Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Satinder K Brar and Version 2 by Sirius Huang.
Perfluorooctanoic acid (PFOA) is a perfluoro compound that contains an eight-carbon perfluoroalkyl chain followed by a carboxylic acid function group. It has found applications in water-resistant coating and is produced either by degrading other long-chain perfluorinated carboxylic acids or fluorotelomer alcohol. PFOA is challenging to further degrade during water treatment processes, leading to its accumulation in natural systems and causing contamination. Research has been conducted to develop several methods for its removal from the water system.
- perfluorooctanoic acid (PFOA)
- perfluorochemicals (PFCs)
- biodegradation
- chemical degradation
- degradation products
- defluorination
1. Background and Characterization of the Perfluoro Compounds
Perfluoro chemicals (PFCs) are man-made chemical compounds that have seen extensive use in various industrial products. They are commonly found in cleaning agents, firefighting foams, and the non-stick coatings on cooking pans [1]. Due to the high bonding energy of the C-F bond, which is approximately 485 kJ/mol in perfluoroalkyl moieties, perfluoro chemicals exhibit strong chemical and thermal stability. This characteristic renders them persistent compounds within the natural environment [2]. When an alkyl chain exceeds eight carbons, it is classified as a long-chain PFC, whereas if it contains fewer than eight carbons, it falls under the category of short-chain PFC. During wastewater treatment processes, long-chain PFCs degrade into shorter-chain products [3]. Other perfluoro compounds, such as fluorotelomer alcohol (FTOH), polyfluoroalkyl phosphates (PAPs), 8:2 fluorotelomer acrylate (8:2 FTAC), and fluorotelomer carboxylates (FTCAs), can also degrade into perfluorooctanoic acid (PFOA) [4]. As chain length decreases, so does toxicity. However, because short-chain PFCs are more hydrophilic than long-chain PFCs, they are more prevalent in natural water systems [5]. In natural water systems, approximately 88.8% of PFC contamination comprises short-chain PFCs [2].
A primary category of compounds in PFCs is referred to as per- and polyfluoroalkyl (PFAS) compounds, as indicated by USEPA. These compounds consist of a variable-length fluorinated alkyl chain followed by a functional group, as depicted in Figure 1. Figure 1 illustrates that there are primarily two types of PFAS: perfluorinated sulfonic acids (PFSAs) and perfluorinated carboxylic acids (PFCAs). PFSA consists of a variable-length fluorinated alkyl chain followed by a sulfonic acid functional group. Upon degradation and defluorination, the long alkyl chain breaks down into short-chain perfluorooctanesulfonic acid (PFOS), which contains eight carbon atoms in the alkyl chain. Another type of PFAS is PFCA, which is formed by various alkyl chains connected to a carboxylic functional group. When long-chain PFCA undergoes degradation, the length of the alkyl chain decreases, and one of the principal degradation products is PFOA [1].
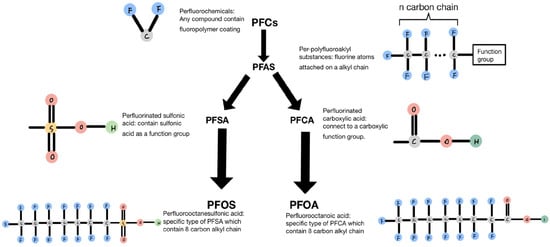
Figure 1.
Classification and characteristics of each compound.
