Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Monika Wujec | -- | 3596 | 2023-11-29 17:23:43 | | | |
| 2 | Rita Xu | Meta information modification | 3596 | 2023-11-30 03:08:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kopystecka, A.; Kozioł, I.; Radomska, D.; Bielawski, K.; Bielawska, A.; Wujec, M. Vaccinium uliginosum and Vaccinium myrtillus. Encyclopedia. Available online: https://encyclopedia.pub/entry/52202 (accessed on 07 February 2026).
Kopystecka A, Kozioł I, Radomska D, Bielawski K, Bielawska A, Wujec M. Vaccinium uliginosum and Vaccinium myrtillus. Encyclopedia. Available at: https://encyclopedia.pub/entry/52202. Accessed February 07, 2026.
Kopystecka, Agnieszka, Ilona Kozioł, Dominika Radomska, Krzysztof Bielawski, Anna Bielawska, Monika Wujec. "Vaccinium uliginosum and Vaccinium myrtillus" Encyclopedia, https://encyclopedia.pub/entry/52202 (accessed February 07, 2026).
Kopystecka, A., Kozioł, I., Radomska, D., Bielawski, K., Bielawska, A., & Wujec, M. (2023, November 29). Vaccinium uliginosum and Vaccinium myrtillus. In Encyclopedia. https://encyclopedia.pub/entry/52202
Kopystecka, Agnieszka, et al. "Vaccinium uliginosum and Vaccinium myrtillus." Encyclopedia. Web. 29 November, 2023.
Copy Citation
Vaccinium uliginosum L. (commonly known as bog bilberry) and Vaccinium myrtillus L. (commonly known as bilberry) are species of the genus Vaccinium (family Ericaceae). The red–purple–blue coloration of blueberries is attributed largely to the anthocyanins found in bilberries. Anthocyanins, known for their potent biological activity as antioxidants, have a significant involvement in the prophylaxis of cancer or other diseases, including those of metabolic origin.
bog bilberry
Vaccinium uliginosum
Vaccinium myrtillus
1. Introduction
Vaccinium uliginosum L. (bog bilberry) and Vaccinium myrtillus L. (bilberry) are species of the genus Vaccinium (family Ericaceae). They are low-growing deciduous shrubs that produce dark purple fruits (berries) which are edible (Figure 1). Commonly called bilberries, their fruits are highly valued as a rich source of anthocyanins, which are naturally occurring compounds. In fresh berries, their content is about 0.5% [1][2][3][4]. In addition to fresh fruit, berries can also be consumed as frozen, dried, juices, jams, and food supplements [5]. It has recently become more popular to consume fermented products made from bilberries [6][7]. In vitro studies have shown that bilberry extracts have an impact on the effects of, among other things, anti-glycation and the scavenging of external radicals. Strong antioxidant properties were also found because of the occurrence of abundant bioactive substances, such as anthocyanins and flavanols [1][8][9]. Thanks to these properties, the supplementation of bilberries can have an impact on health in many cases of diseases. Its known pharmacological effects include vascular regulation, dysentery, antigens, diabetic retinopathy, and potential anti-cancer effects [10][11][12][13][14].

Figure 1. V. myrtillus and V. uliginosum in their natural habitat and the external appearance of their parts (leaves and fruit).
There are many studies on Vaccinium species, but so far there is no comparison of both species, V. uliginosum and V. myrtillus, especially in terms of their biological activity and possible use as functional food. The biological effect of the fruit extract of V. uliginosum is known primarily from both Chinese and European folk medicine.
