Phyllosilicates are common minerals that include the most widely known micas and clay minerals. These minerals are found in several natural environments and have unique physical-chemical features, such as cation exchange capacity (CEC) and surface charge properties. When phyllosilicates are nano-sized, their physical-chemical properties are enhanced from those of the micro-sized counterpart. Because of their unique crystal chemical and physical-chemical features, kinetics, and particle size, nano-sized clay minerals (i.e., kaolinite, montmorillonite/illite) and micas (i.e., muscovite) are of great interest in several fields spanning from environmental applications to engineered materials. This paper aims to overview the recent developments of environmental protection and technological applications employing nano-sized natural micas and clay minerals. Emphasis is given to the role that the unique physical-chemical properties of montmorillonite, vermiculite, kaolinite, and muscovite play in nanoparticle formulations, manufacture, and technical performance.
- phyllosilicates
- nano-sized
- cation exchange capacity
- surface charge
- applications
1. Introduction
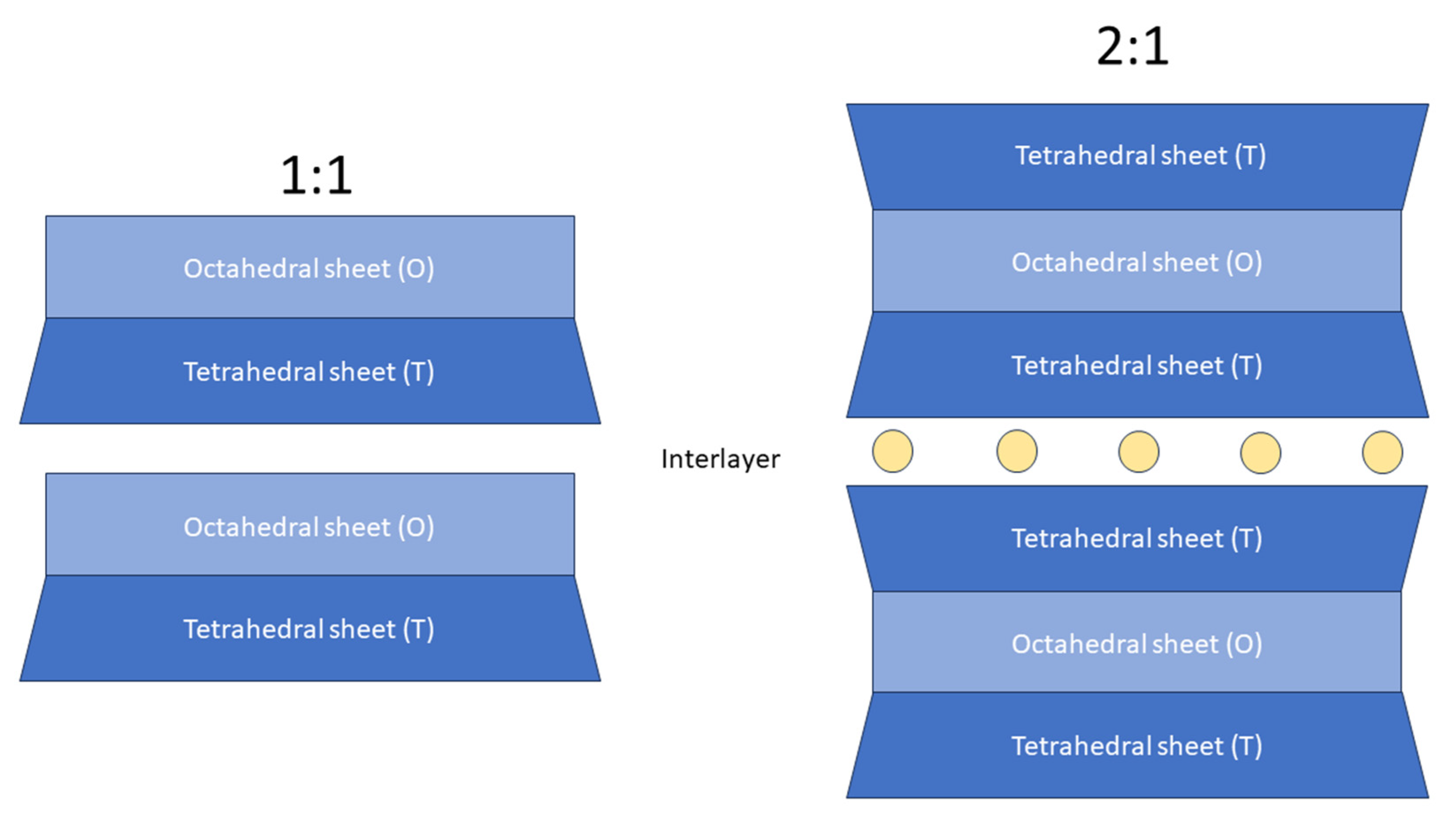
| Mica Group | |||
|---|---|---|---|
| Sheet Type | Layer Stacking | General Formula | |
| Muscovite | Dioctahedral | 2:1 | KAl2(Si3,Al)O10(OH,F)2 |
| Paragonite | Dioctahedral | 2:1 | NaAl2(Si3,Al)O10(OH)2 |
| Margarite | Dioctahedral | 2:1 | CaAl2(Al2Si2)O10(OH)2 |
| Celadonite | Dioctahedral | 2:1 | K(Mg,Fe3+,◽)(Si4O10)(OH)2 |
| Biotite | Trioctahedral | 2:1 | K(Mg,Fe)3AlSi3O10(OH,F)2 |
| Phlogopite | Trioctahedral | 2:1 | KMg3(Si3,Al)O10(OH,F)2 |
| Annite | Trioctahedral | 2:1 | KFe3(AlSi3)O10(OH)2 |
| Lepidolite | Trioctahedral | 2:1 | K(Li,Al)3(Si,Al)4O10(OH,F)2 |
| Zinnwaldite | Trioctahedral | 2:1 | KLiFe2+Al(AlSi3)O10(OH,F)2 |
| Clay group | |||
| Kaolinite | Dioctahedral | 1:1 | Al2Si2O5(OH)4 |
| Halloysite | Dioctahedral | 1:1 | Al2Si2O5(OH)4·2H2O |
| Illite | Dioctahedral | 2:1 | K0.6–0.85(Al,Mg)2(Si,Al)4O10(OH)2 |