Both PFOA and PFOS are common perfluoro compound contaminants that have been widely detected in the environment. This text will specifically focus on PFOA, which exists as a white waxy powder at room temperature. PFOA is utilized in the production of Teflon for non-stick cookware and is also employed in the manufacturing of surfactants such as shampoo and floor wax, taking the form of fluoropolymers and telomer alcohols. Following their use and disposal, these chemical compounds can eventually degrade into PFOA [6]. Due to its solubility in water (9.5 g/L) and its extended half-life in water systems (92 years), PFOA rarely degrades in the natural environment and is often referred to as a ‘forever chemical’ [7]. After PFOA is released from industrial and wastewater treatment plants, some of it is emitted into the atmosphere, while the rest travels through surface-water and groundwater systems [8]. It accumulates within the biota through the food chain and ultimately enters the human body through the consumption of food or drinking water [9]. The research indicates that the aquatic toxicity of PFOA for marine invertebrates is approximately 10–24 ppm, which is several orders of magnitude higher than the detected PFOA concentration in natural systems [10]. However, it has the potential to be transported through the food chain and ultimately bioaccumulate in aquatic life and humans. Recent studies have shown that trace concentrations of PFOA have been detected in human serum worldwide [9]. Typically, it targets sensitive organs such as the liver or kidney. When PFOA combines with albumin, it can reduce glomerular filtration in the kidney or lead to liver hypertrophy or necrosis [11]. The acute toxicity of PFOA in humans is still unknown, but the lethal dose (LD50) in male rats is 175–208 mg/kg [12]. The reference dose (RfD) for PFOA is 20 ng/kg/day, and an overdose can lead to accumulation in the human body, potentially increasing the risk of cancer [11]. To monitor and regulate this emerging contaminant, the US Environmental Protection Agency (USEPA) proposes healthy drinking water advisory levels for PFOA at 70 ng/L. In 2018, Health Canada published a drinking water standard of 200 ug/L for PFOA [13]. PFOA can cause aquatic toxicity, affecting the survival, growth, and reproduction of aquatic flora and fauna, and it can also be harmful to human health [10]. Due to PFOA’s toxicity, there is increasing attention on it as an emerging contaminant. Various methods have been developed to remove these contaminants and control pollutant concentrations to ensure they remain within safe levels.
2. Physical Removal Method and Degradation Methods
Physical removal methods, such as granular activated carbon adsorption, ion exchange resin, membrane filtration, and coagulation, have been frequently documented [1][14][15][16][1,14,15,16]. Recent research has demonstrated that ion exchange resins exhibit high adsorption capacities (525–1500 mg/g) in comparison to granular activated carbon adsorption (41–120 mg/g), and both methods can achieve over 90% removal [17]. These physical removal techniques have been developed at a full scale, making them applicable in drinking water treatment plants for PFOA removal. However, their primary drawback is that they solely extract contaminants from water resources and concentrate them without degradation [18]. Therefore, an additional degradation method or step is necessary to eliminate these compounds and prevent the introduction of secondary contaminants.
There are three primary methods for degrading these compounds. The first is a non-chemical degradation method, such as plasma technology, incineration, sonolysis, and photolysis. These methods require high-energy inputs, such as UV, ultrasound, or heat, to initiate degradation. During the reaction, the input energy can break down the C-F bond and convert PFOA into CO2, free fluoride ions, and less toxic short-chain PFCA. This reaction typically occurs in a high-temperature, high-pressure environment without the involvement of any chemical compounds [19]. The second method is chemical degradation, which involves chemicals like ferric ions or persulfate as catalysts in the reaction. These chemicals lower the activation energy needed for the bond-breaking process, accelerating the degradation reaction [20]. The third degradation method is biological degradation, which represents a newer technology. During biodegradation, microorganisms consume PFOA as a carbon source [21]. Biodegradation boasts lower energy consumption and does not yield unpredictable by-products during the reaction [22].
