
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Theodoros Androutsakos | -- | 2486 | 2023-12-04 14:26:16 | | | |
| 2 | Peter Tang | Meta information modification | 2486 | 2023-12-05 02:34:01 | | |
Video Upload Options
Hepatitis B virus (HBV) infection is a worldwide medical issue with significant morbidity and mortality, as it is the main cause of chronic liver disease and hepatocellular carcinoma (HCC). Both innate and adaptive immune responses play a key role in HBV replication and suppression. The pathophysiological function of interleukins (IL) in the natural course of HBV has gained much attention as a result of the broad use of anti-interleukin agents for a variety of autoimmune diseases and the accompanying risk of HBV reactivation.
1. Introduction
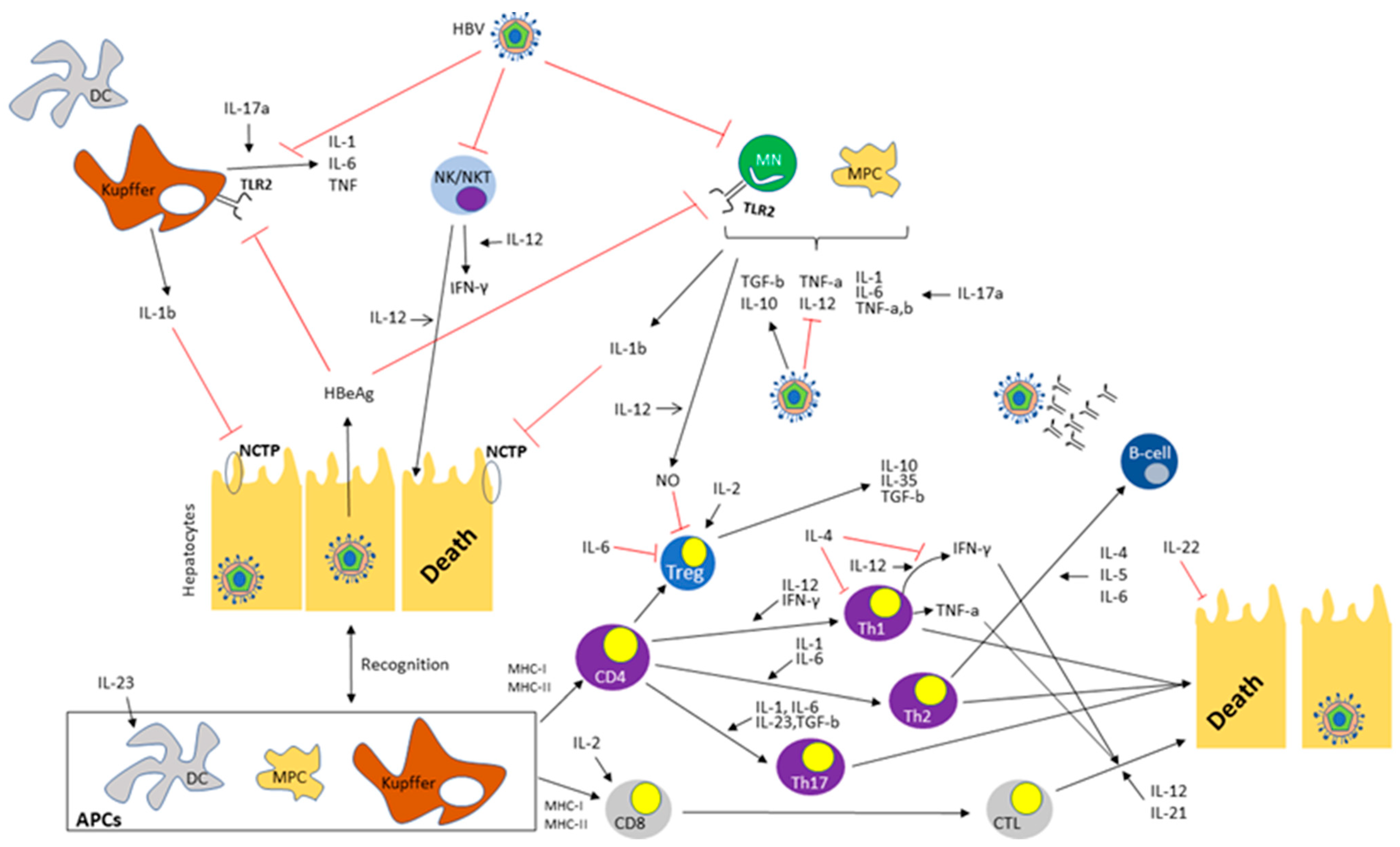
|
Interleukins |
Principal Effects |
Contributions in HBV Infection |
References |
|---|---|---|---|
|
IL-1 |
Proinflammatory |
Inhibition of HBV entry and replication Th2-cell activation |
|
|
IL-2 |
Promotion of effector T-cell differentiation Maintenance of Tregs for suppressive functions |
Regulation of HBV-specific T-cells |
|
|
IL-4 |
Promotion of Th2-cell differentiation (humoral immunity) |
Suppression of Th1-cell response Cessation of HBV replication in specific HCC cell lines |
|
|
IL-5 |
Maturation, differentiation and survival of B-cells |
Probable negative association with HBV replication |
|
|
IL-6 |
Stimulation of CD4+ T, B and Th17-cells Inhibition of Tregs |
Inhibition of HBV entry and replication |
|
|
IL-10 |
Anti-inflammatory |
Enhancement of HBV replication Suppression of HBV-specific T-cell response Mitigation of hepatic injury |
|
|
IL-12 |
Proinflammatory |
Enhancement of HBV-specific CD8+ T-cells in CHB Downregulation of Tregs |
|
|
IL-17 |
Proinflammatory |
Ill-defined role in HBV infection Probable association with CHB, cirrhosis and HCC |
|
|
IL-21 |
Pleiotropic-Immunoregulatory |
Boost of HBV-specific CD8+ T-cells and HBV suppression |
|
|
IL-22 |
Promotion of cellular proliferation, resistance to apoptosis and tissue regeneration Pro-inflammatory |
