Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shaojie Shi | -- | 1232 | 2023-12-05 02:27:04 | | | |
| 2 | Camila Xu | Meta information modification | 1232 | 2023-12-05 02:37:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shi, S.; Wang, H.; Zha, W.; Wu, Y.; Liu, K.; Xu, D.; He, G.; Zhou, L.; You, A. BPH-Resistance Gene Mapping. Encyclopedia. Available online: https://encyclopedia.pub/entry/52350 (accessed on 07 February 2026).
Shi S, Wang H, Zha W, Wu Y, Liu K, Xu D, et al. BPH-Resistance Gene Mapping. Encyclopedia. Available at: https://encyclopedia.pub/entry/52350. Accessed February 07, 2026.
Shi, Shaojie, Huiying Wang, Wenjun Zha, Yan Wu, Kai Liu, Deze Xu, Guangcun He, Lei Zhou, Aiqing You. "BPH-Resistance Gene Mapping" Encyclopedia, https://encyclopedia.pub/entry/52350 (accessed February 07, 2026).
Shi, S., Wang, H., Zha, W., Wu, Y., Liu, K., Xu, D., He, G., Zhou, L., & You, A. (2023, December 05). BPH-Resistance Gene Mapping. In Encyclopedia. https://encyclopedia.pub/entry/52350
Shi, Shaojie, et al. "BPH-Resistance Gene Mapping." Encyclopedia. Web. 05 December, 2023.
Copy Citation
The brown planthopper (Nilaparvata lugens Stål, BPH) is one of the most serious pests that harm rice production. N. lugens soaks up phloem sap by inserting needle-like stylets into the vascular tissue of rice (Oryza sativa L.). Utilizing the inherent resistance has been widely considered as the most cost-effective method for sustainable BPH control. To date, more than 49 BPH-resistance genes/QTLs have been detected and rice varieties containing one or more BPH-resistance genes/QTLs have been developed to reduce the loss of rice yield induced by BPH feeding.
rice
brown planthopper
defense responses
BPH-resistance genes
1. Introduction
The brown planthopper (Nilaparvata lugens Stål, BPH) is a host-specific herbivore that is widespread in Asia, Australia, and the South Pacific islands [1]. N. lugens soaks up phloem sap by inserting needle-like stylets into the vascular tissue of rice (Oryza sativa L.) [2]. Large amounts of BPHs often gathered in groups to harm plants, and caused wilting, yellowing, and even death of rice plants, as well as “hopperburn” in BPH-susceptible rice fields [3]. BPHs are also vectors of various viruses of rice, such as the grassy stunt virus and ragged stunt virus, which were introduced into rice plants during the N. lugens feeding process [4][5][6]. Direct and indirect economic losses induced by BPH feeding in Asia alone exceed hundreds of millions of dollars on an annual basis [7]. Brown planthoppers have become one of the most serious pests that harm rice production [3].
Currently, the application of chemical insecticides remains the major approach to controlling BPH in the field [8]. However, the widespread use of these compounds is hazardous to human health and the environment and has side effects that impact the natural enemies of BPH [9]. In addition, the indiscriminate use of pesticides can promote the emergence of insecticide resistance in BPHs [10][11]. Wu et al. found that the insecticide resistance to different insecticides (including imidacloprid, buprofezin, thiamethoxam, pymetrozine fufprole, chlorpyrifos, sulfoxafor, nitenpyram) of 69 N. lugens populations collected from eight Chinese provinces improved to varying degrees [12]. This led to a significant reduction in the toxicity efficiency against BPH [13][14][15][16]. Therefore, other BPH management strategies that are greener, healthier, and more sustainable must be developed. Utilizing the inherent resistance genes of rice to cultivate resistant rice varieties has been widely considered as the most cost-effective method for sustainable BPH control [17][18][19].
DNA sequence data show that the host of BPHs began to gradually transfer from Leersia to rice approximately 2.5 million years ago [1]. Rice has since evolved sophisticated defense systems to resist BPH infection, and BPHs have evolved various mechanisms to overcome these defenses [1].