3. Chemical Degradation
Chemical degradation is a well-established technology widely utilized in wastewater treatment systems. In most cases, it requires an energy source input, such as UV, electrical power, ultrasound, and microwave energy. With the presence of chemical catalysts, it generates free electrons or free radicals to break down the C-F bond in PFOA for degradation. The most employed chemical degradation methods include photochemical and electrochemical degradation [23][24][26,27]. Other forms of energy input are also used for degradation, including sonochemical degradation, microwave-enhanced degradation, and other chemically catalyzed degradation methods [25][26][27][28,29,30].3.1. Photochemical Degradation
Most of the research on chemical degradation involves using UV as the input energy source to facilitate homogeneous or heterogeneous reactions [28][29][30][31,32,33]. Table 1 shows a selection of photocatalytic degradation studies that focused on low-pressure-to-medium-pressure mercury UV lamps with a wavelength of around 256 nm. This is because PFOA exhibits maximum absorbance at this wavelength, providing optimized energy for breaking down the chemical bonds in PFOA during the degradation process [30][31][33,34]. The UV lamp power varies from 9 W to 200 W, but generally, it utilizes low-power light, typically around 20 W, to maximize energy efficiency. [32][35] used a 15 W UV lamp with a wavelength of 240 nm to perform an anaerobic reduction reaction. Without the presence of chemicals as a reducing agent, the PFOA concentration only reduced by 13.3% in 6 h in an anaerobic environment. However, with the addition of KI as a catalyst, the PFOA concentration dropped to 93.9% in 6 h [32][35]. Table 1 demonstrates that photochemical degradation typically occurs at room temperature under a room atmosphere. The aerobic reaction takes place in a lower pH range, typically from 0.3 to 4.5, while the anaerobic reaction occurs in a higher pH range, usually between 8 and 12 [30][32][33,35]. There are two primary reasons for maintaining the aerobic reaction in an acidic environment. Firstly, the aerobic environment employs oxidation reagents to generate hydroxyl radicals for degradation. However, when the pH exceeds 4, it can render the oxidation agent unstable. For instance, at high pH levels, H2O2 degrades into H2O and O2, reducing the formation of free radicals. The second reason is that the precipitation of Fe(OH)3 forms at high pH, which can obstruct ongoing reactions and significantly impede the degradation performance. Therefore, the reaction should occur in a low-pH environment [33][36]. An experiment conducted by Song et al. (2013) involved reductive defluorination of PFOA under anaerobic conditions. The results indicated that increasing the pH from 8.1 to 10.3 enhanced the defluorination from 58% to 85%. This is because free electrons drive a reduction reaction in the experiment, and the relative quasi-stationary concentration of eaq- (ROSC) is linked to pH. With an increase in pH, more free electrons are available in the solution for defluorination to occur [34][37]. The photocatalyst plays a crucial role in the photochemical degradation of PFOA. Wu et al. (2017) used ZnO as a catalyst that absorbs PFOA for degradation. However, an increase in pH reduces the adsorption of PFOA on the reaction surface and consequently decreases the reaction rate [35][38]. Furthermore, during application, elevated temperatures reduce the surface area of ZnO, leading to a reduction in reaction efficiency [35][38]. TiO2 is another popular catalyst employed in photochemical degradation. It contributes electrons during the reduction reaction and provides a reaction surface [36][39]. The effectiveness of the TiO2 surface is influenced by pH, leading to changes in degradation efficiency [36][39]. Araniti et al. (2015) utilized Mg-aminoclay-coated nanoscale zero-valent iron as a catalyst for PFC degradation. After aging for 3 days, the degradation rate decreased by 15% due to the reduced amount of reactive iron in the coating [37][40]. The main degradation products of PFOA through photochemical degradation are fluoride ions, CO2, and other short-chain PFOA compounds [38][41]. Perfluoroheptanoic acid (PFHpA) is the initial degradation by-product that emerges during the photochemical degradation of PFOA [39][42]. Throughout the degradation process, PFOA undergoes stepwise defluorination and shortens its alkyl chain, resulting in the production of C7 PFHpA [40][43]. Additionally, other short-chain PFCA compounds, such as C6 perfluorohexanoic acid (PFHxA), C5 per-fluoropentanoic acid (PFPeA), and C4 perfluorobutanoic acid (PFBA), have been detected in the final solution, each with a low concentration of approximately nanomoles per liter [34][37]. The degradation efficiency in photochemical degradation ranges from 70% to the complete removal of PFOA, with 30% to 90% defluorination occurring during a reaction time of 4–24 h [32][35]. It is worth noting that the defluorination rate is consistently lower than the PFOA removal rate; Song et al. (2013) achieved up to 100% removal of PFOA within 1 h but only managed a 40% defluorination rate [34][37]. This phenomenon is due to the higher energy of the C-F bond compared to the C-C bond, causing the degradation reaction to undergo chain shortening processes before complete defluorination is achieved.Table 1.
