Phyllosilicates are common minerals that include the most widely known micas and clay minerals. These minerals are found in several natural environments and have unique physical-chemical features, such as cation exchange capacity (CEC) and surface charge properties. When phyllosilicates are nano-sized, their physical-chemical properties are enhanced from those of the micro-sized counterpart. Because of their unique crystal chemical and physical-chemical features, kinetics, and particle size, nano-sized clay minerals (i.e., kaolinite, montmorillonite/illite) and micas (i.e., muscovite) are of great interest in several fields spanning from environmental applications to engineered materials. This paper aims to overview the recent developments of environmental protection and technological applications employing nano-sized natural micas and clay minerals. Emphasis is given to the role that the unique physical-chemical properties of montmorillonite, vermiculite, kaolinite, and muscovite play in nanoparticle formulations, manufacture, and technical performance.
Phyllosilicates (from Greek phyllon, sheet) are the most common minerals in Earth and other planets. Phyllosilicates form a wide group that includes micas and clay minerals (Table 1). Phyllosilicates (also called layer silicates) are hydrous aluminosilicates with a structure arranged in sets of tetrahedral and octahedral sheets which are sandwiched to form a layered structure. The layered structural features provide micas and clay minerals peculiar physical-chemical properties such as cation exchange capacity (CEC), viscous and elastic capability when in contact with water, catalytic capacities, swelling behavior, and low permeability. Because of these numerous properties, phyllosilicates are of great importance in applications and innovations in mineralogy, material science, chemistry, and biomedical engineering.
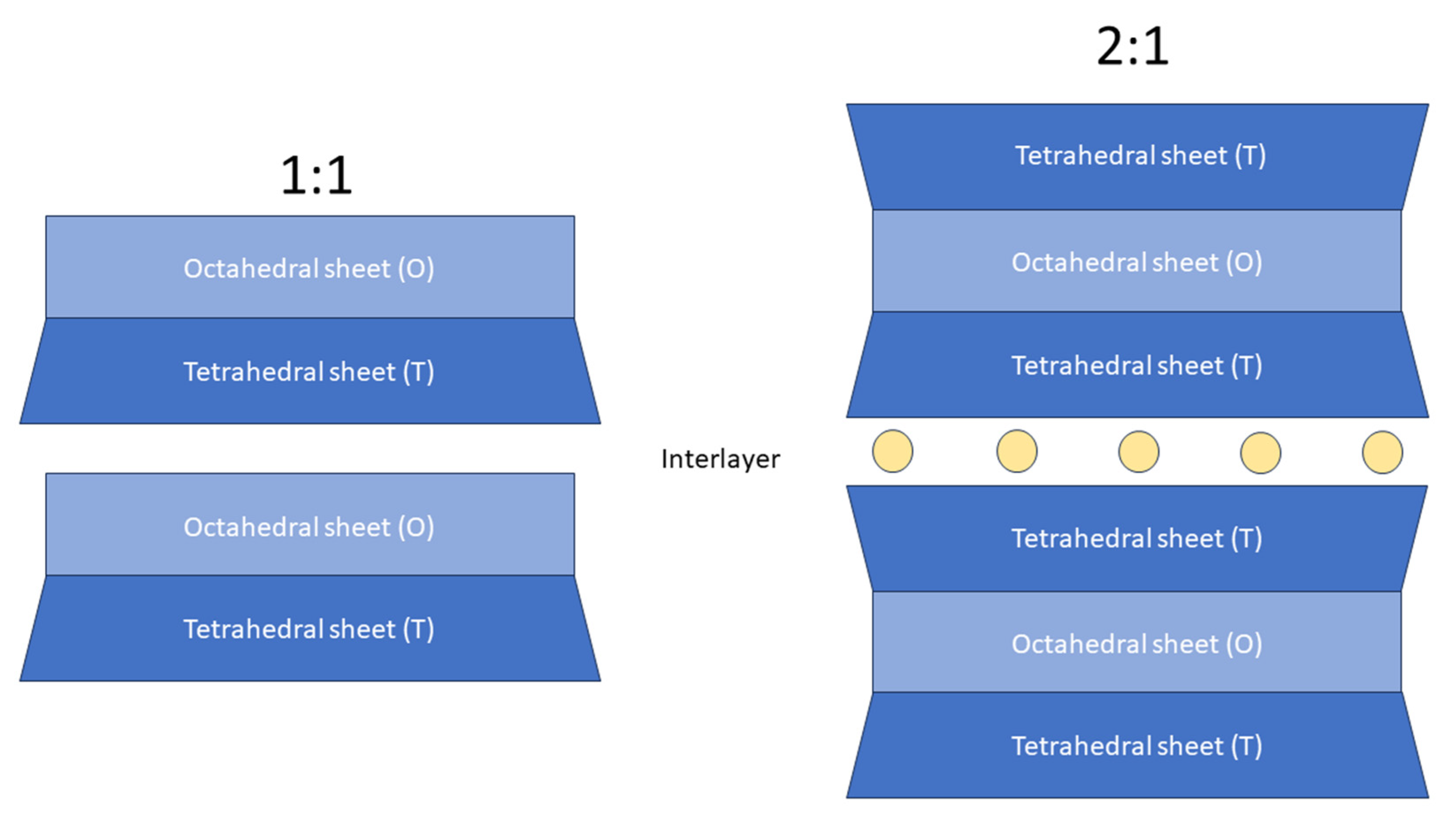
Phyllosilicates are classified based on their structural features: (i) sheet types (i.e., trioctahedral or dioctahedral) and (ii) layer stacking (1:1 or 2:1). The 1:1 (or TO) layer stacking is assembled with one tetrahedral sheet and one octahedral sheet. The 2:1 (or TOT) layer stacking is built with two tetrahedral sheets sandwiching an octahedral sheet
[1][2][3] (
Figure 1). The crystal chemical features of phyllosilicates have been extensively researched in the past decades, often applying X-ray or neutron diffraction methods
