1. Introduction
Hepatitis B virus (HBV) infection is a worldwide healthcare issue with significant morbidity and mortality, and it is the primary cause of chronic liver disease and hepatocellular carcinoma (HCC)
[1][2][1,2]. According to the World Health Organization (WHO), in 2019, 296 million people worldwide were suffering from chronic HBV infection (CHB), with 1.5 million new infections each year, while the HBV burden was estimated at 116 million people in Western Pacific regions, 81 million people in Africa and 14 million people in Europe; in the same year, hepatitis B resulted in approximately 820,000 deaths
[3].
HBV primarily affects hepatocytes and replicates within them. The virus enters the hepatocyte in 2 ways: via a low-affinity binding reaction between hepatitis B surface antigen (HBsAg) and the heparan sulfate proteoglycans (HSPG) on the cell surface and via a high-affinity binding reaction between the N-terminal part of the pre-S1 region of HBsAg and the hepatic bile acid transporter sodium taurocholate co-transporting polypeptide (NTCP), the main HBV hepatocyte receptor
[4]. After entering the hepatocyte, relaxed circular DNA (rcDNA) is transported into the nucleus and converted into covalently closed circular DNA (cccDNA)
[5]. In this way, HBV DNA remains in the hepatocyte, even after successful treatment with nucleos(t)ide analogues. In both chronic and acute infections, HBV induces liver injury mainly in a non-cytopathic way, mediated by the activation of the immune system.
Coordinated innate and adaptive immune responses are important for effective HBV clearance during acute infection. However, when the immune system fails to mount an effective response, viral persistence and consequent CHB occurs.
Interleukins (ILs), as fundamental elements of the immune system, regulate the outcome and characteristics of the adaptive immune response in a key manner. They play a crucial role in cellular communication by being generated and exerting their effects in a wide array of cell types; in this light, they form an intricate regulatory network. Up until now, 40 different ILs have been discovered, having 3 main functions, namely activating and regulating immune cells, transmitting information in a variety of cells, and participating in inflammatory response
[6]. Regarding HBV infection, ILs seem to play an important role in viral persistence as well as in continuous liver damage (
Table 1 and
Figure 1), a role that has gained attention after the wide use of anti-IL agents for a variety of autoimmune diseases.
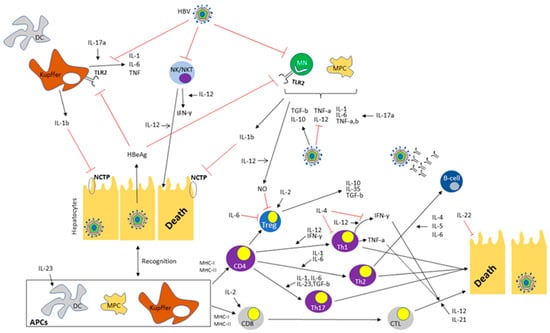
Figure 1. The complex interplay between different cytokines and hepatitis B virus. Black arrows indicate enhancement or secretion. Red arrows indicate suppression. Abbreviations: HBV: Hepatitis B virus, DC: dendritic cell, IL: Interleukin, NK: Natural killer, NKT: Natural killer T-cell, TLR2: Toll-like receptor 2, TNF: Tumor necrosis factor, IFN-γ: Interferon-gamma, TGF-b: Transforming growth factor beta, HbeAg: Hepatitis B envelope antigen, NTCP: Sodium taurocholate co-transporting polypeptide, NO: Nitric oxide, MHC: Major histocompatibility complex, Tregs: Regulatory T-cells, Th1-cells: T helper cells type 1, Th2-cells: T helper cells type 2, Th17-cells: T helper cells type 17, APCs: Antigen presenting cells, CTL: Cytotoxic T-cell.
Table 1.
Interleukins presented in the text with their principal effects in immune response and their role in HBV infection.