2. BPH-Resistance Gene Mapping
The indica cultivar Mudgo, the first BPH-resistant rice germplasm, was identified in 1969 by the International Rice Research Institute [20]. Bph1, the first BPH-resistance gene identified from Mudgo, was mapped on chromosome 12 [21]. In recent decades, more than 49 BPH-resistance genes/QTLs have been detected due to the development of molecular marker technology and methods for evaluating the resistance of rice to BPHs [2][3][22][23][24]. Among these 49 genes/QTLs, 33 (Bph37 from IR64; Bph38(t), Bph33(t), bph19, Bph31, Bph44(t), qBph4.3, Bph33, Bph30, Bph41, Bph40, qBph4.1, Bph3, and qBph4.2 from IR65482-17, qBph4.4, Bph17, and qBph4.2 from Rathu Heenati; Bph27(t), Bph6, Bph44, Bph42, Bph25, and Bph37 from SE382; Bph32, bph4, Bph43, Bph28(t), bph2, bph7, and Bph9 from Kaharamana; and Bph1, Bph26, and Bph9 from Pokkali) were derived from traditional cultivated rice species; the rest were derived from wild rice varieties, including Bph13(t), bph11, qBph3, Bph14, qBph4, and Bph15 from O. officinalis; Bph12 from O. latifolia; Bph35, Bph36, Bph27, and bph29 from O. rufipogon; Bph21 and Bph20(t) from O. minuta; Bph34 from O. nivara; and Bph18 and Bph10 from O. australiensis (Table 1). Rice varieties containing one or more BPH-resistance genes/QTLs have been developed, and the cultivation of these varieties has greatly reduced the loss of rice yield induced by BPH feeding [25].
Table 1. BPH-resistance genes/QTLs discovered in rice.
| Gene | Germplasm | Chromosome | Linked Markers | Reference |
|---|---|---|---|---|
| Bph37 | IR64 | 1L | RM302, YM35 | [26] |
| Bph38(t) | Khazar | 1L | 693369, id1012165 | [27] |
| Bph33(t) | RP2068 | 1L | RM488, RM11522 | [28] |
| Bph13(t) | O. officinalis | 3S | AJ09b, AJ09c | [29] |
| bph19 | AS20-1 | 3S | RM6308, RM3134 | [30] |
| Bph31 | CR2711-76 | 3L | PA26, RM2334 | [31] |
| bph11 | O. officinalis | 3L | G1318 | [32] |
| qBph3 | IR02W101 (O. officinalis) | 3L | t6, f3, c3-14 | [33] |
| Bph14 | B5 (O. officinalis) | 3L | SM1, G1318 | [34] |
| Bph44(t) | IRGC 15344 | 4S | 344-0-6, 344-1-2 | [24] |
| qBph4.3 | Salkathi | 4S | RM551, RM335 | [35] |
| Bph33 | Kolayal, Poliyal | 4S | H99, H101 | [36] |
| Bph30 | AC-1613 | 4S | SSR28, SSR69 | [2] |
| Bph41 | SWD10 | 4S | SWRm_01617, SWRm_01522 | [37] |
| Bph40 | SE232, SE67, C334 | 4S | - | [2] |
| Bph12 | O. latifolia | 4S | RM16459, RM1305 | [3] |
| qBph4.1 | Rathu Heenati | 4S | - | [38] |
| Bph3 | Rathu Heenati | 4S | RHD9, RHC10 | [39] |
| Bph35 | RBPH660 (O. rufipogon) | 4S | PSM16, R4M13 | [40] |
| qBph4.2 | IR65482-17 | 4S | RM261, S1, XC4-27 | [41] |
| Bph15 | B5 (O. officinalis) | 4S | RG1, RG2 | [3] |
| qBph4.4 | Salkathi | 4S | RM335, RM5633 | [35] |
| Bph36 | O. rufipogon | 4L | S13, X48 | [42] |
| qBph4 | IR02W101 (O.officinalis) | 4S | p17, xc4-27 | [33] |
| Bph20(t) | O. minuta | 4S | B42, B44 | [43] |