Regulation of intrahepatic inflammation; tissue protection or liver injury progression depending on disease stage |
[54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69] |
|
IL-23 |
CD4+ T-cell proliferation Enhancement of DC antigen presentation |
Regulation of HBV-related hepatic inflammation |
|
|
IL-35 |
Anti-inflammatoryImmunosuppression |
Stimulation of HBV replication Dampening of cytolytic and non-cytolytic activity of CTLs |
Abbreviations: IL: Interleukin, HBV: Hepatitis B virus, Th2-cells: T helper cells type 2, Tregs: Regulatory T-cells, Th1-cells: T helper cells type 1, HCC: Hepatocellular carcinoma, Th17-cells: T helper cells type 17, CHB: chronic hepatitis B, DC: dendritic cell, CTLs: Cytotoxic T-cells.
2. Interleukin 1
3. Interleukin-2
4. Interleukin-4
5. Interleukin-5
6. Interleukin-6
7. Interleukin-10
8. Interleukin-12
9. Interleukin-17
10. Interleukin-21
11. Interleukin-22
12. Interleukin 23
13. Interleukin 35
References
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555.
- Brody, H. Hepatitis B. Nature 2022, 603, S45.
- The World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 11 November 2023).
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Primers 2018, 4, 18035.
- Schreiner, S.; Nassal, M. A Role for the Host DNA Damage Response in Hepatitis B Virus cccDNA Formation-and Beyond? Viruses 2017, 9, 125.
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O.; et al. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127, 701–721.e70.
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632.
- Xia, Y.; Protzer, U. Control of Hepatitis B Virus by Cytokines. Viruses 2017, 9, 18.
- Ma, Z.; Liu, J.; Wu, W.; Zhang, E.; Zhang, X.; Li, Q.; Zelinskyy, G.; Buer, J.; Dittmer, U.; Kirschning, C.J.; et al. The IL-1R/TLR signaling pathway is essential for efficient CD8(+) T-cell responses against hepatitis B virus in the hydrodynamic injection mouse model. Cell Mol. Immunol. 2017, 14, 997–1008.
- Li, Y.; Zhu, Y.; Feng, S.; Ishida, Y.; Chiu, T.P.; Saito, T.; Wang, S.; Ann, D.K.; Ou, J.J. Macrophages activated by hepatitis B virus have distinct metabolic profiles and suppress the virus via IL-1β to downregulate PPARα and FOXO3. Cell Rep. 2022, 38, 110284.
