Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agronomy
|
Genetics & Heredity
The brown planthopper (Nilaparvata lugens Stål, BPH) is one of the most serious pests that harm rice production. N. lugens soaks up phloem sap by inserting needle-like stylets into the vascular tissue of rice (Oryza sativa L.). Utilizing the inherent resistance has been widely considered as the most cost-effective method for sustainable BPH control. To date, more than 49 BPH-resistance genes/QTLs have been detected and rice varieties containing one or more BPH-resistance genes/QTLs have been developed to reduce the loss of rice yield induced by BPH feeding.
- rice
- brown planthopper
- defense responses
- BPH-resistance genes
1. Introduction
The brown planthopper (Nilaparvata lugens Stål, BPH) is a host-specific herbivore that is widespread in Asia, Australia, and the South Pacific islands [1]. N. lugens soaks up phloem sap by inserting needle-like stylets into the vascular tissue of rice (Oryza sativa L.) [2]. Large amounts of BPHs often gathered in groups to harm plants, and caused wilting, yellowing, and even death of rice plants, as well as “hopperburn” in BPH-susceptible rice fields [3]. BPHs are also vectors of various viruses of rice, such as the grassy stunt virus and ragged stunt virus, which were introduced into rice plants during the N. lugens feeding process [4,5,6]. Direct and indirect economic losses induced by BPH feeding in Asia alone exceed hundreds of millions of dollars on an annual basis [7]. Brown planthoppers have become one of the most serious pests that harm rice production [3].
Currently, the application of chemical insecticides remains the major approach to controlling BPH in the field [8]. However, the widespread use of these compounds is hazardous to human health and the environment and has side effects that impact the natural enemies of BPH [9]. In addition, the indiscriminate use of pesticides can promote the emergence of insecticide resistance in BPHs [10,11]. Wu et al. found that the insecticide resistance to different insecticides (including imidacloprid, buprofezin, thiamethoxam, pymetrozine fufprole, chlorpyrifos, sulfoxafor, nitenpyram) of 69 N. lugens populations collected from eight Chinese provinces improved to varying degrees [12]. This led to a significant reduction in the toxicity efficiency against BPH [13,14,15,16]. Therefore, other BPH management strategies that are greener, healthier, and more sustainable must be developed. Utilizing the inherent resistance genes of rice to cultivate resistant rice varieties has been widely considered as the most cost-effective method for sustainable BPH control [17,18,19].
DNA sequence data show that the host of BPHs began to gradually transfer from Leersia to rice approximately 2.5 million years ago [1]. Rice has since evolved sophisticated defense systems to resist BPH infection, and BPHs have evolved various mechanisms to overcome these defenses [1].
2. BPH-Resistance Gene Mapping
The indica cultivar Mudgo, the first BPH-resistant rice germplasm, was identified in 1969 by the International Rice Research Institute [20]. Bph1, the first BPH-resistance gene identified from Mudgo, was mapped on chromosome 12 [21]. In recent decades, more than 49 BPH-resistance genes/QTLs have been detected due to the development of molecular marker technology and methods for evaluating the resistance of rice to BPHs [2,3,22,23,24]. Among these 49 genes/QTLs, 33 (Bph37 from IR64; Bph38(t), Bph33(t), bph19, Bph31, Bph44(t), qBph4.3, Bph33, Bph30, Bph41, Bph40, qBph4.1, Bph3, and qBph4.2 from IR65482-17, qBph4.4, Bph17, and qBph4.2 from Rathu Heenati; Bph27(t), Bph6, Bph44, Bph42, Bph25, and Bph37 from SE382; Bph32, bph4, Bph43, Bph28(t), bph2, bph7, and Bph9 from Kaharamana; and Bph1, Bph26, and Bph9 from Pokkali) were derived from traditional cultivated rice species; the rest were derived from wild rice varieties, including Bph13(t), bph11, qBph3, Bph14, qBph4, and Bph15 from O. officinalis; Bph12 from O. latifolia; Bph35, Bph36, Bph27, and bph29 from O. rufipogon; Bph21 and Bph20(t) from O. minuta; Bph34 from O. nivara; and Bph18 and Bph10 from O. australiensis (Table 1). Rice varieties containing one or more BPH-resistance genes/QTLs have been developed, and the cultivation of these varieties has greatly reduced the loss of rice yield induced by BPH feeding [25].