| Bph17 | Rathu Heenati | 4S | RM8213, RM5953 | [44] |
| qBph4.2 | Rathu Heenati | 4L | - | [38] |
| Bph27 | O. rufipogon | 4L | RM16766, RM17033 | [42] |
| Bph27(t) | Balamawee | 4L | Q52, Q20 | [45] |
| Bph6 | Swarnalata | 4L | H, Y9 | [46] |
| Bph44 | Balamawee | 4L | Q31, RM17007 | [24] |
| Bph34 | O. nivara | 4L | RM16994, RM17007 | [47] |
| Bph42 | SWD10 | 4L | SWRm_01695, SWRm_00328 | [37] |
| Bph25 | ADR52 | 6S | S00310 | [48] |
| bph29 | O. rufipogon | 6S | BYL8, BID2 | [49] |
| Bph37 | SE382 | 6S | - | [22] |
| Bph32 | Ptb33 | 6S | RM19291, RM8072 | [50] |
| bph4 | Babawee | 6S | RM190, C76A | [51] |
| Bph43 | IRGC 8678 | 11L | 16-22, 16-30 | [23] |
| Bph28(t) | DV85 | 11L | Indel55, Indel66 | [52] |
| bph2 | ASD7 | 12L | RM7102, RM463 | [53] |
| bph7 | T12 | 12L | RM3448, RM313 | [54] |
| Bph10 | O. australiensis | 12L | RG457 | [55] |
| Bph9 | Kaharamana | 12L | RM463, RM5341 | [56] |
| Bph1 | Mudgo | 12L | em5814N, em2802N | [57] |
| Bph26 | ADR52 | 12L | DS72B4, DS173B | [58] |
| Bph18 | O. australiensis | 12L | BIM3, BN162 | [59] |
| Bph9 | Pokkali | 12L | InD2, RsaI | [54] |
| Bph21 | O. minuta | 12L | S12094A, B122 | [43] |
S, short arm of chromosome; L, long arm of chromosome.
Most of the BPH-resistance genes/QTLs identified to date were located on six of twelve chromosomes (chromosomes 1, 3, 4, 6, 11, and 12), and their distribution on chromosomes was clustered (Figure 1). Three genes (bph11, qBph3, and Bph14) were clustered between 35.60 and 35.80 Mb of chromosome 3L [29][32][34]. A total of 21 genes were located on chromosome 4: five genes (Bph44(t), qBph4.3, Bph33, Bph30, and Bph41) were clustered between 0.17 and 1.10 Mb on chromosome 4S [2][24][35][36][37]; 11 genes (Bph40, Bph12, qBph4.1, Bph3, Bph35, qBph4.2, Bph15, Bph36, qBph4, Bph20(t), and Bph17) were clustered between 4.44 and 9.38 Mb on chromosome 4S [2][3][33][38][39][40][41][42][43][44]; and Bph27(t), Bph6, Bph44, Bph34, and Bph42 were clustered on chromosome 4L between 20.60 and 21.80 Mb [24][37][45][46][47]. The Bph37 that from SE382, Bph25, bph29, Bph32 and bph4 were present on chromosome 6S between 0.21 and 1.47 Mb [22][48][49][50][51]. The Bph43 and Bph28(t) genes were clustered between 16.79 and 16.96 Mb of chromosome 11L [23][52]. Some regions of these genes in the same cluster might overlap, indicating that these genes were not the same but were tightly linked, or that they were the same gene. These clustered genes might also constitute different alleles of the same gene that mediate responses to different BPH populations. In the same region on chromosome 12L, a total of eight BPH-resistance genes have been isolated. Sequence alignment revealed that these genes were alleles, and four allelotypes were identified. An assessment of the BPH resistance of the four allelotypes revealed that the resistance to BPH populations conferred by allelotypes of the same resistance gene varies [54].