2. Interleukin 1
Interleukin-1a (IL-1a) and interleukin-1b (IL-1b) belong to pro-inflammatory cytokines, with local and systemic roles, respectively
[7]. When IL-1 binds to its receptor (IL-1R), it induces the production of other pro-inflammatory cytokines, like interleukin-6 (IL-6)
[8].
IL-1 seems to inhibit HBV entry in the hepatocyte by down-regulating the expression of NTCP and blocking cccDNA transcription in 2 different ways: (a) through the inhibitory binding of nuclear factor-kB (NF-kB) to cccDNA and (b) through hepatocytes’ de-differentiation. The latter leads to loss of hepatocyte nuclear factor-4a (HNF4a), a transcription factor controlling HBV gene expression and replication, and thus to suppression of cccDNA transcription
[8].
3. Interleukin-2
Interleukin-2 (IL-2) plays an important role in immune system regulation, as it is involved in T-cell differentiation and regulates immune response and homeostasis. IL-2 stimulation is crucial for the maintenance of T-regulatory (Treg) cells and the differentiation of CD4+ cells into effector T-cell subsets following antigen-mediated activation
[91][97]. In CD8+ cells, IL-2 signals promote effector T-cell generation and differentiation into memory cells
[12]. Interestingly, the use of IL-2 can amplify CD8+ T-cell responses, whereas the application of neutralizing IL-2 specific antibodies induces the expansion of the Treg cell population, thus favoring either immune stimulation or suppression
[12][13][14][15][92][12,13,14,15,98].
Overall, IL-2 seems to be an interesting treatment option for HBV infection, mainly due to the enhancement of anti-viral CD8+ responses.
4. Interleukin-4
Interleukin-4 (IL-4) is a crucial anti-inflammatory cytokine secreted mainly by Th2 cells that leads to proliferation, differentiation and ultimately antibody production by mature B-cells. It shifts the adaptive immune response toward humoral immunity, as it promotes naïve CD4+ cells to differentiate into Th2 cells and inhibits interferon gamma (IFN-γ) production as well as T-helper 1 (Th1) responses
[93][104].
Moreover, a handful of studies have demonstrated the capability of IL-4 to cease HBV replication in specific HCC cell lines
[16][17][16,17].
As IL-4 boosts Th2 and inhibits Th1 immune responses, hence reducing liver inflammation, it could serve as a potential treatment option for HBV infection.
5. Interleukin-5
Interleukin-5 (IL-5) is a cytokine that augments humoral immune response through promotion of maturation, differentiation and survival of B-cells. It is primarily produced by Th2 cells, and secondarily by mast cells, eosinophils and natural killer T-cells (NKT)
[18]. IL-5 plays a vital role in the immune system’s reaction against several infections
[19][94][95][19,109,110], leading to leukocyte expansion and intensification of their activation status
[96][111]. Recently published data show that IL-5 might play a pivotal role in HBV infection.
IL-5 seems to play an important role both in HBV suppression, as well as a predictive marker for treatment virological response; however, more data is required until safe consumptions are reached.
6. Interleukin-6
IL-6 is characterized by pleiotropic functions, involving not only immune response stimulation but also liver homeostasis and protein expression. IL-6 stimulates CD4+ T-cells, leading to antibody production by B-cells and enhancing the differentiation of naive T-cells to T-helper 17 (Th17) cells, while it also inhibits the differentiation of Tregs
[20][21][20,21].
IL-6 also promotes liver regeneration and protects liver cells from injuries caused by immune responses, alcohol and viral infections
[22][23][97][22,23,114].
As explained, IL-6 seems to play a critical role in HBV infection. In this line, physicians should be aware of the possibility of HBV reactivation when treating patients with autoimmune diseases and concurrent HBV infection with anti-IL-6 drugs
[98][119].
