Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Satinder K Brar | -- | 4197 | 2023-11-30 15:28:34 | | | |
| 2 | Sirius Huang | Meta information modification | 4197 | 2023-12-01 02:51:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shu, X.; Pulicharla, R.; Kumar, P.; Brar, S.K. Chemical and Biological Degradation for Perfluorooctanoic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/52240 (accessed on 11 March 2026).
Shu X, Pulicharla R, Kumar P, Brar SK. Chemical and Biological Degradation for Perfluorooctanoic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/52240. Accessed March 11, 2026.
Shu, Xuhan, Rama Pulicharla, Pratik Kumar, Satinder Kaur Brar. "Chemical and Biological Degradation for Perfluorooctanoic Acid" Encyclopedia, https://encyclopedia.pub/entry/52240 (accessed March 11, 2026).
Shu, X., Pulicharla, R., Kumar, P., & Brar, S.K. (2023, November 30). Chemical and Biological Degradation for Perfluorooctanoic Acid. In Encyclopedia. https://encyclopedia.pub/entry/52240
Shu, Xuhan, et al. "Chemical and Biological Degradation for Perfluorooctanoic Acid." Encyclopedia. Web. 30 November, 2023.
Copy Citation
Perfluorooctanoic acid (PFOA) is a perfluoro compound that contains an eight-carbon perfluoroalkyl chain followed by a carboxylic acid function group. It has found applications in water-resistant coating and is produced either by degrading other long-chain perfluorinated carboxylic acids or fluorotelomer alcohol. PFOA is challenging to further degrade during water treatment processes, leading to its accumulation in natural systems and causing contamination. Research has been conducted to develop several methods for its removal from the water system.
perfluorooctanoic acid (PFOA)
perfluorochemicals (PFCs)
biodegradation
chemical degradation
degradation products
defluorination
1. Background and Characterization of the Perfluoro Compounds
Perfluoro chemicals (PFCs) are man-made chemical compounds that have seen extensive use in various industrial products. They are commonly found in cleaning agents, firefighting foams, and the non-stick coatings on cooking pans [1]. Due to the high bonding energy of the C-F bond, which is approximately 485 kJ/mol in perfluoroalkyl moieties, perfluoro chemicals exhibit strong chemical and thermal stability. This characteristic renders them persistent compounds within the natural environment [2]. When an alkyl chain exceeds eight carbons, it is classified as a long-chain PFC, whereas if it contains fewer than eight carbons, it falls under the category of short-chain PFC. During wastewater treatment processes, long-chain PFCs degrade into shorter-chain products [3]. Other perfluoro compounds, such as fluorotelomer alcohol (FTOH), polyfluoroalkyl phosphates (PAPs), 8:2 fluorotelomer acrylate (8:2 FTAC), and fluorotelomer carboxylates (FTCAs), can also degrade into perfluorooctanoic acid (PFOA) [4]. As chain length decreases, so does toxicity. However, because short-chain PFCs are more hydrophilic than long-chain PFCs, they are more prevalent in natural water systems [5]. In natural water systems, approximately 88.8% of PFC contamination comprises short-chain PFCs [2].
A primary category of compounds in PFCs is referred to as per- and polyfluoroalkyl (PFAS) compounds, as indicated by USEPA. These compounds consist of a variable-length fluorinated alkyl chain followed by a functional group, as depicted in Figure 1. Figure 1 illustrates that there are primarily two types of PFAS: perfluorinated sulfonic acids (PFSAs) and perfluorinated carboxylic acids (PFCAs). PFSA consists of a variable-length fluorinated alkyl chain followed by a sulfonic acid functional group. Upon degradation and defluorination, the long alkyl chain breaks down into short-chain perfluorooctanesulfonic acid (PFOS), which contains eight carbon atoms in the alkyl chain. Another type of PFAS is PFCA, which is formed by various alkyl chains connected to a carboxylic functional group. When long-chain PFCA undergoes degradation, the length of the alkyl chain decreases, and one of the principal degradation products is PFOA [1].
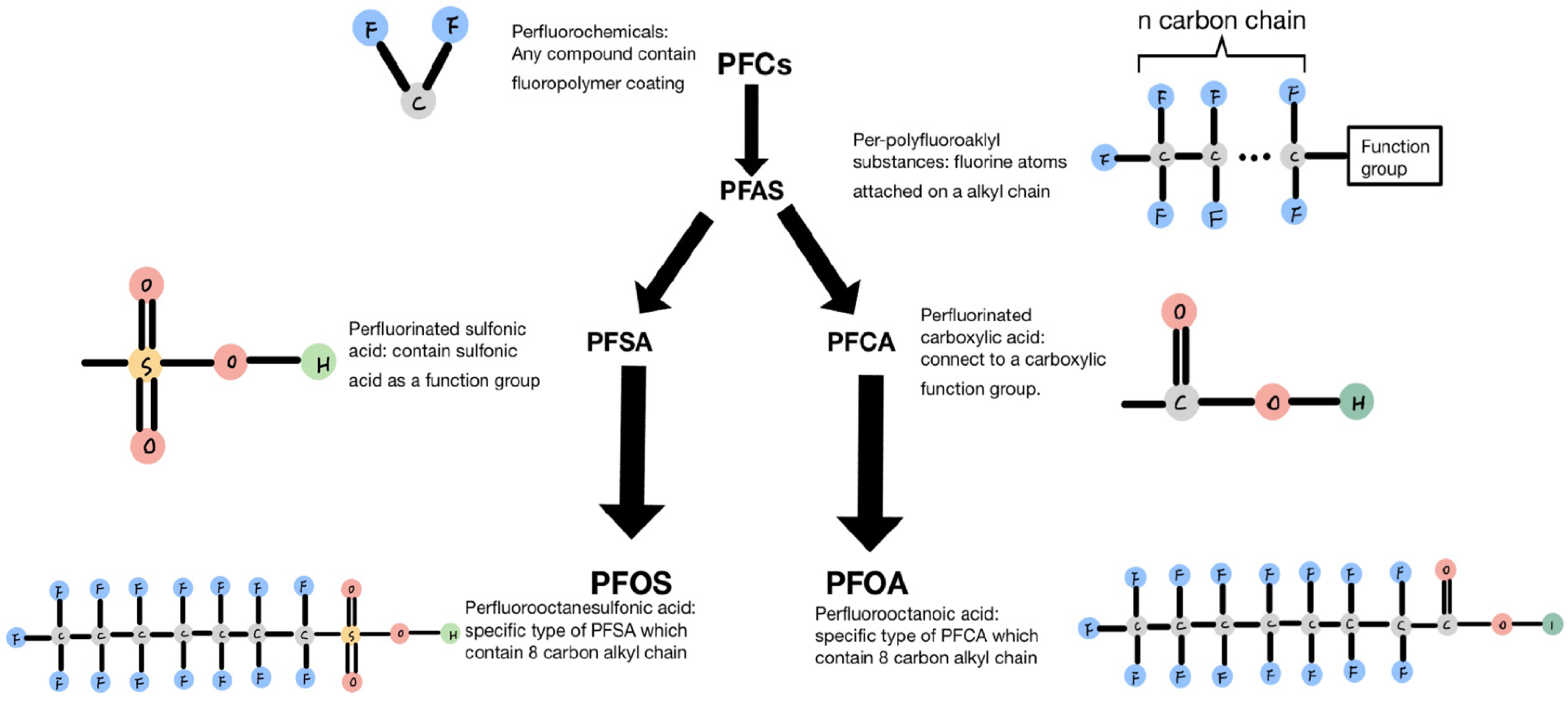
Figure 1. Classification and characteristics of each compound.