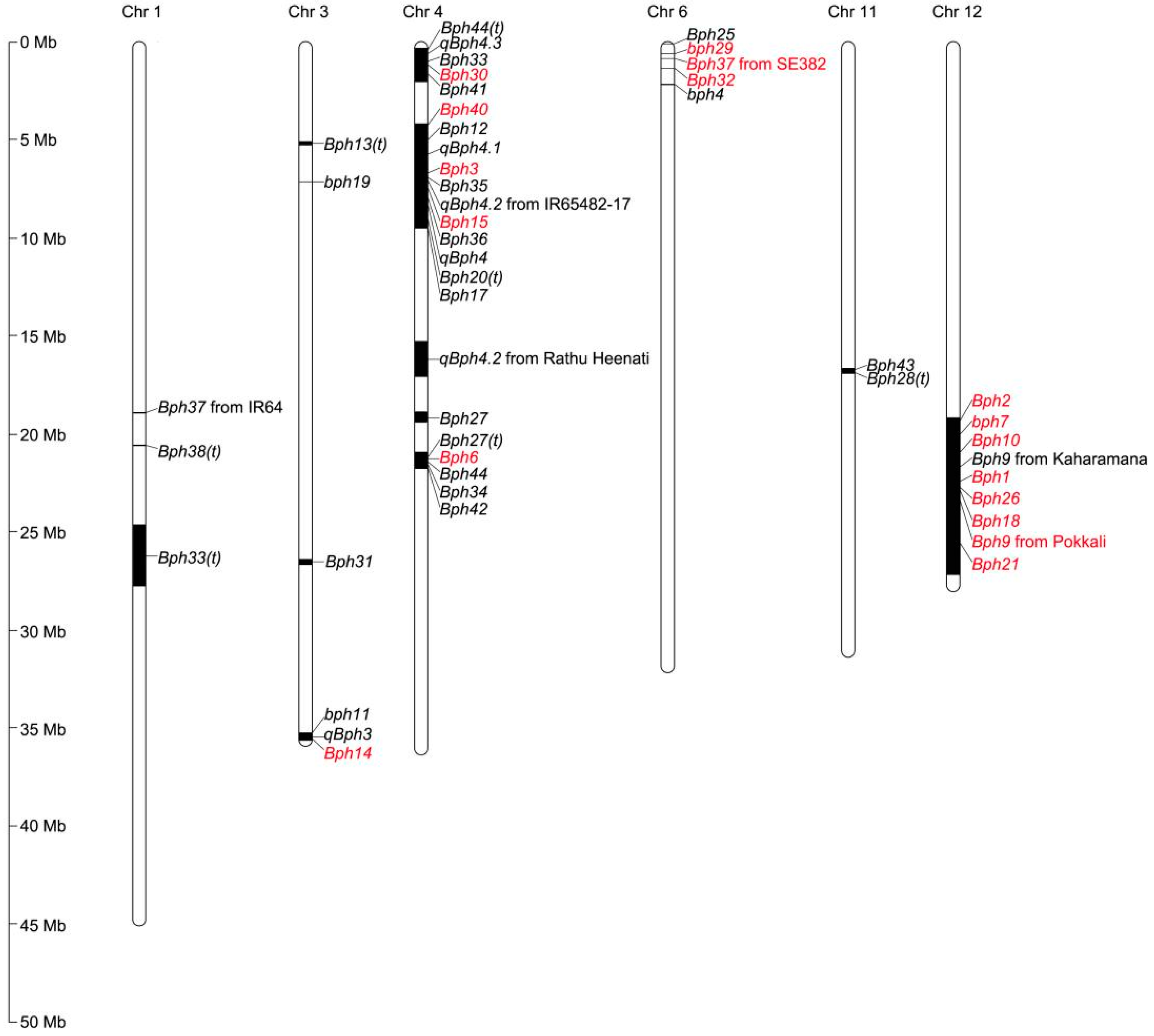
Figure 1. Distribution of BPH-resistance genes/QTLs on rice chromosomes. Numbers on the left indicate the physical distance. Black bars represented the position of BPH-resistance genes/QTLs on rice chromosomes. Red represents genes that have been cloned, and black represents genes that have not been cloned.
References
- Zheng, X.; Zhu, L.; He, G. Genetic and molecular understanding of host rice resistance and Nilaparvata lugens adaptation. Curr. Opin. Insect Sci. 2021, 45, 14–20.