Table 1. BPH-resistance genes/QTLs discovered in rice.
| Gene | Germplasm | Chromosome | Linked Markers | Reference |
|---|---|---|---|---|
| Bph37 | IR64 | 1L | RM302, YM35 | [26] |
| Bph38(t) | Khazar | 1L | 693369, id1012165 | [27] |
| Bph33(t) | RP2068 | 1L | RM488, RM11522 | [28] |
| Bph13(t) | O. officinalis | 3S | AJ09b, AJ09c | [29] |
| bph19 | AS20-1 | 3S | RM6308, RM3134 | [30] |
| Bph31 | CR2711-76 | 3L | PA26, RM2334 | [31] |
| bph11 | O. officinalis | 3L | G1318 | [32] |
| qBph3 | IR02W101 (O. officinalis) | 3L | t6, f3, c3-14 | [33] |
| Bph14 | B5 (O. officinalis) | 3L | SM1, G1318 | [34] |
| Bph44(t) | IRGC 15344 | 4S | 344-0-6, 344-1-2 | [24] |
| qBph4.3 | Salkathi | 4S | RM551, RM335 | [35] |
| Bph33 | Kolayal, Poliyal | 4S | H99, H101 | [36] |
| Bph30 | AC-1613 | 4S | SSR28, SSR69 | [2] |
| Bph41 | SWD10 | 4S | SWRm_01617, SWRm_01522 | [37] |
| Bph40 | SE232, SE67, C334 | 4S | - | [2] |
| Bph12 | O. latifolia | 4S | RM16459, RM1305 | [3] |
| qBph4.1 | Rathu Heenati | 4S | - | [38] |
| Bph3 | Rathu Heenati | 4S | RHD9, RHC10 | [39] |
| Bph35 | RBPH660 (O. rufipogon) | 4S | PSM16, R4M13 | [40] |
| qBph4.2 | IR65482-17 | 4S | RM261, S1, XC4-27 | [41] |
| Bph15 | B5 (O. officinalis) | 4S | RG1, RG2 | [3] |
| qBph4.4 | Salkathi | 4S | RM335, RM5633 | [35] |
| Bph36 | O. rufipogon | 4L | S13, X48 | [42] |
| qBph4 | IR02W101 (O.officinalis) | 4S | p17, xc4-27 | [33] |
| Bph20(t) | O. minuta | 4S | B42, B44 | [43] |
| Bph17 | Rathu Heenati | 4S | RM8213, RM5953 | [44] |
| qBph4.2 | Rathu Heenati | 4L | - | [38] |
| Bph27 | O. rufipogon | 4L | RM16766, RM17033 | [42] |
| Bph27(t) | Balamawee | 4L | Q52, Q20 | [45] |
| Bph6 | Swarnalata | 4L | H, Y9 | [46] |
| Bph44 | Balamawee | 4L | Q31, RM17007 | [24] |
| Bph34 | O. nivara | 4L | RM16994, RM17007 | [47] |
| Bph42 | SWD10 | 4L | SWRm_01695, SWRm_00328 | [37] |
| Bph25 | ADR52 | 6S | S00310 | [48] |
| bph29 | O. rufipogon | 6S | BYL8, BID2 | [49] |
| Bph37 | SE382 | 6S | - | [22] |
| Bph32 | Ptb33 | 6S | RM19291, RM8072 | [50] |
| bph4 | Babawee | 6S | RM190, C76A | [51] |
| Bph43 | IRGC 8678 | 11L | 16-22, 16-30 | [23] |
| Bph28(t) | DV85 | 11L | Indel55, Indel66 | [52] |
| bph2 | ASD7 | 12L | RM7102, RM463 | [53] |
| bph7 | T12 | 12L | RM3448, RM313 | [54] |
| Bph10 | O. australiensis | 12L | RG457 | [55] |
| Bph9 | Kaharamana | 12L | RM463, RM5341 | [56] |
| Bph1 | Mudgo | 12L | em5814N, em2802N | [57] |
| Bph26 | ADR52 | 12L | DS72B4, DS173B | [58] |
| Bph18 | O. australiensis | 12L | BIM3, BN162 | [59] |
| Bph9 | Pokkali | 12L | InD2, RsaI | [54] |
| Bph21 | O. minuta | 12L | S12094A, B122 | [43] |
S, short arm of chromosome; L, long arm of chromosome.