Both PFOA and PFOS are common perfluoro compound contaminants that have been widely detected in the environment. This text will specifically focus on PFOA, which exists as a white waxy powder at room temperature. PFOA is utilized in the production of Teflon for non-stick cookware and is also employed in the manufacturing of surfactants such as shampoo and floor wax, taking the form of fluoropolymers and telomer alcohols. Following their use and disposal, these chemical compounds can eventually degrade into PFOA [6]. Due to its solubility in water (9.5 g/L) and its extended half-life in water systems (92 years), PFOA rarely degrades in the natural environment and is often referred to as a ‘forever chemical’ [7]. After PFOA is released from industrial and wastewater treatment plants, some of it is emitted into the atmosphere, while the rest travels through surface-water and groundwater systems [8]. It accumulates within the biota through the food chain and ultimately enters the human body through the consumption of food or drinking water [9]. The research indicates that the aquatic toxicity of PFOA for marine invertebrates is approximately 10–24 ppm, which is several orders of magnitude higher than the detected PFOA concentration in natural systems [10]. However, it has the potential to be transported through the food chain and ultimately bioaccumulate in aquatic life and humans. Recent studies have shown that trace concentrations of PFOA have been detected in human serum worldwide [9]. Typically, it targets sensitive organs such as the liver or kidney. When PFOA combines with albumin, it can reduce glomerular filtration in the kidney or lead to liver hypertrophy or necrosis [11]. The acute toxicity of PFOA in humans is still unknown, but the lethal dose (LD50) in male rats is 175–208 mg/kg [12]. The reference dose (RfD) for PFOA is 20 ng/kg/day, and an overdose can lead to accumulation in the human body, potentially increasing the risk of cancer [11]. To monitor and regulate this emerging contaminant, the US Environmental Protection Agency (USEPA) proposes healthy drinking water advisory levels for PFOA at 70 ng/L. In 2018, Health Canada published a drinking water standard of 200 ug/L for PFOA [13]. PFOA can cause aquatic toxicity, affecting the survival, growth, and reproduction of aquatic flora and fauna, and it can also be harmful to human health [10]. Due to PFOA’s toxicity, there is increasing attention on it as an emerging contaminant. Various methods have been developed to remove these contaminants and control pollutant concentrations to ensure they remain within safe levels.
2. Physical Removal Method and Degradation Methods
Physical removal methods, such as granular activated carbon adsorption, ion exchange resin, membrane filtration, and coagulation, have been frequently documented [1][14][15][16]. Recent research has demonstrated that ion exchange resins exhibit high adsorption capacities (525–1500 mg/g) in comparison to granular activated carbon adsorption (41–120 mg/g), and both methods can achieve over 90% removal [17]. These physical removal techniques have been developed at a full scale, making them applicable in drinking water treatment plants for PFOA removal. However, their primary drawback is that they solely extract contaminants from water resources and concentrate them without degradation [18]. Therefore, an additional degradation method or step is necessary to eliminate these compounds and prevent the introduction of secondary contaminants.
There are three primary methods for degrading these compounds. The first is a non-chemical degradation method, such as plasma technology, incineration, sonolysis, and photolysis. These methods require high-energy inputs, such as UV, ultrasound, or heat, to initiate degradation. During the reaction, the input energy can break down the C-F bond and convert PFOA into CO2, free fluoride ions, and less toxic short-chain PFCA. This reaction typically occurs in a high-temperature, high-pressure environment without the involvement of any chemical compounds [19]. The second method is chemical degradation, which involves chemicals like ferric ions or persulfate as catalysts in the reaction. These chemicals lower the activation energy needed for the bond-breaking process, accelerating the degradation reaction [20]. The third degradation method is biological degradation, which represents a newer technology. During biodegradation, microorganisms consume PFOA as a carbon source [21]. Biodegradation boasts lower energy consumption and does not yield unpredictable by-products during the reaction [22].
3. Chemical Degradation
Chemical degradation is a well-established technology widely utilized in wastewater treatment systems. In most cases, it requires an energy source input, such as UV, electrical power, ultrasound, and microwave energy. With the presence of chemical catalysts, it generates free electrons or free radicals to break down the C-F bond in PFOA for degradation. The most employed chemical degradation methods include photochemical and electrochemical degradation [23][24]. Other forms of energy input are also used for degradation, including sonochemical degradation, microwave-enhanced degradation, and other chemically catalyzed degradation methods [25][26][27].