Photochemical degradation of perfluorooctanoic acid (PFOA).
| Wavelength (nm) | Power | PFOA Initial Concentration (mg/L) | Other Chemical Content | Conditions | Degradation Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| 254 | 200 W | 140–1387 | 34 mg/L of H2 | ||||||
| Ti/SnO2-Sb/PbO2 | 10 | 100 | O2 0.48 MPa O2 |
Room temperature | 10 mg/L NaClO4 pH 0.8 |
25 °C pH 5 5 mm distance0.674 mg/h Up to100% removal |
60 mg/h 91% removal 77.4% defluorination[23][26] |
||
| [ | 24 | ] | [ | 27 | ] | 220–460 | 200 W | 559 | 9600 mg/L S2O |
4. Biological Degradation
Biological degradation represents an emerging technology when compared to chemical degradation, and research in this field remains rather limited. Only a handful of research articles have been published, with a specific focus on the biodegradation of PFOA. Previous research has demonstrated the thermodynamic favourability of dehalogenation reactions and the maturity of microbial dechlorination technology. This indicates that bacteria can indeed derive energy from breaking down perfluoro compounds [6]. However, the majority of biodegradation research centers on the degradation of fluorotelomer alcohols (FTOH), which ultimately yield PFOA as a final product without further breakdown [6][56][6,59]. Furthermore, it has been reported that biodegradation typically requires at least one hydrogen atom to be attached to the alkyl chain to initiate the reaction. In the case of PFOA, however, the alkyl chain only features fluorine atoms, forming high-energy C-F bonds (485 kJ/mol), which render them even more challenging to degrade compared to other perfluorinated compounds, such as fluorotelomer alcohols [57][60]. Schröder et al. (2004) employed sewage-treatment-plant sludge as a bacterial seed in a closed-loop system to treat PFOA for 28 days [58][59][61,62]. In recent years, scientists have managed to isolate and acclimate Pseudomonas Parafulv and Acidimicrobium sp A6 strains from PFOA-enriched environments, allowing them to perform biological degradation reactions using PFOA as a carbon source. Bacteria exhibit a certain level of tolerance to PFOA concentrations; when the concentration of PFOA reaches 500 ppm, the bacterial strain reaches a maximum population of 0.175 OD600 and achieves a degradation rate of 30% over 72 h [2]. However, if the concentration exceeds this threshold, both the OD600 and degradation rate begin to decline due to the inhibitory effects of PFOA on bacterial growth. In Table 3, biodegradation can occur either aerobically or anaerobically under culturing conditions of 30 °C and 150 rpm. However, the majority of experiments are conducted in anaerobic environments [60][63]. The primary reason for biological degradation occurring in an anaerobic environment rather than an aerobic one is the highly oxidized nature of PFOA. It struggles to donate electrons for further oxidation reactions in an aerobic environment [22]. In anaerobic conditions, PFOA can preferentially accept electrons to undergo reduction reactions, leading to no removal of PFOA under Liou’s aerobic degradation conditions but achieving 67% removal in Huang’s anaerobic degradation experiment [60][63]. Table 3 presents the anaerobic degradation of PFOA carried out by an Acidimicrobium sp A6 strain in an acidic environment with a pH range of 4.5–5.5. There has only one aerobic experiment, conducted by Yi et al. (2016), in a neutral environment using Pseudomonas parafulva for degradation. During their experiment, Yi et al. (2016) supplemented yeast extract and glucose to support metabolism. This can be used during the acclimation and isolation stages to boost bacterial populations during the degradation processes and increase the final removal rate of PFOA [2].Table 34.