- Yang, C.Y.; Kuo, T.H.; Ting, L.P. Human hepatitis B viral e antigen interacts with cellular interleukin-1 receptor accessory protein and triggers interleukin-1 response. J. Biol. Chem. 2006, 281, 34525–34536.
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190.
- Gaffen, S.L.; Liu, K.D. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004, 28, 109–123.
- Malek, T.R. The biology of interleukin-2. Annu. Rev. Immunol. 2008, 26, 453–479.
- Zhong, S.; Zhang, T.; Tang, L.; Li, Y. Cytokines and Chemokines in HBV Infection. Front. Mol. Biosci. 2021, 8, 805625.
- Lin, S.J.; Shu, P.Y.; Chang, C.; Ng, A.K.; Hu, C.P. IL-4 suppresses the expression and the replication of hepatitis B virus in the hepatocellular carcinoma cell line Hep3B. J. Immunol. 2003, 171, 4708–4716.
- Yao, Y.; Li, J.; Lu, Z.; Tong, A.; Wang, W.; Su, X.; Zhou, Y.; Mu, B.; Zhou, S.; Li, X.; et al. Proteomic analysis of the interleukin-4 (IL-4) response in hepatitis B virus-positive human hepatocelluar carcinoma cell line HepG2.2.15. Electrophoresis 2011, 32, 2004–2012.
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463–485.
- Vimali, J.; Yong, Y.K.; Murugesan, A.; Vishnupriya, K.; Ashwin, R.; Daniel, E.A.; Balakrishnan, P.; Raju, S.; Rosmawati, M.; Velu, V.; et al. Plasma interleukin-7 correlation with human immunodeficiency virus RNA and CD4+ T cell counts, and interleukin-5 with circulating hepatitis B virus DNA may have implications in viral control. Front. Med. 2022, 9, 1019230.
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835.
- Lan, T.; Chang, L.; Wu, L.; Yuan, Y.F. IL-6 Plays a Crucial Role in HBV Infection. J. Clin. Transl. Hepatol. 2015, 3, 271–276.
- Kuo, T.M.; Hu, C.P.; Chen, Y.L.; Hong, M.H.; Jeng, K.S.; Liang, C.C.; Chen, M.L.; Chang, C. HBV replication is significantly reduced by IL-6. J. Biomed. Sci. 2009, 16, 41.
- Li, X.; Liu, X.; Tian, L.; Chen, Y. Cytokine-Mediated Immunopathogenesis of Hepatitis B Virus Infections. Clin. Rev. Allergy Immunol. 2016, 50, 41–54.
- Hösel, M.; Quasdorff, M.; Wiegmann, K.; Webb, D.; Zedler, U.; Broxtermann, M.; Tedjokusumo, R.; Esser, K.; Arzberger, S.; Kirschning, C.J.; et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 2009, 50, 1773–1782.
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765.
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218.
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor. Rev. 2010, 21, 331–344.
- Brooks, D.G.; Trifilo, M.J.; Edelmann, K.H.; Teyton, L.; McGavern, D.B.; Oldstone, M.B. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006, 12, 1301–1309.
- Bachmann, M.F.; Wolint, P.; Walton, S.; Schwarz, K.; Oxenius, A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007, 37, 1502–1512.
- Das, A.; Ellis, G.; Pallant, C.; Lopes, A.R.; Khanna, P.; Peppa, D.; Chen, A.; Blair, P.; Dusheiko, G.; Gill, U.; et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 2012, 189, 3925–3935.
- Gong, Y.; Zhao, C.; Zhao, P.; Wang, M.; Zhou, G.; Han, F.; Cui, Y.; Qian, J.; Zhang, H.; Xiong, H.; et al. Role of IL-10-Producing Regulatory B Cells in Chronic Hepatitis B Virus Infection. Dig. Dis. Sci. 2015, 60, 1308–1314.
- Liu, Y.; Cheng, L.S.; Wu, S.D.; Wang, S.Q.; Li, L.; She, W.M.; Li, J.; Wang, J.Y.; Jiang, W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci 2016, 130, 907–919.
- Huang, A.; Zhang, B.; Yan, W.; Wang, B.; Wei, H.; Zhang, F.; Wu, L.; Fan, K.; Guo, Y. Myeloid-derived suppressor cells regulate immune response in patients with chronic hepatitis B virus infection through PD-1-induced IL-10. J. Immunol. 2014, 193, 5461–5469.