3.1. Photochemical Degradation
Most of the research on chemical degradation involves using UV as the input energy source to facilitate homogeneous or heterogeneous reactions [28][29][30]. Table 1 shows a selection of photocatalytic degradation studies that focused on low-pressure-to-medium-pressure mercury UV lamps with a wavelength of around 256 nm. This is because PFOA exhibits maximum absorbance at this wavelength, providing optimized energy for breaking down the chemical bonds in PFOA during the degradation process [30][31]. The UV lamp power varies from 9 W to 200 W, but generally, it utilizes low-power light, typically around 20 W, to maximize energy efficiency. [32] used a 15 W UV lamp with a wavelength of 240 nm to perform an anaerobic reduction reaction. Without the presence of chemicals as a reducing agent, the PFOA concentration only reduced by 13.3% in 6 h in an anaerobic environment. However, with the addition of KI as a catalyst, the PFOA concentration dropped to 93.9% in 6 h [32].
Table 1 demonstrates that photochemical degradation typically occurs at room temperature under a room atmosphere. The aerobic reaction takes place in a lower pH range, typically from 0.3 to 4.5, while the anaerobic reaction occurs in a higher pH range, usually between 8 and 12 [30][32]. There are two primary reasons for maintaining the aerobic reaction in an acidic environment. Firstly, the aerobic environment employs oxidation reagents to generate hydroxyl radicals for degradation. However, when the pH exceeds 4, it can render the oxidation agent unstable. For instance, at high pH levels, H2O2 degrades into H2O and O2, reducing the formation of free radicals. The second reason is that the precipitation of Fe(OH)3 forms at high pH, which can obstruct ongoing reactions and significantly impede the degradation performance. Therefore, the reaction should occur in a low-pH environment [33]. An experiment conducted by Song et al. (2013) involved reductive defluorination of PFOA under anaerobic conditions. The results indicated that increasing the pH from 8.1 to 10.3 enhanced the defluorination from 58% to 85%. This is because free electrons drive a reduction reaction in the experiment, and the relative quasi-stationary concentration of eaq- (ROSC) is linked to pH. With an increase in pH, more free electrons are available in the solution for defluorination to occur [34].
The photocatalyst plays a crucial role in the photochemical degradation of PFOA. Wu et al. (2017) used ZnO as a catalyst that absorbs PFOA for degradation. However, an increase in pH reduces the adsorption of PFOA on the reaction surface and consequently decreases the reaction rate [35]. Furthermore, during application, elevated temperatures reduce the surface area of ZnO, leading to a reduction in reaction efficiency [35]. TiO2 is another popular catalyst employed in photochemical degradation. It contributes electrons during the reduction reaction and provides a reaction surface [36]. The effectiveness of the TiO2 surface is influenced by pH, leading to changes in degradation efficiency [36]. Araniti et al. (2015) utilized Mg-aminoclay-coated nanoscale zero-valent iron as a catalyst for PFC degradation. After aging for 3 days, the degradation rate decreased by 15% due to the reduced amount of reactive iron in the coating [37].
The main degradation products of PFOA through photochemical degradation are fluoride ions, CO2, and other short-chain PFOA compounds [38]. Perfluoroheptanoic acid (PFHpA) is the initial degradation by-product that emerges during the photochemical degradation of PFOA [39]. Throughout the degradation process, PFOA undergoes stepwise defluorination and shortens its alkyl chain, resulting in the production of C7 PFHpA [40]. Additionally, other short-chain PFCA compounds, such as C6 perfluorohexanoic acid (PFHxA), C5 per-fluoropentanoic acid (PFPeA), and C4 perfluorobutanoic acid (PFBA), have been detected in the final solution, each with a low concentration of approximately nanomoles per liter [34]. The degradation efficiency in photochemical degradation ranges from 70% to the complete removal of PFOA, with 30% to 90% defluorination occurring during a reaction time of 4–24 h [32]. It is worth noting that the defluorination rate is consistently lower than the PFOA removal rate; Song et al. (2013) achieved up to 100% removal of PFOA within 1 h but only managed a 40% defluorination rate [34]. This phenomenon is due to the higher energy of the C-F bond compared to the C-C bond, causing the degradation reaction to undergo chain shortening processes before complete defluorination is achieved.
Table 1. Photochemical degradation of perfluorooctanoic acid (PFOA).
| Wavelength (nm) | Power | PFOA Initial Concentration (mg/L) | Other Chemical Content | Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|---|---|
| 254 | 200 W | 140–1387 | 34 mg/L of H2O2 0.48 MPa O2 |
Room temperature pH 0.8 |
0.674 mg/h Up to100% removal |
[23] |
| 220–460 | 200 W | 559 | 9600 mg/L S2O82− | 0.3 pH 0.48 MPa O2 25 °C |
139.5 mg/h Up to 100% removal |
[38] |
| 254 | 15 W | 40 | 500 mg/L b-Ga2O3 | Anaerobic | 5.3 mg/h 40% removal 15% defloration |
[41] |
| 254 | 15 W | 10.35 | 16 mg/L–133 mg/L KI | 9 pH Room temperature anoxic |
1.62 mg/h 93.9% removal 89% defluorination |
[32] |
| 254 and 185 | 23 W | 4.14 | 12 mg/L NaIO4 | 1.8 mg/h 87% removal 25% defluorination |
[42] | |
| C | 6 W | 50 | 500 mg/L TiO2 | pH4 25 °C |
8.3 mg/h Up to 100% removal 50% defluorination |
[36] |
| 254 | 9 W | 8.2 | 1020 mg/L H2O2 152 mg/L FeSO4 |
25 °C pH 3 |
1.5 mg/h 58% defluorination 93% removal |
[33] |
| 254 | 10 W | 8.3 | 960 mg/L SO42− | Anaerobic pH 10.3 |
2.7 mg/h 80% defluorination Up to 100% removal |
[34] |
| 254 | 23 W | 19.8 | 1140 mg/L FeCl3 270 mg/L H2C2O4 |
Room temperature pH 2.4 anoxic |
3.8 mg/h 80% removal 20% defluorination |
[30] |
| 254 | 28 W | 10 | 25 mg/h O3 1 L/min air 2000 mg/L ZnO |
pH 4.5 25 °C |
1.75 mg/h 70% removal |
[35] |
| 210–280 | 75 W | 0.11 0.7 L/min |
2 L/min UV ozonated air | 0.00033 mg/h Up to 100%removal |
[43] | |
| 254 | 18 W | 10 | 1260 mg/L Na2SO3 | pH 12 20 °C |
10 mg/h 93% defluorination Up to 100% removal |
[44] |
| 350–780 | 500 W | 0.5 | BiOCl/Zn-Al hydrotalcite 500 mg/L | pH 2 | 90% removal 0.075 mg/L |
[40] |
| 254 | 23 W | 5 | 2420 mg/L Fe(NO3) 2000 mg/L Fe2(SO4)3 2550 mg/L NaNO3 |
pH2.4 Room temperature Anaerobic |
92% removal | [39] |
| 225–300 | 450 W | 100 | Zn0.5Cu0.5Fe2O4 8300 mg/L Oxalic acid 9000 mg/L |
pH 2.03 27 °C |
15 mg/h 30% removal |
[45] |
3.2. Electrochemical Degradation
Electrochemical degradation utilizes boron-doped diamond and Ti-SnO2 electrodes submerged in an electrolyte containing ion-exchange reagents, such as NaClO4, Na2SO4, NH4OH, and NH4Cl, for PFOA degradation [24][46][47]. This process generates free electrons on the electrode side, which then react with PFOA to produce free fluoride ions and CO2. Table 2 illustrates that the current density during electrical chemical degradation typically varies from 10 to 50 mA/cm2. Higher current densities remove PFOA more quickly but consume more energy [24]. Witt et al. (2020) experimented with various current densities during the degradation process to strike a balance between energy consumption and defluorination performance. During the first hour, they used 50 mA/cm2 to initiate PFOA degradation, then switched to 5 mA/cm2 for long-term defluorination, ultimately resulting in 30% energy savings [46].