[1][2][4][5][6][7].
Figure 1. Schematic representation of phyllosilicate structural features. Yellow circles represent interlayer cations (commonly Na+, K+, Ca2+).
Micas and clay minerals (also called hydrous phyllosilicates) contain two-dimensional tetrahedral polymerization T
2O
5 (T = Si, Al, Fe
3+, etc.). Clay minerals are isostructural with micas. As in micas, clay minerals’ tetrahedral (T) sheets form a hexagonal pattern and individual octahedra are generally filled with Al
3+, Mg
2+, and Fe
2+ cations and share edges with oxygens and hydroxyl anion groups
[6][7]. As in micas, clay minerals’ octahedral (O) sheet can be distinguished as dioctahedral and trioctahedral. Compared to clay minerals where the interlayer can be partially occupied or can host water molecules, micas interlayer is usually fully occupied by K
+, Na
+, or Ca
2+ in place of alkali metals
[2]. Clay minerals are grouped in non-swelling (e.g., kaolinite, illite) and swelling clays (e.g., vermiculite and smectite). It is worth noticing that many clay minerals are of finer grain sizes and lower crystallinities than most of micas. For this reason, micas are used as an approximation for the physical-chemical properties and performance of clay minerals’ surfaces
[8].
Table 1. Endmembers of the common rock-forming mica and clay minerals groups. Data from
[1][9].
Phyllosilicates are common minerals in several oxidizing environments. Micas are widespread platy minerals found in igneous, sedimentary, and metamorphic rocks. Micas form also as hydrothermal alteration products from the hydrolysis of feldspars
[10]. Clay minerals form through the alteration of minerals such as feldspars and some ferromagnesian minerals (i.e., olivine, pyroxenes, amphiboles, etc.). The original minerals can be weathered in situ through chemical leaching caused by meteoric and ground waters and drainage. Feldspars commonly alter first to montmorillonite because the alkaline alteration environment. Montmorillonite is stable in arid climates. However, in presence of moisture, chemical leaching, and good drainage, montmorillonite alters first to halloysite and then to kaolinite. Kaolinite alters in gibbsite, if further chemical weathering occurs
[11]. The alteration series of ferromagnesian minerals is: olivine → pyroxene → amphibole → biotite → chlorite → muscovite → illite. Muscovite and illite (hydro-muscovite) are commonly found in soils and residual deposits indicating a long period of weathering
[11].