- Xu, L.; Yin, W.; Sun, R.; Wei, H.; Tian, Z. Kupffer cell-derived IL-10 plays a key role in maintaining humoral immune tolerance in hepatitis B virus-persistent mice. Hepatology 2014, 59, 443–452.
- Hyodo, N.; Nakamura, I.; Imawari, M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2004, 135, 462–466.
- Hyodo, N.; Tajimi, M.; Ugajin, T.; Nakamura, I.; Imawari, M. Frequencies of interferon-gamma and interleukin-10 secreting cells in peripheral blood mononuclear cells and liver infiltrating lymphocytes in chronic hepatitis B virus infection. Hepatol. Res. 2003, 27, 109–116.
- Wang, K.; Wu, Z.B.; Ye, Y.N.; Liu, J.; Zhang, G.L.; Su, Y.J.; He, H.L.; Zheng, Y.B.; Gao, Z.L. Plasma Interleukin-10: A Likely Predictive Marker for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Hepat. Mon. 2014, 14, e19370.
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146.
- Jia, Z.; Ragoonanan, D.; Mahadeo, K.M.; Gill, J.; Gorlick, R.; Shpal, E.; Li, S. IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Front. Immunol. 2022, 13, 952231.
- Xiong, S.Q.; Lin, B.L.; Gao, X.; Tang, H.; Wu, C.Y. IL-12 promotes HBV-specific central memory CD8+ T cell responses by PBMCs from chronic hepatitis B virus carriers. Int. Immunopharmacol. 2007, 7, 578–587.
- Brahmachari, S.; Pahan, K. Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J. Immunol. 2009, 183, 2045–2058.
- Schurich, A.; Pallett, L.J.; Lubowiecki, M.; Singh, H.D.; Gill, U.S.; Kennedy, P.T.; Nastouli, E.; Tanwar, S.; Rosenberg, W.; Maini, M.K. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013, 9, e1003208.
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54.
- Huang, Z.; van Velkinburgh, J.C.; Ni, B.; Wu, Y. Pivotal roles of the interleukin-23/T helper 17 cell axis in hepatitis B. Liver Int. 2012, 32, 894–901.
- Arababadi, M.K.; Bidaki, M.Z.; Kennedy, D. IL-17A in hepatitis B infection: Friend or foe? Arch. Virol. 2014, 159, 1883–1888.
- Mehta, D.S.; Wurster, A.L.; Grusby, M.J. Biology of IL-21 and the IL-21 receptor. Immunol. Rev. 2004, 202, 84–95.
- Monteleone, G.; Pallone, F.; Macdonald, T.T. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth Factor. Rev. 2009, 20, 185–191.
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Res 2016, 5.
- Mesas-Fernández, A.; Bodner, E.; Hilke, F.J.; Meier, K.; Ghoreschi, K.; Solimani, F. Interleukin-21 in autoimmune and inflammatory skin diseases. Eur. J. Immunol. 2023, 53, e2250075.
- Li, L.; Liu, M.; Cheng, L.W.; Gao, X.Y.; Fu, J.J.; Kong, G.; Feng, X.; Pan, X.C. HBcAg-specific IL-21-producing CD4+ T cells are associated with relative viral control in patients with chronic hepatitis B. Scand. J. Immunol. 2013, 78, 439–446.
- Li, J.; Ren, W.; Ma, W.; Zhang, J.; Shi, J.; Qin, C. Interleukin-21 responses in patients with chronic hepatitis B. J. Interferon Cytokine Res. 2015, 35, 134–142.
- Li, Y.; Tang, L.; Hou, J. Role of interleukin-21 in HBV infection: Friend or foe? Cell Mol. Immunol. 2015, 12, 303–308.
- Tang, L.; Chen, C.; Gao, X.; Zhang, W.; Yan, X.; Zhou, Y.; Guo, L.; Zheng, X.; Wang, W.; Yang, F.; et al. Interleukin 21 Reinvigorates the Antiviral Activity of Hepatitis B Virus (HBV)-Specific CD8+ T Cells in Chronic HBV Infection. J. Infect. Dis. 2019, 219, 750–759.