As depicted in Table 2, in most of the studies, the reactions occurred at room temperature in a pH 5 acid solution. The optimal electrode separation distance falls within the range of 5–25 mm, dependent on the reacting current density. When the current density remains constant, an increase in the electrode separation distance results in a decrease in the efficiency of the reaction [48]. The degradation of PFOA requires free radicals. Ma et al. (2015) demonstrated that the degradation of PFOA decreased when the pH increased from 5 to 11 or decreased to 3, primarily due to the blocking of radical formation [47]. Table 2 illustrates that most of the electrochemical degradation processes occur in an acidic environment, typically within the pH range of 3 to 5.
However, in certain special conditions, such as in the treatment of wastewater or landfill leachate, the reaction still occurs in a natural environment. Nevertheless, it necessitates a higher current density, and the degradation efficiency is lower compared to the acidic environment [49][50]. A higher initial concentration of PFOA typically leads to an increased reaction rate. However, due to the limitations of ion exchange at the anode, if the initial PFOA concentration surpasses 100 ppm, it can also impede the reaction. This is because elevated levels of PFOA generate numerous short-chain by-products, such as C7 PFHxA, and a higher concentration of these by-products can deplete the available radicals and extinguish the reaction [47].
Table 2. Electrical chemical degradation of perfluorooctanoic acid (PFOA).
| Electrodes | Current Density (mA/cm2) | PFOA Initial Concentration (mg/L) | Chemical Reagent | Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|---|---|
| Ti/SnO2-Sb/PbO2 | 10 | 100 | 10 mg/L NaClO4 | 25 °C pH 5 5 mm distance |
60 mg/h 91% removal 77.4% defluorination |
[24] |
| Ti/SnO2-Sb/Yb-PbO2 | 20 | 100 | 12,240 mg/L Na2SO4 | pH 5 25 °C 500 rpm 5 mm distance |
38 mg/h 95% removal 75% defluorination |
[47] |
| B and N codoped diamond | 4 | 50 | 6122 mg/L Na2SO4 | pH 4.8 2.5 cm distance |
25 mg/h Up to 100% removal 80% defluorination in 3 h |
[51] |
| Boron-doped diamond | 0.6 | 1000 | 12,240 mg/L NaClO4 1.5% TiO2 |
1 cm distance 600 mW/cm2 UV at 254 nm |
166 mg/h 50% removal |
[28] |
| Boron-doped diamond | 21.4 | 5.5 | 1500 mg/L Na2SO4 | 2 cm distance 6.27–8.53 pH |
2.75 mg/h Up to 100% removal 40% defluorination |
[49] |
| Boron-doped diamond | 75 | 0.1 | 17.466 mg/L K2HPO4 250 μL/L H3PO4 |
pH 7.03–7.29 20–25 °C 2.5 cm distance |
0.0125 mg/h | [50] |
| Boron-doped diamond | 50 | 1.19 | 6.25% NH4OH 2% CH3OH |
Anaerobic | 0.29 mg/h | [46] |
| Ti and boron-doped diamond | 5 V/SHE | 19.8 | 2840 mg/L Na2SO4 | 25 °C 3.5 cm pH 4 anaerobic |
Up to 100% removal 90% defluorination 3.96 mg/h |
[52] |
| Boron-doped diamond and stainless steel |
10 | 20 | 10% Na2SO4 | pH 3 50 °C 1 cm distance 700 rpm |
Up to 100% removal 70% defluorination 10 mg/h |
[48] |
Similar to photochemical degradation, electrochemical degradation also required 1–4 h to achieve 95% removal of PFOA, along with a 75% defluorination, as reported by Ma et al. in 2015. The degradation products are reminiscent of those produced through photochemical degradation, including short-chain PFCAs like PFHpA, PFHxA, PFPeA, and PFBA. As a result of a stepwise degradation process, intermediate products, such as C7 PFCA, reach their maximum concentrations shortly after the reaction begins. However, as the experiment continues, these intermediate concentrations gradually decrease and are further degraded into even shorter-chain PFCA compounds, such as PHFpA, PFHxA, and PFBA, as documented by Ma et al. in 2015.
3.3. Other Degradation Methods
In addition to photochemical and electrochemical degradation methods, a few other techniques are employed for PFOA degradation. These methods include sonochemical, microwave-enhanced, and other chemical-catalyzed degradation methods. Unlike UV light or electrical currents, which serve as energy sources, these chemical degradation processes utilize substances like Na2S2O8, argon gas, or vitamin B13 as catalysts. They may combine with various forms of energy or even operate without additional energy input to facilitate degradation.