7. Interleukin-10
Interleukin-10 (IL-10) plays a unique role in the anti-inflammatory regulation of the immune system. Its main cellular sources are monocytes/macrophages, as well as Th1, Th2, Th17 and Tregs. IL-10 is, to a lesser extent, produced by B-cells, dendritic cells (DC) and NKT cells
[25][26][27][99][25,26,27,120]. IL-10 diminishes the secretion of pro-inflammatory cytokines and mediators and regulates the expression of cell surface molecules by myeloid cell subsets. In addition, it inhibits antigen presentation, thus acting as a potent anti-inflammatory cytokine, inducing immune tolerance and viral persistence
[25][26][27][28][29][99][25,26,27,28,29,120].
As anticipated, IL-10 plays a pivotal role in HBV infection; in CHB patients, higher levels of IL-10 have been consistently noted
[28][30][31][28,30,31]. Specifically, IL-10 enhances HBV replication and suppresses HBV-specific CD8+ T-cell immune responses, providing a defensive mechanism to mitigate immune-mediated hepatic injury that ultimately results in an increase in viral load and HBV infection persistence
[30][31][32][33][34][35][36][30,31,32,33,34,35,36]. The importance of IL-10 in alleviating hepatic injury is shown in a study by Wang et al., where, in patients with HBV-ACLF, a decrease in IL-10 serum levels after HBV exacerbation was noted
[37].
Overall, IL-10 seems to be pivotal in HBV replication and suppression of the anti-viral immune response; however, its anti-inflammatory role could be protective against the possibility of viral-related hepatotoxicity leading to liver failure.
8. Interleukin-12
IL-12, primarily produced by DC and phagocytes, serves as an immunomodulatory and pro-inflammatory cytokine
[38][39][40][38,39,40]. IL-12 stimulates IFN-γ production, regulates the differentiation of Th1 cells, activates NK cells, and enhances their proliferation and cytotoxicity. Consequently, it acts as a crucial bridge connecting innate and adaptive immunity
[38][39][40][38,39,40]. IL-12 secretion by DC subsets in response to various pathogens is dependent on different regulation of genes encoding IL-12, TLR expression, and cross-regulation between the different DC subsets, involving cytokines such as IL-10 and type I IFN
[38]. Moreover, IL-12 down-regulates Tregs through the stimulation of nitric oxide production by antigen presenting cells (APCs), like macrophages
[41][100][41,127]. Due to its close relation with lymphocyte activation, IL-12 is considered a potent anticancer agent with a significant role in T-cell-mediated cytolysis of cancer cells and malignant antigen presentation
[101][102][128,129].
Due to its physiological actions, IL-12 has been used as an anti-viral drug for HBV. Cavanaugh et al. reported that treatment of HBV-infected mice with IL-12 interrupted HBV replication, probably through the induction of TNF-α and IFN α/β and γ
[103][135]. Similarly, in patients with CHB, the use of IL-12 was correlated with significantly lower HBV DNA concentrations at the end of treatment, as well as during the 24 weeks of follow-up
[104][105][136,137]. In a study by Rigopoulou et al., IL-12 was combined with lamivudine as a CHB treatment. Even though this combination regimen showed better anti-viral activity than lamivudine monotherapy, HBV DNA increased after lamivudine discontinuation
[106][138]. Finally, Yang et al. suggested that increased serum IL-12 after 48 weeks of treatment with entecavir maleate contributed to an increased probability of HBeAg seroconversion
[107][139].
9. Interleukin-17
The interleukin-17 (IL-17) family comprises six members (IL-17A to IL-17F) that mediate their biological functions by binding to receptors IL-17RA and IL-17RE, which form receptor complexes and initiate downstream signaling events in the IL-17 signaling pathway
[108][140]. The most studied IL-17 family members are IL-17A and IL-17F. These interleukins promote their biological activities by binding to the heterodimeric receptor complex composed of IL-17RA and IL-17RC or by forming a ternary complex with IL-17RA and IL-17RC
[43][108][109][43,140,141]. IL-17A induces several pro-inflammatory responses, mainly the production of TNF-α and β, IL-1β, IL-6 and other pro-inflammatory cytokines from Kupffer cells, DCs, hepatic stellate cells (HSC) and monocytes
[23][43][23,43]. IL-17 seems to play a pivotal role in host defense against various fungal, bacterial and viral infections, including influenza, human immunodeficiency (HIV) and HCV viruses
[110][111][112][142,143,144]. Moreover, IL-17 has an established role in many autoimmune diseases, especially ankylosing spondylitis, psoriasis and psoriatic arthritis; anti-IL-17 monoclonal antibodies are, in fact, broadly used in treatment of these diseases
[113][114][115][145,146,147].