- Wolk, K.; Witte, E.; Witte, K.; Warszawska, K.; Sabat, R. Biology of interleukin-22. Semin. Immunopathol. 2010, 32, 17–31.
- Zenewicz, L.A.; Flavell, R.A. Recent advances in IL-22 biology. Int. Immunol. 2011, 23, 159–163.
- Zenewicz, L.A. IL-22 Binding Protein (IL-22BP) in the Regulation of IL-22 Biology. Front. Immunol. 2021, 12, 766586.
- Wang, L.Y.; Yang, X.Y.; Wu, Y.P.; Fan, Y.C. IL-22-producing CD3 + CD8- T cells increase in immune clearance stage of chronic HBV infection and correlate with the response of Peg-interferon treatment. Clin. Immunol. 2023, 250, 109320.
- Zhang, Y.; Cobleigh, M.A.; Lian, J.Q.; Huang, C.X.; Booth, C.J.; Bai, X.F.; Robek, M.D. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011, 141, 1897–1906.
- Zheng, W.P.; Zhang, B.Y.; Shen, Z.Y.; Yin, M.L.; Cao, Y.; Song, H.L. Biological effects of bone marrow mesenchymal stem cells on hepatitis B virus in vitro. Mol. Med. Rep. 2017, 15, 2551–2559.
- Zhao, J.; Zhang, Z.; Luan, Y.; Zou, Z.; Sun, Y.; Li, Y.; Jin, L.; Zhou, C.; Fu, J.; Gao, B.; et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014, 59, 1331–1342.
- Feng, D.; Kong, X.; Weng, H.; Park, O.; Wang, H.; Dooley, S.; Gershwin, M.E.; Gao, B. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012, 143, 188–198.e187.
- Park, O.; Wang, H.; Weng, H.; Feigenbaum, L.; Li, H.; Yin, S.; Ki, S.H.; Yoo, S.H.; Dooley, S.; Wang, F.S.; et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 2011, 54, 252–261.
- Zhang, H.; Yan, X.; Yang, C.; Zhan, Q.; Fu, Y.; Luo, H.; Luo, H. Intrahepatic T helper 17 cells recruited by hepatitis B virus X antigen-activated hepatic stellate cells exacerbate the progression of chronic hepatitis B virus infection. J. Viral Hepat. 2020, 27, 1138–1149.
- Wei, X.; Wang, J.P.; Hao, C.Q.; Yang, X.F.; Wang, L.X.; Huang, C.X.; Bai, X.F.; Lian, J.Q.; Zhang, Y. Notch Signaling Contributes to Liver Inflammation by Regulation of Interleukin-22-Producing Cells in Hepatitis B Virus Infection. Front. Cell Infect. Microbiol. 2016, 6, 132.
- Zhang, J.; Liu, Z.; Liu, L.; Huang, M.; Huang, Y. Th22/IL-22 mediates the progression of HBV-related hepatocellular carcinoma via STAT3. Cytotechnology 2022, 74, 203–216.
- Shi, J.; Wang, Y.; Wang, F.; Zhu, Z.; Gao, Y.; Zhang, Q.; Du, Z. Interleukin 22 is related to development and poor prognosis of hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 855–864.
- Schwarzkopf, K.; Rüschenbaum, S.; Barat, S.; Cai, C.; Mücke, M.M.; Fitting, D.; Weigert, A.; Brüne, B.; Zeuzem, S.; Welsch, C.; et al. IL-22 and IL-22-Binding Protein Are Associated With Development of and Mortality From Acute-on-Chronic Liver Failure. Hepatol. Commun. 2019, 3, 392–405.
- Cobleigh, M.A.; Robek, M.D. Protective and pathological properties of IL-22 in liver disease: Implications for viral hepatitis. Am. J. Pathol. 2013, 182, 21–28.
- Gao, Y.H.; Li, Q.Q.; Wang, C.G.; Sun, J.; Wang, X.M.; Li, Y.J.; He, X.T.; Xu, H.Q.; Niu, J.Q. The role of IL22 polymorphisms on liver cirrhosis in patients with hepatitis B virus: A case control study. Medicine 2019, 98, e17867.
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.C. Interleukin-23: A key cytokine in inflammatory diseases. Ann. Med. 2011, 43, 503–511.
- Floss, D.M.; Moll, J.M.; Scheller, J. IL-12 and IL-23-Close Relatives with Structural Homologies but Distinct Immunological Functions. Cells 2020, 9, 2184.