| Talc | Dioctahedral | 2:1 | Mg3Si4O10(OH)2 |
| Pyrophyllite | Dioctahedral | 2:1 | Al2Si4O10(OH)2 |
| Palygorskite | Dioctahedral | 2:1 | (Mg,Al)2Si4O10(OH)·4(H2O) |
| Sepiolite | Trioctahedral | 2:1 | Mg4Si6O15(OH)2·6H2O |
| Nontronite | Dioctahedral | 2:1 | Na0.3Fe3+2(Si,Al)4O10(OH)2·n(H2O) |
| Montmorillonite | Dioctahedral | 2:1 | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O |
| Vermiculite | Either trioctahedral or dioctahedral | 2:1 | Mg0.7(Mg,Fe2+,Al)6(Si,Al)8O20(OH)4·8H2O |
| Glauconite | Dioctahedral | 2:1 | (K,Na)(Fe3+Al,Mg)2(Si,Al)4O10(OH)2 |
| Chlorite | Either trioctahedral or dioctahedral | 2:1:1 | (Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6 |
References
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Chapter 2—Structure and Mineralogy of Clay Minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 5, pp. 19–86. ISBN 1572-4352.
- Brigatti, M.F.; Malferrari, D.; Laurora, A.; Elmi, C. Structure and Mineralogy of Layer Silicates: Recent Perspectives and New Trends. In Layered Mineral Structures and Their Application in Advanced Technologies; Brigatti, M.F., Mottana, A., Eds.; Mineralogical Society of Great Britain and Ireland: Middlesex, UK, 2011; Volume 11, pp. 1–71. ISBN 978-0-903056-29-8.
- Brigatti, M.F.; Guggenheim, S.J. Mica Crystal Chemistry and the Influence of Pressure, Temperature, and Solid Solution on Atomistic Models. Rev. Mineral. Geochem. 2002, 46, 1–97.
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 21–81. ISBN 978-0-08-099364-5.
- Brindley, G.W.; Brown, G. (Eds.) Crystal Structures of Clay Minerals and Their X-ray Identification; Mineralogical Society of Great Britain and Ireland: Middlesex, UK, 1980; ISBN 978-0-903056-08-3.
- Brown, G. Structure, Crystal Chemistry, and Origin of the Phyllosilicate Minerals Common in Soil Clays. In Soil Colloids and Their Associations in Aggregates; De Boodt, M.F., Hayes, M.H.B., Herbillon, A., De Strooper, E.B.A., Tuck, J.J., Eds.; Springer: Boston, MA, USA, 1990; pp. 7–38. ISBN 978-1-4899-2611-1.