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732.
- Muduli, L.; Pradhan, S.K.; Mishra, A.; Bastia, D.N.; Samal, K.C.; Agrawal, P.K.; Dash, M. Understanding brown planthopper resistance in rice: Genetics, biochemical and molecular breeding approaches. Rice Sci. 2021, 28, 532–546.
- Chang, X.; Wang, F.; Fang, Q.; Chen, F.; Yao, H.; Gatehouse, A.; Ye, G. Virus-induced plant volatiles mediate the olfactory behaviour of its insect vectors. Plant Cell Environ. 2021, 44, 2700–2715.
- Sarao, P.S.; Sahi, G.K.; Neelam, K.; Mangat, G.S.; Patra, B.C.; Singh, K. Donors for resistance to brown planthopper Nilaparvata lugens Stål from wild rice species. Rice Sci. 2016, 23, 219–224.
- Feng, C.; Wan, Z.; Lan, L.; Le, K. Rice responses and resistance to planthopper-borne viruses at transcriptomic and proteomic levels. Curr. Issues Mol. Biol. 2015, 19, 43–52.
- Min, S.; Lee, S.W.; Choi, B.R.; Lee, S.H.; Kwon, D.H. Insecticide resistance monitoring and correlation analysis to select appropriate insecticides against Nilaparvata lugens (Stål), a migratory pest in Korea. J. Asia-Pac. Entomol. 2014, 17, 711–716.
- Lu, K.; Chen, X.; Liu, W.; Zhang, Z.; Wang, Y.; You, K.; Li, Y.; Zhang, R.; Zhou, Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens Stål: Its response to temperature and insecticide stresses. Pestic. Biochem. Phys. 2017, 42, 102–110.
- Alam, M.J.; Das, G. Toxicity of insecticides to predators of rice brown planthopper: Wolf spider and carabid beetle. J. Sci. Food Agric. 2020, 3, 9–13.
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429.
- Ge, L.Q.; Huang, L.J.; Yang, G.Q.; Song, Q.S.; Stanley, D.; Gurr, G.M.; Wu, J.C. Molecular basis for insecticide-enhanced thermotolerance in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Mol. Ecol. 2013, 22, 5624–5634.
- Wu, S.; Zeng, B.; Zheng, C.; Mu, X.; Zhang, Y.; Hu, J.; Zhang, S.; Gao, C.; Shen, J. The evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) of China in the period 2012–2016. Sci. Rep. 2018, 8, 4586.
- Zeng, Q.; Yu, C.; Chang, X.; Wan, Y.; Ba, Y.; Li, C.; Lv, H.; Guo, Z.; Cai, T.; Ren, Z.; et al. CeO2 nanohybrid as a synergist for insecticide resistance management. Chem. Eng. J. 2022, 446, 137074.
- Jin, R.; Wang, Y.; He, B.; Zhang, Y.; Cai, T.; Wan, H.; Jin, B.R.; Li, J. Activator protein-1 mediated CYP6ER1 overexpression in the clothianidin resistance of Nilaparvata lugens (Stål). Pest Manag. Sci. 2021, 77, 4476–4482.
- Liao, X.; Jin, R.; Zhang, X.; Ali, E.; Mao, K.; Xu, P.; Li, J.; Wan, H. Characterization of sulfoxaflor resistance in the brown planthopper, Nilaparvata lugens (Stål). Pest Manag. Sci. 2019, 75, 1646–1654.
- Jin, R.; Mao, K.; Liao, X.; Xu, P.; Li, Z.; Ali, E.; Wan, H.; Li, J. Overexpression of CYP6ER1 associated with clothianidin resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 154, 39–45.
- Sharma, H.C. Host plant resistance to insects: Modern approaches and limitations. Plant Prot. Assoc. India 2007, 35, 179–184.
- Gurr, G.M.; Liu, J.; Read, D.; Catindig, J.; Cheng, J.A.; Lan, L.P.; Heong, K.L. Parasitoids of Asian rice planthopper (Hemiptera: Delphacidae) pests and prospects for enhancing biological control by ecological engineering. Ann. Appl. Biol. 2015, 158, 149–176.