- Wang, Q.; Zhou, J.; Zhang, B.; Tian, Z.; Tang, J.; Zheng, Y.; Huang, Z.; Tian, Y.; Jia, Z.; Tang, Y.; et al. Hepatitis B virus induces IL-23 production in antigen presenting cells and causes liver damage via the IL-23/IL-17 axis. PLoS Pathog. 2013, 9, e1003410.
- Xia, L.; Tian, D.; Huang, W.; Zhu, H.; Wang, J.; Zhang, Y.; Hu, H.; Nie, Y.; Fan, D.; Wu, K. Upregulation of IL-23 expression in patients with chronic hepatitis B is mediated by the HBx/ERK/NF-κB pathway. J. Immunol. 2012, 188, 753–764.
- Wang, Q.; Zheng, Y.; Huang, Z.; Tian, Y.; Zhou, J.; Mao, Q.; Wu, Y.; Ni, B. Activated IL-23/IL-17 pathway closely correlates with increased Foxp3 expression in livers of chronic hepatitis B patients. BMC Immunol. 2011, 12, 25.
- Bao, S.; Zheng, J.; Li, N.; Huang, C.; Chen, M.; Cheng, Q.; Li, Q.; Lu, Q.; Zhu, M.; Ling, Q.; et al. Role of interleukin-23 in monocyte-derived dendritic cells of HBV-related acute-on-chronic liver failure and its correlation with the severity of liver damage. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 147–155.
- Khanam, A.; Trehanpati, N.; Sarin, S.K. Increased interleukin-23 receptor (IL-23R) expression is associated with disease severity in acute-on-chronic liver failure. Liver Int. 2019, 39, 1062–1070.
- Olson, B.M.; Sullivan, J.A.; Burlingham, W.J. Interleukin 35: A key mediator of suppression and the propagation of infectious tolerance. Front. Immunol. 2013, 4, 315.
- Yang, L.; Jia, S.; Shao, X.; Liu, S.; Zhang, Q.; Song, J.; Wang, W.; Jin, Z. Interleukin-35 modulates the balance between viral specific CD4(+)CD25(+)CD127(dim/-) regulatory T cells and T helper 17 cells in chronic hepatitis B virus infection. Virol. J. 2019, 16, 48.
- Shao, X.; Ma, J.; Jia, S.; Yang, L.; Wang, W.; Jin, Z. Interleukin-35 Suppresses Antiviral Immune Response in Chronic Hepatitis B Virus Infection. Front. Cell Infect. Microbiol. 2017, 7, 472.
- Teng, D.K.; Liu, Y.; Lv, Y.F.; Wang, L.; Zhang, W.; Wang, J.P.; Li, Y. Elevated interleukin-35 suppresses liver inflammation by regulation of T helper 17 cells in acute hepatitis B virus infection. Int. Immunopharmacol. 2019, 70, 252–259.
- Tang, Y.; Ma, T.; Jia, S.; Zhang, Q.; Liu, S.; Qi, L.; Yang, L. The Mechanism of Interleukin-35 in Chronic Hepatitis B. Semin. Liver Dis. 2021, 41, 516–524.
- Tao, N.N.; Gong, R.; Chen, X.; He, L.; Ren, F.; Yu, H.B.; Chen, J.; Ren, J.H. Interleukin-35 stimulates hepatitis B virus transcription and replication by targeting transcription factor HNF4α. J. Gen. Virol. 2018, 99, 645–654.
- Li, X.; Zhu, Q.; Ye, B.; Zhu, C.; Dong, Y.; Ni, Q. JNK/c-Jun pathway activation is essential for HBx-induced IL-35 elevation to promote persistent HBV infection. J. Clin. Lab. Anal. 2023, 37, e24860.
- Liu, F.; Tong, F.; He, Y.; Liu, H. Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin. Immunol. 2011, 139, 1–5.
- Liu, Y.; Luo, Y.; Zhu, T.; Jiang, M.; Tian, Z.; Tang, G.; Liang, X. Regulatory B Cells Dysregulated T Cell Function in an IL-35-Dependent Way in Patients with Chronic Hepatitis B. Front. Immunol. 2021, 12, 653198.