Altogether, the role of IL-17 in HBV infection is still uncertain, even though IL-17 has been associated with HBV-related liver injury.
10. Interleukin-21
Interleukin-21 (IL-21) is an immunoregulatory cytokine—secreted predominantly by follicular helper T (Tfh), Th17 and NKT cells
[116][117][156,157]—that contributes to immune system regulation in a pleiotropic manner depending on its microenvironmental conditions
[46]. IL-21 mainly controls maturation, activation and proliferation of CD4+, CD8+ T-cells and B cells, having a pivotal role in many autoimmune and inflammatory diseases
[47][48][49][47,48,49].
Overall, IL-21 seems to play a critical role in host defense against HBV infection by enhancing both humoral and adaptive immune responses. Interestingly, a handful of studies have shown that IL-21 could also serve as a biomarker for HBeAg seroconversion.
11. Interleukin-22
Interleukin-22 (IL-22), a member of the IL-10 family, controls tissue responses to inflammation. IL-22 is mainly secreted by T-helper 22 (Th22), Th17 and Th1 cells, as well as γδT cells, cytotoxic T-cell subsets and NKT cells. IL-22 targets and regulates tissue cells to protect them from damage and to induce their regeneration
[54][55][56][54,55,56].
As far as the role of IL-22 as a treatment option in CHB is concerned, Wang et al. showed that IL-22 producing CD3+ CD8-T-cells were suppressed after 48 weeks of treatment with peg-interferon
[57]. In addition, Hao et al. found a notable decrease in IL-22 levels after 48 weeks of telbivudine treatment in a cohort of 24 CHB patients
[118][175], suggesting that IL-22 could be used as a biomarker for disease response to treatment.
Collectively, the exact role of IL-22 in HBV infection is still unknown, since IL-22 seems to exhibit both pro- and anti-inflammatory effects, as already shown in other diseases
[119][176].
12. Interleukin 23
Interleukin-23 (IL-23) is a heterodimeric cytokine belonging to the IL-12 cytokine family. The main biological functions of IL-23 consist of promoting CD4+ T-cell proliferation and inducing IFN-γ and IL-12 production, thus enhancing DC antigen presentation
[23][70][71][23,70,71].
As opposed to other cytokines, like IL-22, the role of IL-23 in HBV infection seems to be quite straightforward; IL-23 production is enhanced by various HBV proteins and its overproduction leads to severe hepatic inflammation and consequent necrosis.
13. Interleukin 35
Interleukin-35 (IL-35) is a relatively new identified member of the IL-12 family, primarily secreted by Treg and regulatory B-cells (Breg)
[120][121][178,179]. Its predominant action is immunosuppression, which is achieved through the inhibition of T-cell proliferation and effector functions
[77][122][77,180]. IL-35 has a crucial role in immune-related diseases and in the maintenance of immunological tolerance
[120][123][124][125][126][178,181,182,183,184]. Given the crucial role of Treg in blocking effective immune responses against HBV, IL-35 could be a very important contributor in CHB and even used as a therapeutic target
[127][185]. Moreover, IL-35 seems to play a pivotal role in regulating the virus-specific Treg/Th17 balance
[78]. The latter function constitutes this interleukin, an active contributor in the control of liver inflammation, by dampening antiviral immune responses and thus suppressing inflammatory responses
[79][80][81][79,80,81].
Taken together, the above data support the multifaceted emerging effect of IL-35 on viral persistence and immune tolerance during HBV infection. Future investigation is essential for an in-depth understanding of IL-35′s immune regulation mechanisms, which may yield new immunotherapeutic strategies.