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822.
- Pathak, M.; Cheng, C.; Fortuno, M. Resistance to Nephotettix impicticeps and Nilaparvata lugens in varieties of rice. Nature 1969, 223, 502–504.
- Hirabayashi, H.; Ogawas, T. RFLP mapping of Bph-1 (brown planthopper resistance gene) in rice. Jpn. J. Breed. 1995, 45, 369–371.
- Zhou, C.; Zhang, Q.; Chen, Y.; Huang, J.; Guo, Q.; Li, Y.; Wang, W.; Qiu, Y.; Guan, W.; Zhang, J.; et al. Balancing selection and wild gene pool contribute to resistance in global rice germplasm against planthopper. J. Integr. Plant Biol. 2021, 63, 1695–1711.
- Simon, E.V.; Hechanova, S.L.; Hernandez, J.E.; Li, C.P.; Tülek, A.; Ahn, E.K.; Jairin, J.; Choi, I.R.; Sundaram, R.M.; Jena, K.K.; et al. Available cloned genes and markers for genetic improvement of biotic stress resistance in rice. Front. Plant Sci. 2023, 14, 1247014.
- Kiswanto, I.; Soetopo, L.; Adiredjo, A.L. Identification of novel candidate of brown planthopper resistance gene Bph44 in rice (Oryza sativa L.). Genome 2022, 65, 505–511.
- Liu, M.; Fan, F.; He, S.; Guo, Y.; Chen, G.; Li, N.; Li, N.; Yuan, H.; Si, F.; Yang, F.; et al. Creation of elite rice with high-yield, superior-quality and high resistance to brown planthopper based on molecular design. Rice 2022, 15, 17.
- Yang, M.; Cheng, L.; Yan, L.; Shu, W.; Wang, X.; Qiu, Y. Mapping and characterization of a quantitative trait locus resistance to the brown planthopper in the rice variety IR64. Hereditas 2019, 156, 22.
- Balachiranjeevi, C.H.; Prahalada, G.D.; Mahender, A.; Jamaloddin, M.; Sevilla, M.A.L.; Marfori-Nazarea, C.M.; Vinarao, R.; Sushanto, U.; Baehaki, S.E.; Li, Z.; et al. Identification of a novel locus, BPH38(t), conferring resistance to brown planthopper (Nilaparvata lugens Stål.) using early backcross population in rice (Oryza sativa L.). Euphytica 2019, 215, 185.
- Naik, S.B.; Divya, D.; Sahu, N.; Sundaram, R.M.; Sarao, P.S.; Singh, K.; Lakshmi, V.J.; Bentur, J.S. A new gene Bph33(t) conferring resistance to brown planthopper (BPH), Nilaparvata lugens (Stål) in rice line RP2068-18-3-5. Euphytica 2018, 214, 53.
- Renganayaki, K.; Fritz, A.K.; Sadasivam, S.; Pammi, S.; Harrington, S.E.; McCouch, S.R.; Kumar, S.M.; Reddy, A.S. Mapping and progress toward map-based cloning of brown planthopper biotype-4 resistance gene introgressed from Oryza officinalis into cultivated rice, O. sativa. Crop Sci. 2002, 42, 2112–2117.
- Chen, J.; Wang, L.; Pang, X.; Pan, Q. Genetic analysis and fine mapping of a rice brown planthopper (Nilaparvata lugens Stål) resistance gene bph19(t). Mol. Genet. Genom. 2006, 275, 321–329.
- Prahalada, G.D.; Shivakumar, N.; Lohithaswa, H.C.; Gowda, D.K.S.; Ramkumar, G.; Kim, S.R.; Ramachandra, C.; Hittalmani, S.; Mohapatra, T.; Jena, K.K. Identification and fine mapping of a new gene, BPH31 conferring resistance to brown planthopper biotype 4 of India to improve rice, Oryza sativa L. Rice 2017, 10, 41.