- Yang, K.; Pan, Y.; Yan, B. Interleukin-35 induced by hepatitis B virus inhibits viral replication and viral antigen secretion in hepatocytes. Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi 2022, 38, 776–780.
- Zhou, Y.; Zhang, H.; Li, Y. IL-35 expression in peripheral blood CD4(+) T cells from chronic hepatitis B virus-infected patients directly correlates with virus load. Cytokine 2015, 73, 169–175.
- Li, X.; Liu, X.; Wang, W. IL-35: A Novel Immunomodulator in Hepatitis B Virus-Related Liver Diseases. Front. Cell Dev. Biol. 2021, 9, 614847.
- Li, X.; Tian, L.; Dong, Y.; Zhu, Q.; Wang, Y.; Han, W.; Liu, X.; Ni, Q.; Chen, Y.; Li, L. IL-35 inhibits HBV antigen-specific IFN-γ-producing CTLs in vitro. Clin Sci 2015, 129, 395–404.
- Dong, Y.; Li, X.; Yu, Y.; Lv, F.; Chen, Y. JAK/STAT signaling is involved in IL-35-induced inhibition of hepatitis B virus antigen-specific cytotoxic T cell exhaustion in chronic hepatitis B. Life Sci. 2020, 252, 117663.
- Abbas, A.K.; Trotta, E.; Simeonov, D.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482.
- Hsieh, E.W.; Hernandez, J.D. Clean up by aisle 2: Roles for IL-2 receptors in host defense and tolerance. Curr. Opin. Immunol. 2021, 72, 298–308.
- Keegan, A.D.; Leonard, W.J.; Zhu, J. Recent advances in understanding the role of IL-4 signaling. Fac. Rev. 2021, 10, 71.
- Strestik, B.D.; Olbrich, A.R.M.; Hasenkrug, K.J.; Dittmer, U. The role of IL-5, IL-6 and IL-10 in primary and vaccine-primed immune responses to infection with Friend retrovirus (Murine leukaemia virus). J. Gen. Virol. 2001, 82, 1349–1354.
- Malla, N.; Fomda, B.A.; Thokar, M.A. Serum cytokine levels in human ascariasis and toxocariasis. Parasitol. Res. 2006, 98, 345–348.
- Broughton, S.E.; Dhagat, U.; Hercus, T.R.; Nero, T.L.; Grimbaldeston, M.A.; Bonder, C.S.; Lopez, A.F.; Parker, M.W. The GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immunol. Rev. 2012, 250, 277–302.
- Giraldez, M.D.; Carneros, D.; Garbers, C.; Rose-John, S.; Bustos, M. New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 787–803.
- Katelani, S.; Fragoulis, G.E.; Bakasis, A.D.; Pouliakis, A.; Nikiphorou, E.; Atzeni, F.; Androutsakos, T. HBV reactivation in patients with rheumatoid arthritis treated with anti-interleukin-6: A systematic review and meta-analysis. Rheumatology 2023, 62, SI252–SI259.
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418.
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167.
- Nguyen, H.M.; Guz-Montgomery, K.; Saha, D. Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy. Cells 2020, 9, 400.
- van Herpen, C.M.; van der Voort, R.; van der Laak, J.A.; Klasen, I.S.; de Graaf, A.O.; van Kempen, L.C.; de Vries, I.J.; Boer, T.D.; Dolstra, H.; Torensma, R.; et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int. J. Cancer 2008, 123, 2354–2361.
- Cavanaugh, V.J.; Guidotti, L.G.; Chisari, F.V. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 1997, 71, 3236–3243.
- Zeuzem, S.; Carreño, V. Interleukin-12 in the treatment of chronic hepatitis B and C. Antivir. Res. 2001, 52, 181–188.
- Carreño, V.; Zeuzem, S.; Hopf, U.; Marcellin, P.; Cooksley, W.G.; Fevery, J.; Diago, M.; Reddy, R.; Peters, M.; Rittweger, K.; et al. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J. Hepatol. 2000, 32, 317–324.
- Rigopoulou, E.I.; Suri, D.; Chokshi, S.; Mullerova, I.; Rice, S.; Tedder, R.S.; Williams, R.; Naoumov, N.V. Lamivudine plus interleukin-12 combination therapy in chronic hepatitis B: Antiviral and immunological activity. Hepatology 2005, 42, 1028–1036.