In sonochemical degradation, argon gas and ozone are introduced during sonolysis. This process degrades PFOA by generating bubbles under ultrasound. When these bubbles rupture, they create high temperatures at the interface, enabling interface pyrolysis. Argon gas, characterized by a high polytopic index, exhibits greater heat flux during transitions between different thermodynamic states. Consequently, the addition of argon gas during degradation can elevate the interface temperature and accelerate the reaction rate [25]. Sonochemical degradation employs a 200–250 W ultrasound generator operating at a frequency of 200–612 kHz to facilitate a first-order reaction with a rate of 0.032 min−1 [25][53]
The reaction temperature typically falls within the range of 10–20 °C, and the reaction occurs in an acidic solution. However, this method has several limitations. Firstly, it can only effectively degrade PFOA at low concentrations (10–100 ppb of PFOA). Secondly, when treating contaminated water sources such as landfill groundwater, it can be susceptible to the influence of volatile compounds like acetone, diisopropyl ether, and butanone, as these compounds can lower the interfacial temperature and hinder the formation of OH radicals [53].
Researchers have also observed that microwaves enhance degradation in sonochemical processes. In the study of Li et al. (2021), a 300 W microwave oven was used to generate a 2450 MHz microwave, heating specific compounds. This reaction, conducted under high temperatures in an acidic environment, produced free radicals, resulting in the removal of up to 80% of PFOA in just 4 h [27]. During sonochemical degradation, chemicals such as H2O2 are added to initiate Fenton-like processes or Na2S2O8 to produce SO4− ions, which then engage in persulfate oxidation reactions [27][54]. However, similar to photochemical reactions, the degradation rate is significantly affected by pH and temperature and can also be hampered by the presence of Cl- ions [27]. Another notable drawback of this reaction is its high energy consumption, approximately 458 kWh/mol, owing to the dissipation of energy as heat during the reaction [26].
Some chemical degradation processes do not require additional power input to initiate degradation. For instance, [26] introduced a method that employs vitamin B13 as an electron transporter in combination with CuSO4 and NaBH4, resulting in the formation of zero-valence copper to expedite the reaction [26]. By adding 45 nM of titanium citrate as a reducing agent, they achieved a 65% removal of PFOA within 18 h [26]. In another approach, researchers utilized KMnO4 in combination with HCl for PFOA degradation. However, this method, although effective, required an extended period of up to 6 months to remove 90% of contaminants [55]. Comparing the experiment of Fang et al. (2016) with other chemical degradation methods reveals that even without the need for additional power input, degradation can occur. Nevertheless, due to the stubborn stability of PFOA, the reaction rate remains relatively low. Balancing energy consumption with degradation efficiency is a potential avenue for future research in chemical degradation.
4. Biological Degradation
Biological degradation represents an emerging technology when compared to chemical degradation, and research in this field remains rather limited. Only a handful of research articles have been published, with a specific focus on the biodegradation of PFOA. Previous research has demonstrated the thermodynamic favourability of dehalogenation reactions and the maturity of microbial dechlorination technology. This indicates that bacteria can indeed derive energy from breaking down perfluoro compounds [6]. However, the majority of biodegradation research centers on the degradation of fluorotelomer alcohols (FTOH), which ultimately yield PFOA as a final product without further breakdown [6][56]. Furthermore, it has been reported that biodegradation typically requires at least one hydrogen atom to be attached to the alkyl chain to initiate the reaction. In the case of PFOA, however, the alkyl chain only features fluorine atoms, forming high-energy C-F bonds (485 kJ/mol), which render them even more challenging to degrade compared to other perfluorinated compounds, such as fluorotelomer alcohols [57].
Schröder et al. (2004) employed sewage-treatment-plant sludge as a bacterial seed in a closed-loop system to treat PFOA for 28 days [58][59]. In recent years, scientists have managed to isolate and acclimate Pseudomonas Parafulv and Acidimicrobium sp A6 strains from PFOA-enriched environments, allowing them to perform biological degradation reactions using PFOA as a carbon source. Bacteria exhibit a certain level of tolerance to PFOA concentrations; when the concentration of PFOA reaches 500 ppm, the bacterial strain reaches a maximum population of 0.175 OD600 and achieves a degradation rate of 30% over 72 h [2]. However, if the concentration exceeds this threshold, both the OD600 and degradation rate begin to decline due to the inhibitory effects of PFOA on bacterial growth.
In Table 3, biodegradation can occur either aerobically or anaerobically under culturing conditions of 30 °C and 150 rpm. However, the majority of experiments are conducted in anaerobic environments [60]. The primary reason for biological degradation occurring in an anaerobic environment rather than an aerobic one is the highly oxidized nature of PFOA. It struggles to donate electrons for further oxidation reactions in an aerobic environment [22]. In anaerobic conditions, PFOA can preferentially accept electrons to undergo reduction reactions, leading to no removal of PFOA under Liou’s aerobic degradation conditions but achieving 67% removal in Huang’s anaerobic degradation experiment [60]. Table 3 presents the anaerobic degradation of PFOA carried out by an Acidimicrobium sp A6 strain in an acidic environment with a pH range of 4.5–5.5. There has only one aerobic experiment, conducted by Yi et al. (2016), in a neutral environment using Pseudomonas parafulva for degradation. During their experiment, Yi et al. (2016) supplemented yeast extract and glucose to support metabolism. This can be used during the acclimation and isolation stages to boost bacterial populations during the degradation processes and increase the final removal rate of PFOA [2].
