in patients with chronic rhinosinusitis post endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2016, 6, 568–572.
- Thamboo, A.; Manji, J.; Szeitz, A.; Santos, R.D.; Hathorn, I.; Gan, E.C.; Alsaleh, S.; Javer, A.R. The safety and efficacy of short-term budesonide delivered via mucosal atomization device for chronic rhinosinusitis without nasal polyposis. Int. Forum Allergy Rhinol. 2014, 4, 397–402.
- Habib, A.-R.R.; Thamboo, A.; Manji, J.; Santos, R.C.D.; Gan, E.C.; Anstead, A.; Javer, A.R. The effect of head position on the distribution of topical nasal medication using the Mucosal Atomization Device: A cadaver study. Int. Forum Allergy Rhinol. 2013, 3, 958–962.
- Cannady, S.B.; Batra, P.S.; Citardi, M.J.; Lanza, D.C. Comparison of Delivery of Topical Medications to the Paranasal Sinuses via “Vertex-to-floor” Position and Atomizer Spray after FESS. Otolaryngol.-Head Neck Surg. 2005, 133, 735–740.
- Exhalation Delivery Systems. Optinose. Available online: https://www.optinose.com/exhalation-delivery-systems/technical-overview/ (accessed on 1 December 2022).
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62.
- Sher, M.R.; Mair, E.A.; Messina, J.; Carothers, J.; Mahmoud, R.; Djupesland, P.G. EXHANCE-3: A Phase 3, Three-Month Study of Safety and Efficacy of Fluticasone Propionate Exhalation Delivery System (FLU-EDS) in Patients with Chronic Rhinosinusitis with (CRSwNP) and without Nasal Polyps (CRSsNP). J. Allergy Clin. Immunol. 2017, 139, AB66.
- Vlckova, I.; Navratil, P.; Kana, R.; Pavlicek, P.; Chrbolka, P.; Djupesland, P.G. Effective treatment of mild-to-moderate nasal polyposis with fluticasone delivered by a novel device. Rhinol. J. 2009, 47, 419–426.
- Leopold, D.A.; Elkayam, D.; Messina, J.C.; Kosik-Gonzalez, C.; Djupesland, P.G.; Mahmoud, R.A. Navigate II: Randomized, double-blind trial of the exhalation delivery system with fluticasone for nasal polyposis. J. Allergy Clin. Immunol. 2019, 143, 126–134.e5.
- Kuan, E.C.; Kovacs, A.J.; Workman, A.D.; Bosso, J.V.; Adappa, N.D. Efficacy of fluticasone exhalation delivery system in the management of chronic rhinosinusitis: What is the evidence? Int. Forum Allergy Rhinol. 2019, 9, S16–S21.
- Rezaeian, A. Effect of Intranasal Insulin on Olfactory Recovery in Patients with Hyposmia: A Randomized Clinical Trial. Otolaryngol.-Head Neck Surg. 2018, 158, 1134–1139.