The widespread distribution in various Earth’s crust environments and their peculiar physical-chemical properties support the impact that phyllosilicates have in different technological areas, spanning from environmental applications to nanotechnology. As raw materials, micas are used as flakes and powders in the production of fillers, cosmetics, pigments, industrial coatings, and plastics. Micas have also been used as a substrate for supporting carbon films for the study of molecular and nanoscale systems. Clays as raw materials are used in numerous industrial branches, especially in paper production, polymers, chemical and agrichemical sectors, pigments, ceramic and cement industries. Clays are also applied as fillers in pharmaceuticals, cosmetics, oil industry, civil engineering, and environmental protection projects.
Nanomaterials are rising as top materials in various fields and industries owing to their nanoscale unique performances. Mineral physical-chemical properties, kinetics, and reactivity vary as a function of particle size when smaller than a few nanometers to several tens of nanometers
[12]. Nanoparticles, nanostructures, and nanosystems are developed using specific crystal chemical features by modifying their size, shape, and composition
[13]. Crystal chemical properties of nano-sized natural phyllosilicates lead to their different behavior in environmental and industrial applications. Therefore, a solid crystal chemical characterization is fundamental to shed light on nanoparticles’ layer charge, size, and other physical-chemical properties (e.g., colloidal suspensions, growth, adsorption, and dissolution) that are targeted in nanotechnology. Nano-sized particles show remarkable mechanical, rheological, thermal, optical, electrochemical, biodegradable, biocompatible properties that differ from those of the original micro-sized counterpart. Nanomaterials are also synthesized to enhance certain properties that can be used in applications spanning from medicine to electronics as well as energy, water, and food conservation
[13][14].
Nanoclays can be defined as nanoscale clay minerals sizing from about 100 nm to a few μm. Nano-sized clay minerals include smectite (vermiculite and montmorillonite), kaolinite, and illite depending on their chemical composition and applications
[15]. Nanoclays consist of TOT layer stacks of about 10 nm in size. Thus, they have approximately 657 m
2/g specific surface area compared to the micro-sized clays
[16]. Nanoclays are low-cost, and nontoxic to human health or the environment. Physical, thermal, and chemical processes can produce nano-sized clays with enhanced adsorption capacity and mechanical strength
[17]. Clay minerals as nano-sized units to produce nanomaterials are known as 21st-century green materials
[18].
Several studies focus on using nano-sized microporous phyllosilicates owing to their high surface area, adsorption and desorption capacity, and catalytic features. The nano-sized units of clay minerals are applied to prepare functional materials shaped in nanorods, nanofibers, nanotubes, and nanosheets. Nanoclays are incorporated as matrix modifying agents to fabricate several functional green materials
[13][18][19][20][21][22]. Kaolinite can be exfoliated into few-layered nanosheets and reassembled into functional materials with macroscopic controllable size and thus unique physical and mechanical properties
[23]. Mixed-layer clay minerals (e.g., montmorillonite/illite/chlorite) can be dispersed into polymer matrices forming nanocomposites used for drug delivery carrier, food packaging, advanced rubber and polymers
[24]. Nano-sized clay minerals are mixed with organic dyes for preparing Maya Blue-like pigments
[25].
This article aims to summarize the state-of-the-art of recent technological and environmental applications employing nano-sized natural micas and clay minerals with an emphasis on the importance of their physical-properties in the development of these applications. The first part of the paper outlines the methods to produce nano-sized phyllosilicates and sample preparation of phyllosilicates for X-ray powder diffraction to minimize the destruction of their structure and enhance the physical and mechanical properties. The second part of the paper reports on the most recent research progresses of technological applications employing unique physical-chemical features of the most common nano-sized phyllosilicates (montmorillonite, vermiculite, kaolinite, and muscovite).