Table 3. Biological degradation of perfluorooctanoic acid (PFOA).
| Sources of Bacteria | Initial PFOA Concentration | Reaction Environment | Chemical Supplement | Degradation Efficiency | Reference |
|---|---|---|---|---|---|
| Pseudomonas parafulva | 500 mg/L | pH 7 30 °C 160 rpm |
1000 mg/L yeast extract 2% inoculum 5000 mg/L NH4NO3 2000 mg/L NaCl 1000 mg/L KH2PO4 1000 mg/L K2HPO4 500 g/L MgSO4·7H2O 50 mg/L CaCl2·2H2O |
48% removal In 5 day |
[2] |
| Acidimicrobium sp. Strain A6 | 0.1 mg/L | Anaerobic 4.5 pH 30 °C 150 rpm |
1260 mg/L Fe2O3·0.5H2O 150 mg/L NH4Cl 25 mg/L (NH4)2SO4 20 mg/L NaHCO3 71 mg/L KHCO3 7.523 mg/L KH2PO4 101 mg/L MgSO4·7H2O 58.8 mg/L CaCl2·2H2O 1 mL trace element 1 mL vitamin solution |
60% removal In 100 d |
[60] |
| Acidimicrobium sp. Strain A6 | 10 mg/L | 25 °C Anaerobic pH 4.5–5 |
506 mg/L Fe2O3·0.5H2O 177 mg/L NH4Cl 77.9 mg/L (NH4)2SO4 19.8 mg/L NaHCO3 71 mg/L KHCO3 9 mg/L KH2PO4 100 mg/L MgSO3·7H2O 60 mg/LCaCl2·2H2O 1 mg/L trace element 1 mL/L vitamin solution |
67.7% removal in 150 d | [61] |
| Acidimicrobium sp. Strain A6 | 100 mg/L | Anaerobic pH 5–5.5 240 rpm Room temperature stainless steel as cathode and graphite plate as anode. |
203.3 mg/L NH4Cl 79.28 mg/L (NH4)2PO4 20.16 mg/L NaHCO3 71 mg/L KHCO3 8.98 mg/L KH2PO4 101 mg/L MgSO4·7H2O 45.5 mg/L CaCl2 55.24 mg/L AQDS 1 mL/L vitamin supplement |
77% removal in 18 d | [57] |
The most significant degradation occurs in an anaerobic environment using a closed-loop system for treating PFOA-containing wastewater, achieving up to 100% removal within 28 days [58]. However, it does not indicate the defluorination rate during the reaction. Recent research employed Acidimicrobium sp A6 Strain for anaerobic degradation, achieving a maximum defluorination rate of 63% over a 100-day reaction period [60]. When researchers substituted iron with electrolysis cells, similar degradation results were achieved, removing 60–70% of PFOA within a shorter timeframe of 18 days [57]. Nevertheless, all these experiments were conducted on a laboratory scale in a monoculturing environment and were not directly used for wastewater treatment.
When the initial PFOA concentration increases from 0.1 to 200 ppm, the consumption rate of the reduction reagent does not change significantly. This indicates that the reaction rate is barely affected by PFOA at low initial concentrations [60]. In aerobic reactions, when the concentration increases from 0 to 500 ppm, the bacterial population also increases, showing that higher PFOA levels can stimulate bacterial growth. However, due to the toxicity of PFOA, microbacteria exhibit a certain tolerance to PFOA concentrations in the culturing media. When the concentration of PFOA exceeds 500 ppm, the reaction rate decreases because the toxicity of PFOA inhibits bacterial growth [2].
To maintain proper bacterial growth in a monoculture environment, it is necessary to add other chemical compounds to the culturing solution. Carbon, nitrogen, and phosphorus are the primary elements required by bacteria for building their cells. Therefore, studies have utilized NH4Cl, (NH4)2SO4, and NH4NO3 as nitrogen sources and KH2PO4 and K2HPO4 as phosphate sources [61][62]. Regarding the carbon source, some studies used PFOA, intending for bacteria to consume it as an energy source during metabolism. However, adding glucose as a co-metabolism compound can also stimulate bacterial growth and increase the degradation rate of PFOA from 30% to 45% [2]. Calcium and magnesium are essential elements for maintaining proper bacterial metabolism, and they have been added in the form of MgSO4 and CaCl2. Additionally, other trace elements and vitamin solutions have been introduced into this reaction. Lastly, alkalinity can act as a buffer for long-term culturing periods. Therefore, most of the experiments shown in Table 3 included NaHCO3 and KHCO3 as additional alkalinity sources.
References
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Nguyen, T.A.H. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation. J. Water Process Eng. 2020, 36, 101393.
- Yi, L.; Chai, L.; Xie, Y.; Peng, Q. Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet. Mol. Res. 2016, 15, gmr.15028043.
- Canada, Health. “Government of Canada.” Long-Chain Perfluorocarboxylic Acids (PFCAs), Their Salts, and Their Precursors—Information Sheet—Canada.ca. 19 May 2023. Available online: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/chemicals-glance/long-chain-perfluorocarboxylic-acids-pfcas-salts-precursors.html. (accessed on 10 September 2023).
- Dinglasan, M.J.A.; Ye, Y.; Edwards, E.A.; Mabury, S.A. Fluorotelomer Alcohol Biodegradation Yields Poly- and Perfluorinated Acids. Environ. Sci. Technol. 2004, 38, 2857–2864.
- Eref. The History of PFAS: PFAS Carbon Chain Length—It Is Just a Number or Is It? 10 April 2023. Available online: https://www.waste360.com/pfas-pfoas/pfas-carbon-chain-length-it-just-number-or-it (accessed on 10 September 2023).
- Parsons, J.R.; Sáez, M.; Dolfing, J.; De Voogt, P. Biodegradation of Perfluorinated Compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71.
- USEPA. What Are PFCs and How Do They Relate to Per- and Polyfluoroalkyl Substances (PFASs)? 7 October 2016. Available online: https://19january2017snapshot.epa.gov/pfas/what-are-pfcs-and-how-do-they-relate-and-polyfluoroalkyl-substances-pfass_.html (accessed on 10 November 2022).
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic Acid (PFOA), an Emerging Drinking Water Contaminant: A Critical Review of Recent Literature. Environ. Res. 2012, 116, 93–117.
- Liu, J.; Avendaño, S.M. Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ. Int. 2013, 61, 98–114.
- Hayman, N.T.; Rosen, G.; Colvin, M.A.; Conder, J.; Arblaster, J.A. Aquatic toxicity evaluations of PFOS and PFOA for five standard marine. Chemosphere 2021, 273, 129699.