- Hirabayashi, H.; Angeles, E.R.; Kaji, R.; Ogawa, T.; Brar, D.S.; Khush, G.S. Identification of a brown planthopper resistance gene derived from O. officinalis using molecular markers in rice. Breed. Sci. 1998, 48, 82.
- Hu, J.; Xiao, C.; Cheng, M.; Gao, G.; Zhang, Q.; He, Y. Fine mapping and pyramiding of brown planthopper resistance genes QBph3 and QBph4 in an introgression line from wild rice O. officinalis. Mol. Breed. 2015, 35, 3.
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168.
- Mohanty, S.K.; Panda, R.S.; Mohapatra, S.L.; Nanda, A.; Behera, L.; Jena, M.; Sahu, R.K.; Sahu, S.C.; Mohapatra, T. Identification of novel quantitative trait loci associated with brown planthopper resistance in the rice landrace Salkathi. Euphytica 2017, 213, 38.
- Hu, J.; Chang, X.; Zou, L.; Tang, W.; Wu, W. Identification and fine mapping of Bph33, a new brown planthopper resistance gene in rice (Oryza sativa L.). Rice 2018, 11, 55.
- Tan, H.; Palyam, S.; Gouda, J.; Kumar, P.P.; Chellian, S.K. Identification of two QTLs, BPH41 and BPH42, and their respective gene candidates for brown planthopper resistance in rice. Sci. Rep. 2022, 12, 18538.
- Kamolsukyeunyong, W.; Ruengphayak, S.; Chumwong, P.; Kusumawati, L.; Chaichoompu, E.; Jamboonsri, W.; Saensuk, C.; Phoonsiri, K.; Toojinda, T.; Vanavichit, A. Identification of spontaneous mutation for broad-spectrum brown planthopper resistance in a large, long-term fast neutron mutagenized rice population. Rice 2019, 12, 16.
- Liu, Y.; Wu, H.; Chen, H.; Liu, Y.; He, J.; Kang, H.; Sun, Z.; Pan, G.; Wang, Q.; Hu, J.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015, 33, 301–305.
- Zhang, Y.; Qin, G.; Ma, Q.; Wei, M.; Yang, X.; Ma, Z.; Liang, H.; Liu, C.; Li, Z.; Liu, F.; et al. Identification of a major resistance locus Bph35 to brown planthopper in rice (Oryza sativa L.). Rice Sci. 2020, 27, 237–245.
- Hu, J.; Xiao, C.; Cheng, M.; Gao, G.; Zhang, Q.; He, Y. A new finely mapped Oryza australiensis-derived QTL in rice confers resistance to brown planthopper. Gene 2015, 561, 132–137.
- Li, Z.; Xue, Y.; Zhou, H.; Li, Y.; Usman, B.; Jiao, X.; Wang, X.; Liu, F.; Qin, B.; Li, R.; et al. High-resolution mapping and breeding application of a novel brown planthopper resistance gene derived from wild rice (Oryza rufipogon Griff). Rice 2019, 12, 41.
- Rahman, M.L.; Jiang, W.Z.; Chu, S.H.; Qiao, Y.L.; Ham, T.H.; Woo, M.O.; Lee, J.; Khanam, M.S.; Chin, J.H.; Jeung, J.U.; et al. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor. Appl. Genet. 2009, 119, 1237–1246.
- Sun, L.; Su, C.; Wang, C.; Zhai, H.; Wan, J. Mapping of a major resistance gene to the brown planthopper in the rice cultivar Rathu Heenati. Breed. Sci. 2005, 55, 391–396.
- He, J.; Liu, Y.; Liu, Y.; Jiang, L.; Wu, H.; Kang, H.; Liu, S.; Chen, L.; Liu, X.; Cheng, X.; et al. High-resolution mapping of brown planthopper (BPH) resistance gene Bph27(t) in rice (Oryza sativa L.). Mol. Breed. 2013, 31, 549–557.
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306.