Biological degradation of perfluorooctanoic acid (PFOA).
| Sources of Bacteria | Initial PFOA Concentration | Reaction Environment | Chemical Supplement | Degradation Efficiency | Reference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pseudomonas parafulva | 500 mg/L | pH 7 30 °C 160 rpm |
1000 mg/L yeast extract 2% inoculum 5000 mg/L NH4NO3 2000 mg/L NaCl 1000 mg/L KH2PO4 1000 mg/L K2HPO4 500 g/L MgSO4·7H2O 50 mg/L CaCl2·2H2O |
48% removal In 5 day |
[2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | 2− | 0.3 pH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ] | Ti/SnO2-Sb/Yb-PbO2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acidimicrobium sp. Strain A6 | 0.1 mg/L | Anaerobic 4.5 pH 30 °C 150 rpm |
1260 mg/L Fe2O3·0.5H2O | 0.48 MPa O | 202 25 °C |
139.5 mg/h Up to 100% removal |
100 [ |
12,240 mg/L Na2SO4 | 150 mg/L NH4Cl 25 mg/L (NH4)2SO4 20 mg/L NaHCO3 71 mg/L KHCO3 7.523 mg/L KH2PO4 101 mg/L MgSO4·7H2O 58.8 mg/L CaCl2·2H2O 1 mL trace element 1 mL vitamin solutionpH 5 25 °C 500 rpm 5 mm distance |
60% removal In 100 d38][41] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38 mg/h | 95% removal | 75% defluorination | [ | 47 | [60][63]][50] | 254 | 15 W | 40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acidimicrobium sp. Strain A6 | 10 mg/L | 25 °C Anaerobic | 500 mg/L b-Ga2O3 | pH 4.5–5 | 506 mg/L Fe2O3·0.5HAnaerobic | 2O 177 mg/L NH4Cl 77.9 mg/L (NH4)2SO4 19.8 mg/L NaHCO3 71 mg/L KHCO3 9 mg/L KH2PO4 100 mg/L MgSO3·7H2O 60 mg/LCaCl2·2H2O 1 mg/L trace element 1 mL/L vitamin solution5.3 mg/h 40% removal 15% defloration |
[41][44] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 254 | 15 W | 10.35 | 16 mg/L–133 mg/L KI | 9 pH Room temperature anoxic |
1.62 mg/h 93.9% removal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B and N codoped diamond | 4 | 50 | 6122 mg/L Na2SO4 | pH 4.8 2.5 cm distance |
25 mg/h Up to 100% removal 80% defluorination in 3 h |
[51 | 67.7% removal in 150 d | [61][64]][54] | 89% defluorination |
[32][35] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boron-doped diamond | 0.6 | 1000 | 12,240 mg/L NaClO4 1.5% TiO2 |
1 cm distance 600 mW/cm2 UV at 254 nm |
166 mg/h 50% removal |
[28] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acidimicrobium sp. Strain A6 | 100 mg/L | Anaerobic pH 5–5.5 240 rpm Room temperature stainless steel as cathode and graphite plate as anode. |
203.3 mg/L NH4Cl 79.28 mg/L (NH4)2PO4 20.16 mg/L NaHCO3 71 mg/L KHCO3 8.98 mg/L KH2PO4 101 mg/L MgSO4·7H2O 45.5 mg/L CaCl2 55.24 mg/L AQDS 1 mL/L vitamin supplement | [ | 31 | ] | 77% removal in 18 d | [57][ | 254 and 185 | 23 W | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 60 | ] | Boron-doped diamond4.14 | 12 mg/L NaIO4 | 1.8 mg/h 87% removal 25% defluorination |
[42][ | 21.445] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.5 | 1500 mg/L Na | 2 | SO4 | 2 cm distance 6.27–8.53 pH |
2.75 mg/h Up to 100% removal 40% defluorination |
[49][52] | C | 6 W | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boron-doped diamond | 75 | 500 mg/L TiO | 2 | 0.1 | 17.466 mg/L K2HPO4 250 μL/L H3PO4pH4 25 °C |
8.3 mg/h | pH 7.03–7.29 20–25 °C