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 1.
- Olson, C.T.; Andersen, M.E. The Acute Toxicity of Perfluorooctanoic and Perfluorodecanoic Acids in Male Rats and Effects on Tissue Fatty Acids. Toxicol. Appl. Pharmacol. 1983, 70, 362–372.
- Goldhaber, S. On PFAS Guidelines, Oh Canada! 14 February 2023. Available online: https://www.acsh.org/news/2023/02/14/pfas-guidelines-oh-canada-16882#:~:text=Canada’s%20goal%20is%20to%20eventually,total%20PFAS%20in%20September%202022 (accessed on 10 September 2023).
- Lenka, S.P.; Kah, M.; Padhye, L.P. A Review of the Occurrence, Transformation, and Removal of Poly- and Perfluoroalkyl Substances (PFAS) in Wastewater Treatment Plants. Water Res. 2021, 199, 117187.
- Speth, T. Speech. PFAS Treatment in Drinking Water and Wastewater—State of the Science. Speech presented at the PFAS Science Webinars for EPA Region 1 and State & Tribal Partners. 16 September 2020. Available online: https://www.epa.gov/research-states/pfas-treatment-drinking-water-and-wastewater-state-science#:~:text=It%20is%20currently%20known%20that,and%20high%2Dpressure%20membrane%20systems (accessed on 10 September 2023).
- Xiao, F.; Simcik, M.F.; Gulliver, J.S. Mechanisms for removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation. Water Res. 2013, 47, 49–56.
- Zhang, D.; Zhang, W.; Liang, Y. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution—A review. Sci. Total. Environ. 2019, 694, 133606.
- Saltwroks. Treating Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) Contaminated Wastewater and Landfill Leachate. 4 October 2019. Available online: https://www.saltworkstech.com/articles/pfas-contaminated-wastewater-and-landfill-leachate/ (accessed on 15 September 2023).
- Longendyke, G.K.; Katel, S.; Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: A comprehensive review. Environ. Sci. Process Impacts 2021, 24, 196–208.
- Deng, Y.; Liang, Z.; Lu, X.; Chen, D.; Li, Z.; Wang, F. The degradation mechanisms of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) by different chemical methods: A critical review. Chemosphere 2021, 283, 131168.
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O.N. Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538.
- Liou, J.-C.; Szostek, B.; DeRito, C.; Madsen, E. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183.
- Hori, H.; Hayakawa, E.; Einaga, H.; Kutsuna, S.; Koike, K.; Ibusuki, T.; Kiatagawa, H.; Arakawa, R. Decomposition of Environmentally Persistent Perfluorooctanoic Acid in Water by Photochemical Approaches. Environ. Sci. Technol. 2004, 38, 6118–6124.
- Lin, H.; Niu, J.; Ding, S.; Zhang, L. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2eSb, Ti/SnO2eSb/PbO2 and Ti/SnO2eSb/MnO2 anodes. Water Res. 2012, 46, 2281–2289.
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical Decomposition of Perfluorooctane Sulfonate and Perfluorooctanoic Acid. Environ. Sci. Technol. 2005, 39, 3388–3392.
- Lee, Y.-C.; Chen, Y.-P.; Chen, M.-J.; Kuo, J.; Lo, S.-L. Reductive defluorination of perfluorooctanoic acid by titanium(III) citrate with vitamin B12 and copper nanoparticles. J. Hazard. Mater. 2017, 340, 336–343.
- Lee, Y.; Lo, S.; Kuo, J.; Hsieh, C. Decomposition of perfluorooctanoic acid by microwave-activated persulfate: Effects of temperature, pH, and chlorideions. Front. Environ. Sci. Eng. 2012, 6, 17–25.
- Ochiai, T.; Moriyama, H.; Nakata, K.; Murakami, T.; Koide, Y.; Fujishima, A. Electrochemical and Photocatalytic Decomposition of Perfluorooctanoic Acid with a Hybrid Reactor Using a Boron-doped Diamond Electrode and TiO2 Photocatalyst. Chem. Lett. 2011, 40, 682–683.
- Giri, R.R.; Ozaki, H.; Morigaki, T.; Taniguchi, S.; Takanami, R. UV photolysis of perfluorooctanoic acid (PFOA) in dilute aqueous solution. Water Sci. Technol. 2011, 63, 276–282.
- Wang, Y.; Zhang, P. Enhanced photochemical decomposition of environmentally persistent perfluorooctanoate by coexisting ferric ion and oxalate. Environ. Sci. Pollut. Res. 2016, 23, 9660–9668.
- Rayne, S.; Forest, K. Perfluoroalkyl sulfonic and carboxylic acids: A critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. J. Environ. Sci. Health Part A 2009, 44, 1145–1199.
- Qu, Y.; Zhang, C.; Li, F.; Chen, J.; Zhou, Q. Photo-reductive defluorination of perfluorooctanoic acid in water. Water Res. 2010, 44, 2939–2947.
- Tang, H.; Xiang, Q.; Lei, M.; Yan, J.; Zhu, L.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 2012, 184, 156–162.
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydratedelectrons in a sulfite-mediated UV photochemical system. Journal Hazard. Mater. 2013, 262, 332–338.
- Wu, D.; Li, X.; Tang, Y.; Lu, P.; Chen, W.; Xu, X.; Li, L. Mechanism Insight of PFOA Degradation by Zno Assisted-Photocatalytic Ozonation: Efficiency and Intermediates. Chemosphere 2017, 180, 247–252.
- Cho, I.-H. Degradation and Reduction of Acute Toxicity of Environmentally Persistent Perfluorooctanoic Acid (PFOA) Using VUV Photolysis and TiO2 Photocatalysis in Acidic and Basic Aqueous Solutions. Toxicol. Environ. Chem. 2011, 93, 925–940.
- Arvaniti, O.S.; Hwang, Y.; Andersen, H.R.; Stasinakis, A.S.; Thomaidis, N.S.; Aloupi, M. Reductive degradation of perfluorinated compounds in water using Mg-aminoclay coated nanoscale zero valent iron. Chem. Eng. J. 2015, 262, 133–139.