- Yang, J.; Guo, R.; Yan, D.; Lu, H.; Zhang, H.; Ye, P.; Jin, L.; Diao, H.; Li, L. Plasma Level of ADAMTS13 or IL-12 as an Indicator of HBeAg Seroconversion in Chronic Hepatitis B Patients Undergoing m-ETV Treatment. Front. Cell Infect. Microbiol. 2020, 10, 335.
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602.
- Goepfert, A.; Lehmann, S.; Wirth, E.; Rondeau, J.M. The human IL-17A/F heterodimer: A two-faced cytokine with unique receptor recognition properties. Sci. Rep. 2017, 7, 8906.
- Hamada, H.; Garcia-Hernandez Mde, L.; Reome, J.B.; Misra, S.K.; Strutt, T.M.; McKinstry, K.K.; Cooper, A.M.; Swain, S.L.; Dutton, R.W. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009, 182, 3469–3481.
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32ra36.
- Rowan, A.G.; Fletcher, J.M.; Ryan, E.J.; Moran, B.; Hegarty, J.E.; O’Farrelly, C.; Mills, K.H. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J. Immunol. 2008, 181, 4485–4494.
- Batalla, A.; Coto, E.; González-Lara, L.; González-Fernández, D.; Gómez, J.; Aranguren, T.F.; Queiro, R.; Santos-Juanes, J.; López-Larrea, C.; Coto-Segura, P. Association between single nucleotide polymorphisms IL17RA rs4819554 and IL17E rs79877597 and Psoriasis in a Spanish cohort. J. Dermatol. Sci. 2015, 80, 111–115.
- Atzeni, F.; Carriero, A.; Boccassini, L.; D’Angelo, S. Anti-IL-17 Agents in the Treatment of Axial Spondyloarthritis. Immunotargets Ther. 2021, 10, 141–153.
- Ogdie, A.; Coates, L.C.; Gladman, D.D. Treatment guidelines in psoriatic arthritis. Rheumatology 2020, 59, i37–i46.
- Tangye, S.G. Advances in IL-21 biology—Enhancing our understanding of human disease. Curr. Opin. Immunol. 2015, 34, 107–115.
- Asao, H. Interleukin-21 in Viral Infections. Int. J. Mol. Sci. 2021, 22, 9521.
- Hao, C.; Wang, J.; Kang, W.; Xie, Y.; Zhou, Y.; Ma, L.; Peng, M.; Bai, X.; Lian, J.; Jia, Z. Kinetics of Th17 cytokines during telbivudine therapy in patients with chronic hepatitis B. Viral Immunol. 2013, 26, 336–342.
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 2148.
- Xiang, X.G.; Xie, Q. IL-35: A potential therapeutic target for controlling hepatitis B virus infection. J. Dig. Dis. 2015, 16, 1–6.
- Choi, J.K.; Egwuagu, C.E. Interleukin 35 Regulatory B Cells. J. Mol. Biol. 2021, 433, 166607.
- Jiang, H.; Zhang, T.; Yan, M.X.; Wu, W. IL-35 inhibits CD8(+) T cells activity by suppressing expression of costimulatory molecule CD28 and Th1 cytokine production. Transl. Cancer Res. 2019, 8, 1319–1325.
- Shen, P.; Roch, T.; Lampropoulou, V.; O’Connor, R.A.; Stervbo, U.; Hilgenberg, E.; Ries, S.; Dang, V.D.; Jaimes, Y.; Daridon, C.; et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014, 507, 366–370.
- Wang, R.X.; Yu, C.R.; Dambuza, I.M.; Mahdi, R.M.; Dolinska, M.B.; Sergeev, Y.V.; Wingfield, P.T.; Kim, S.H.; Egwuagu, C.E. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 2014, 20, 633–641.
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101.
- Yang, C.; Lei, L.; Pan, J.; Zhao, C.; Wen, J.; Qin, F.; Dong, F.; Wei, W. Altered CD4+ T cell and cytokine levels in peripheral blood and skin samples from systemic sclerosis patients and IL-35 in CD4+ T cell growth. Rheumatology 2022, 61, 794–805.
- Tavakolpour, S. Inhibition of regulatory cells as a possible cure of chronically hepatitis B virus infected patients. Immunol. Lett. 2016, 171, 70–71.