3.2. Electrochemical DegradationElectrochemical degradation utilizes boron-doped diamond and Ti-SnO2 electrodes submerged in an electrolyte containing ion-exchange reagents, such as NaClO4, Na2SO4, NH4OH, and NH4Cl, for PFOA degradation [24][46][47][27,49,50]. This process generates free electrons on the electrode side, which then react with PFOA to produce free fluoride ions and CO2. Table 2 illustrates that the current density during electrical chemical degradation typically varies from 10 to 50 mA/cm2. Higher current densities remove PFOA more quickly but consume more energy [24][27]. Witt et al. (2020) experimented with various current densities during the degradation process to strike a balance between energy consumption and defluorination performance. During the first hour, they used 50 mA/cm2 to initiate PFOA degradation, then switched to 5 mA/cm2 for long-term defluorination, ultimately resulting in 30% energy savings [46][49]. As depicted in Table 2, in most of the studies, the reactions occurred at room temperature in a pH 5 acid solution. The optimal electrode separation distance falls within the range of 5–25 mm, dependent on the reacting current density. When the current density remains constant, an increase in the electrode separation distance results in a decrease in the efficiency of the reaction [48][51]. The degradation of PFOA requires free radicals. Ma et al. (2015) demonstrated that the degradation of PFOA decreased when the pH increased from 5 to 11 or decreased to 3, primarily due to the blocking of radical formation [47][50]. Table 2 illustrates that most of the electrochemical degradation processes occur in an acidic environment, typically within the pH range of 3 to 5. However, in certain special conditions, such as in the treatment of wastewater or landfill leachate, the reaction still occurs in a natural environment. Nevertheless, it necessitates a higher current density, and the degradation efficiency is lower compared to the acidic environment [49][50][52,53]. A higher initial concentration of PFOA typically leads to an increased reaction rate. However, due to the limitations of ion exchange at the anode, if the initial PFOA concentration surpasses 100 ppm, it can also impede the reaction. This is because elevated levels of PFOA generate numerous short-chain by-products, such as C7 PFHxA, and a higher concentration of these by-products can deplete the available radicals and extinguish the reaction [47][50].Table 2. Electrical chemical degradation of perfluorooctanoic acid (PFOA).
3.3. Other Degradation MethodsIn addition to photochemical and electrochemical degradation methods, a few other techniques are employed for PFOA degradation. These methods include sonochemical, microwave-enhanced, and other chemical-catalyzed degradation methods. Unlike UV light or electrical currents, which serve as energy sources, these chemical degradation processes utilize substances like Na2S2O8, argon gas, or vitamin B13 as catalysts. They may combine with various forms of energy or even operate without additional energy input to facilitate degradation. In sonochemical degradation, argon gas and ozone are introduced during sonolysis. This process degrades PFOA by generating bubbles under ultrasound. When these bubbles rupture, they create high temperatures at the interface, enabling interface pyrolysis. Argon gas, characterized by a high polytopic index, exhibits greater heat flux during transitions between different thermodynamic states. Consequently, the addition of argon gas during degradation can elevate the interface temperature and accelerate the reaction rate [25][28]. Sonochemical degradation employs a 200–250 W ultrasound generator operating at a frequency of 200–612 kHz to facilitate a first-order reaction with a rate of 0.032 min−1 |