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S.; Kiatagawa, H.; Arakawa, R. Efficient Decomposition of Environmentally Persistent Perfluorocarboxylic Acids by Use of Persulfate as a Photochemical Oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388.
- Yuan, Y.; Feng, L.; Xie, N.; Zhang, L.; Gong, J. Rapid photochemical decomposition of perfluorooctanoic acid mediated by a comprehensive effect of nitrogen dioxide radicals and Fe3+/Fe2+ redox cycle. J. Hazard. Mater. 2020, 388, 121730.
- Yang, Y.; Zheng, Z.; Yang, M.; Chen, J.; Li, C.; Zhang, C.; Zhang, X. In-situ fabrication of a spherical-shaped Zn-Al hydrotalcite with BiOCl and study on its enhanced photocatalytic mechanism for perfluorooctanoic acid removal performed with a response surface methodology. J. Hazard. Mater. 2020, 399, 123070.
- Zhao, B.; Zhang, P. Photocatalytic Decomposition of Perfluorooctanoic Acid with β-Ga2O3 Wide Bandgap Photocatalyst. Catal. Commun. 2009, 10, 1184–1187.
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical Decomposition of Perfluorooctanoic Acid in Aqueous Periodate with VUV and UV Light Irradiation. J. Hazard. Mater. 2010, 179, 1143–1146.
- Dai, X.; Xie, Z.; Dorian, B.; Gray, S.; Zhang, J. Comparative Study of Pfas Treatment by UV, UV/Ozone, and Fractionations with Air and Ozonated Air. Environ. Sci. Water Res. Technol. 2019, 5, 1897–1907.
- Bentel, M.J.; Liu, Z.; Yu, Y.; Gao, J.; Men, Y.; Liu, J. Enhanced Degradation of Perfluorocarboxylic Acids (Pfcas) by UV/Sulfite Treatment: Reaction Mechanisms and System Efficiencies at Ph 12. Environ. Sci. Technol. Lett. 2020, 7, 351–357.
- Verma, S.; Mezgebe, B.; Sahle-Demessie, E.; Nadagouda, M.N. Photooxidative decomposition and defluorination of perfluorooctanoic acid (PFOA) using an innovative technology of UVevis/ZnxCu1-xFe2O4/oxalic acid. Chemosphere 2021, 280, 130660.
- Witt, S.; Rancis, N.; Ensch, M.; Maldonado, V. Electrochemical Destruction of ‘Forever Chemicals’: The Right Solution at the Right Time. Electrochem. Soc. Interface 2020, 29, 73.
- Ma, Q.; Liu, L.; Cui, W.; Li, R.; Song, T.; Cui, Z. Electrochemical degradation of perfluorooctanoicacid (PFOA) by Yb-doped Ti/SnO2–Sb/PbO2 anodesand determination of the optimal conditions. Poyal Soc. Chem. 2015, 5, 84856–84864.
- Zeidabadi, F.A.; Esfahani, E.B.; McBeath, S.T.; Dubrawski, K.L.; Mohseni, M. Electrochemical degradation of PFOA and its common alternatives: Assessment of key parameters, roles of active species, and transformation pathway. Chemosphere 2023, 315, 137743.
- Uwayezu, J.N.; Carabante, I.; Lejon, T.; van Hees, P.; Karlsson, P.; Hollman, P.; Kumpiene, J. Electrochemical degradation of per- and poly-fluoroalkyl substances using boron-doped diamond electrodes. J. Environ. Manag. 2021, 290, 112573.
- Pierpaoli, M.; Szopińska, M.; Wilk, B.K.; Sobaszek, M.; Łuczkiewicz, A.; Bogdanowicz, R.; Fudala-Książek, S. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J. Hazard. Mater. 2021, 403, 123606.
- Liu, Y.; Fan, X.; Quan, X.; Fan, Y.; Chen, S.; Zhao, X. Enhanced Perfluorooctanoic Acid Degradation by Electrochemical. Environ. Sci. Technol. 2019, 53, 5195–5201.
- Wan, Z.; Cao, L.; Huang, W.; Zheng, D.; Li, G.; Zhang, F. Enhanced electrochemical destruction of perfluorooctanoic acid (PFOA) aided by overlooked cathodically produced bubbles. Environ. Sci. Technol. Lett. 2022, 10, 111–116.
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of peerfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: Environmental matrix effects. Environ. Sci. Technol. 2008, 42, 8057–8063.
- Li, S.; Zhang, G.; Zhang, W.; Zheng, H.; Zhu, W.; Sun, N.; Zheng, Y.; Wang, P. Microwave enhanced Fenton-like process for degradation of perfluorooctanoic acid (PFOA) using Pb-BiFeO3/rGO as heterogeneous catalyst. Chem. Eng. J. 2017, 326, 756–764.
- Fanga, C.; Megharaja, M.; Naidu, R. Breakdown of PFOA, PFOS and 6:2FTS Using Acidic Potassium Permanganate as Oxidant. Austin Environ. Sci. 2016, 1, 1005.
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 602040.
- Ruiz-Urigüen, M.; Shuai, W.; Huang, S.; Jaffé, P.R. Biodegradation of PFOA in microbial electrolysis cells by Acidimicrobiaceae sp. strain A6. Chemosphere 2022, 292, 133506.
- Meesters, R.J.W.; Schröder, H.F. Perfluorooctane sulfonate—A quite mobile anionic anthropogenic surfactant, ubiquitously found in the environment. Water Sci. Technol. 2004, 50, 235–242.
- Schröder, H.F. Determination of fluorinated surfactants and their metabolites insewage sludge samples by liquid chromatography with massspectrometry and tandem mass spectrometry after pressurisedliquid extraction and separation on fluorine-modifiedreversed-phase sorbent. J. Chromatogr. 2003, 1020, 131–151.
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419.
- Huang, S.; Sima, M.; Long, Y.; Messenger, C.; Jaffé, P.R. Anaerobic degradation of perfluorooctanoic acid (PFOA) in biosolids by Acidimicrobium sp. strain A6. J. Hazard. Mater. 2021, 424, 127699.
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2017, 344, 950–957.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
01 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