- Guggenheim, S.; Martin, R.T. Definition of Clay and Clay Mineral: Joint Report of the Aipea Nomenclature and CMS Nomenclature Committees. Clays Clay Miner. 1995, 43, 255–256.
- Elmi, C.; Guggenheim, S.; Gieré, R. Surface Crystal Chemistry of Phyllosilicates Using X-ray Photoelectron Spectroscopy: A Review. Clays Clay Miner. 2016, 64, 537–551.
- Sinha Ray, S. 1—An Overview of Pure and Organically Modified Clays. In Clay-Containing Polymer Nanocomposites; Sinha Ray, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–24. ISBN 978-0-444-59437-2.
- Alderton, D. Micas. In Encyclopedia of Geology, 2nd ed.; Alderton, D., Elias, S.A., Eds.; Academic Press: Oxford, UK, 2021; pp. 326–333. ISBN 978-0-08-102909-1.
- Carroll, D. Clay Minerals: A Guide to Their X-ray Identification; Special Paper; Geological Society of America: Boulder, CO, USA, 1970; ISBN 978-0-8137-2126-2.
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, Mineral Nanoparticles, and Earth Systems. Science 2008, 319, 1631–1635.
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177.
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, Incidental, and Engineered Nanomaterials and Their Impacts on the Earth System. Science 2019, 363, eaau8299.
- Kirbiyik Kurukavak, Ç. Thermal and Morphological Characterization of Bionanocomposites. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-803581-8.
- Majid, M.S.A.; Ridzuan, M.J.M.; Lim, K.H. 6—Effect of Nanoclay Filler on Mechanical and Morphological Properties of Napier/Epoxy Composites. In Interfaces in Particle and Fibre Reinforced Composites; Goh, K.L., Aswathi, M.k., De Silva, R.T., Thomas, S., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2020; pp. 137–162. ISBN 978-0-08-102665-6.
- Guan, H.; Zhao, Y. 9—Decontamination Application of Nanoclays. In Clay Nanoparticles; Cavallaro, G., Fakhrullin, R., Pasbakhsh, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–224. ISBN 978-0-12-816783-0.
- Wang, W.; Wang, A. Nanoscale Clay Minerals for Functional Ecomaterials: Fabrication, Applications, and Future Trends. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 2409–2490. ISBN 978-3-319-68255-6.
- Kausar, A. 7—Flame Retardant Potential of Clay Nanoparticles. In Clay Nanoparticles; Cavallaro, G., Fakhrullin, R., Pasbakhsh, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–184. ISBN 978-0-12-816783-0.
- Sinha Ray, S.; Bousmina, M. Biodegradable Polymers and Their Layered Silicate Nanocomposites: In Greening the 21st Century Materials World. Prog. Mater. Sci. 2005, 50, 962–1079.
- Theng, B.K.G.; Churchman, G.J.; Gates, W.P.; Yuan, G. Organically Modified Clays for Pollutant Uptake and Environmental Protection. In Soil Mineral Microbe-Organic Interactions: Theories and Applications; Huang, Q., Huang, P.M., Violante, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 145–174. ISBN 978-3-540-77686-4.
- Xu, P.; Qi, G.; Lv, D.; Niu, D.; Yang, W.; Bai, H.; Yan, X.; Zhao, X.; Ma, P. Enhanced Flame Retardancy and Toughness of Eco-Friendly Polyhydroxyalkanoate/Bentonite Composites Based on in Situ Intercalation of P-N-Containing Hyperbranched Macromolecules. Int. J. Biol. Macromol. 2023, 232, 123345.
- Cheng, H.; Zhou, Y.; Liu, Q. 6—Kaolinite Nanomaterials: Preparation, Properties and Functional Applications. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–334. ISBN 978-0-12-814533-3.
- Deng, H.; Wu, Y.; Shahzadi, I.; Liu, R.; Yi, Y.; Li, D.; Cao, S.; Wang, C.; Huang, J.; Su, H. 8—Nanomaterials From Mixed-Layer Clay Minerals: Structure, Properties, and Functional Applications. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 365–413. ISBN 978-0-12-814533-3.
- Dong, J.; Zhang, J. 13—Maya Blue Pigments Derived From Clay Minerals. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 627–661. ISBN 978-0-12-814533-3.