Most of the BPH-resistance genes/QTLs identified to date were located on six of twelve chromosomes (chromosomes 1, 3, 4, 6, 11, and 12), and their distribution on chromosomes was clustered (Figure 1). Three genes (bph11, qBph3, and Bph14) were clustered between 35.60 and 35.80 Mb of chromosome 3L [29,32,34]. A total of 21 genes were located on chromosome 4: five genes (Bph44(t), qBph4.3, Bph33, Bph30, and Bph41) were clustered between 0.17 and 1.10 Mb on chromosome 4S [2,24,35,36,37]; 11 genes (Bph40, Bph12, qBph4.1, Bph3, Bph35, qBph4.2, Bph15, Bph36, qBph4, Bph20(t), and Bph17) were clustered between 4.44 and 9.38 Mb on chromosome 4S [2,3,33,38,39,40,41,42,43,44]; and Bph27(t), Bph6, Bph44, Bph34, and Bph42 were clustered on chromosome 4L between 20.60 and 21.80 Mb [24,37,45,46,47]. The Bph37 that from SE382, Bph25, bph29, Bph32 and bph4 were present on chromosome 6S between 0.21 and 1.47 Mb [22,48,49,50,51]. The Bph43 and Bph28(t) genes were clustered between 16.79 and 16.96 Mb of chromosome 11L [23,52]. Some regions of these genes in the same cluster might overlap, indicating that these genes were not the same but were tightly linked, or that they were the same gene. These clustered genes might also constitute different alleles of the same gene that mediate responses to different BPH populations. In the same region on chromosome 12L, a total of eight BPH-resistance genes have been isolated. Sequence alignment revealed that these genes were alleles, and four allelotypes were identified. An assessment of the BPH resistance of the four allelotypes revealed that the resistance to BPH populations conferred by allelotypes of the same resistance gene varies [54].
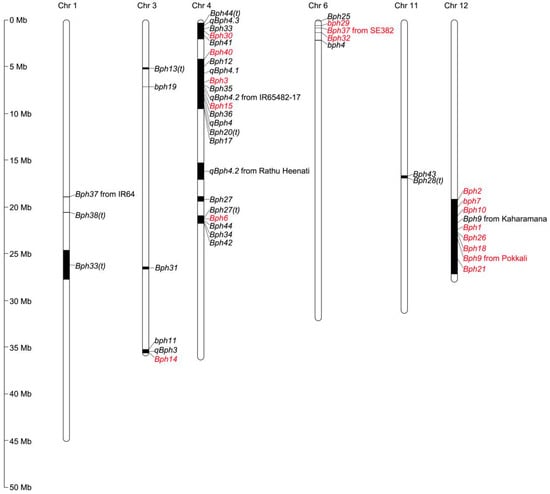
Figure 1. Distribution of BPH-resistance genes/QTLs on rice chromosomes. Numbers on the left indicate the physical distance. Black bars represented the position of BPH-resistance genes/QTLs on rice chromosomes. Red represents genes that have been cloned, and black represents genes that have not been cloned.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242316959
This entry is offline, you can click here to edit this entry!