- Kumar, K.; Sarao, P.S.; Bhatia, D.; Neelam, K.; Kaur, A.; Mangat, G.S.; Brar, D.S.; Singh, K. High-resolution genetic mapping of a novel brown planthopper resistance locus, Bph34 in Oryza sativa L. × Oryza nivara (Sharma & Shastry) derived interspecific F2 population. Theor. Appl. Genet. 2018, 131, 1163–1171.
- Myint, K.K.M.; Fujita, D.; Matsumura, M.; Sonoda, T.; Yoshimura, A.; Yasui, H. Mapping and pyramiding of two major genes for resistance to the brown planthopper (Nilaparva talugens ) in the rice cultivar ADR52. Theor. Appl. Genet. 2012, 124, 495–504.
- Wang, Y.; Cao, L.; Zhang, Y.; Cao, C.; Liu, F.; Huang, F.; Qiu, Y.; Li, R.; Luo, X. Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J. Exp. Bot. 2015, 66, 6035–6045.
- Ren, J.; Gao, F.; Wu, X.; Lu, X.; Zeng, L.; Lv, J.; Su, X.; Luo, H.; Ren, G. Bph32, a novel gene encoding an unknown SCR domain-containing protein, confers resistance against the brown planthopper in rice. Sci. Rep. 2016, 6, 37645.
- Kawaguchi, M.; Murata, K.; Ishii, T.; Takumi, S.; Mori, N.; Nakamura, C. Assignment of a brown planthopper (Nilaparvata lugens Stål) resistance gene bph4 to the rice chromosome 6. Breed. Sci. 2001, 51, 13–18.
- Wu, H.; Liu, Y.; He, J.; Liu, Y.; Jiang, L.; Liu, L.; Wang, C.; Cheng, X.; Wan, J. Fine mapping of brown planthopper (Nilaparvata lugens Stål) resistance gene Bph28(t) in rice (Oryza sativa L.). Mol. Breed. 2014, 33, 909–918.
- Sun, L.; Wang, C.; Su, C.; Liu, Y.; Zhai, H.; Wan, J. Mapping and marker-assisted selection of a brown planthopper resistance gene bph2 in rice (Oryza sativa L.). Acta Genet. Sin. 2006, 33, 717–723.
- Zhao, Y.; Huang, J.; Wang, Z.; Jing, S.; Wang, Y.; Ouyang, Y.; Cai, B.; Xin, X.; Liu, X.; Zhang, C.; et al. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. USA 2016, 113, 12850–12855.
- Ishii, T.; Brar, D.S.; Multani, D.S.; Khush, G.S. Molecular tagging of genes for brown planthopper resistance and earliness introgressed from Oryza australiensis into cultivated rice O. sativa. Genome 1994, 37, 217–221.
- Su, C.; Zhai, H.; Wang, C.; Sun, L.; Wan, J. SSR mapping of brown planthopper resistance gene Bph9 in Kaharamana, an indica rice (Oryza sativa L.). Acta Genet. Sin. 2006, 33, 262–268.
- Sharma, P.N.; Ketipearachchi, Y.; Murata, K.; Torii, A.; Takumi, S.; Mori, N.; Nakamura, C. RFLP/AFLP mapping of a brown planthopper (Nilaparvata lugens Stål) resistance gene Bph1 in rice. Euphytica 2002, 129, 109–117.
- Tamura, Y.; Hattori, M.; Yoshioka, H.; Yoshioka, M.; Takahashi, A.; Wu, J.; Sentoku, N.; Yasui, H. Map-based cloning and characterization of a brown planthopper resistance gene BPH26 from Oryza sativa L. ssp. indica cultivar ADR52. Sci. Rep. 2014, 4, 5872.
- Ji, H.; Kim, S.R.; Kim, Y.H.; Suh, J.P.; Park, H.M.; Sreenivasulu, N.; Misra, G.; Kim, S.M.; Hechanova, S.L.; Kim, H.; et al. Map-based cloning and characterization of the BPH18 gene from wild rice conferring resistance to brown planthopper (BPH) insect pest. Sci. Rep. 2016, 6, 34376.
More
Information
Subjects:
Agronomy; Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
546
Revisions:
2 times
(View History)
Update Date:
05 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