2. Occurrence
Most V. myrtillus and V. uliginosum are mainly acquired from their native habitats [15][16]. These members of the Ericaceae family grow best in humid and moderate climates. Mountains and high mountains are the most common habitats in their southernmost distribution [17]. V. myrtillus is found in European mountains and forests, while V. uliginosum grows in areas of Asia, Europe, and North America [18]. V. uliginosum, V. myrtillus, and V. vitis-idaea are the species that grow on the Iberian Peninsula. Observations of V. uliginosum on Portugal’s mainland suggest fragmented populations and uncertain survival in the uppermost parts of Serra da Estrela. Serra da Estrela as well as Serra da Freita both have fragmented populations of V. myrtillus, but the latter is more plentiful in northern Portugal’s mountains [19]. Bilberry (Vaccinium myrtillus L.) is the most important economically wild berry in Northern Europe, and it is also extensively used in juice and food production. The bog bilberry is used to a lesser extent, but it is widespread in northern areas [20]. Compared to cultivated species, wild berries have a more complex chemical composition [18]. A very important aspect is also climate and weather conditions, which determine the content of the various bioactive substances (phenolic acids, anthocyanins, etc.) in blueberries [21]. In turn, the qualitative–quantitative composition of phenolic compounds in bilberries depends on the plant parts used, growth stage, and genetic factors [22][23]. For this reason, buyers are interested in the origin of the berries, as those from specific areas or countries often have a higher price. As spectrophotometers are quick and easy to use, they are highly suitable for commercial purposes, especially for evaluating berry quality [24][25][26].
A study by Urbanaviciene and Dalia et al. determined the physicochemical properties, as well as the levels of total anthocyanins (TAC) and polyphenols (TPC) present in V. myrtillus populations, which occur in areas of Northern Europe (Lithuania, Latvia, Finland, and Norway), along with their ability to scavenge free radicals. In the investigation, V. myrtillus had pH values ranging from 2.94 to 3.47. Approximately 232.7 to 475.5 mg/100 g of fresh weight (FW) were obtained from the investigated V. myrtillus samples. The content of TPC was the highest in Norway and the lowest in Lithuania and varied between 452–902 mg/100 g FW. According to the study, the antioxidant capacity of V. myrtillus oscillated between 60.9 and 106.0 mol TE/g FW, with the lowest value in populations from Lithuania and the highest from Norway [27]. The main ingredients that make up more than 50% of the Lithuanian bilberry water extract are cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, delphinidin-3-O-galactoside, peonidin-3-O-glucoside, petunidin-3-O-glucoside, delphinidin glycosides, and cyanidin [28]. According to Szakiel et al., the content of triterpenoids in the leaves of V. myrtillus from wild habitats varies significantly depending on its location in Poland and Finland. Polish leaves were significantly richer in lupeol, and friedelin was only found on Finnish leaves, while taraxasterol was only found on leaves of plants from Poland. Polish leaves contained more than three times as much 2α-hydroxyursolic and 2α-hydroxyoleanolic acids as Finnish leaves, but they had similar levels of oleanolic and ursolic acids [29].
3. V. uliginosum and V. myrtillus Composition
Blueberry composition depends on the genotype of the plant [30][31][32]. V. uliginosum berries contain many anthocyanins and flavonols. V. uliginosum has a characteristic profile of flavonols and anthocyanins compared to other berries of the Vaccinium family, which can be used to distinguish bog bilberry from V. myrtillus [33]. V. myrtillus seeds and oils contain natural antioxidants, anti-inflammatory, anti-atherosclerotic, and anticancer compounds, such as tocochromanols, carotenoids, flavonoids, phytosterols, and phenolic acids [34][35]. The caloric energy intake of fresh bilberries is approximately 45 kcal/100 g. They consist of water (84%), carbohydrates (9.6%), proteins (0.7%), fats (0.4%), and fibers (about 3.5%) [36]. This is compared to dry bilberry, which has 395 kcal/100 g and contains 94% carbohydrates, 3% proteins, and 1.5% fats [37]. The pH value of the bog bilberry’s berry (V. uliginosum L.) was relatively high (pH = 3.5), and their titratable acidity, in turn, was moderate (1 g of citric acid/100 g). The main identified soluble sugar was fructose (concentration of 2138 ± 149 mg/100 g FW), while glucose was the second in amount (concentration of 1664 ± 121 mg/100 g FW) [38].
3.1. Polyphenols
Polyphenols are a group of naturally occurring compounds found in various plant foods, including berries from the Vaccinium genus (Figure 2).
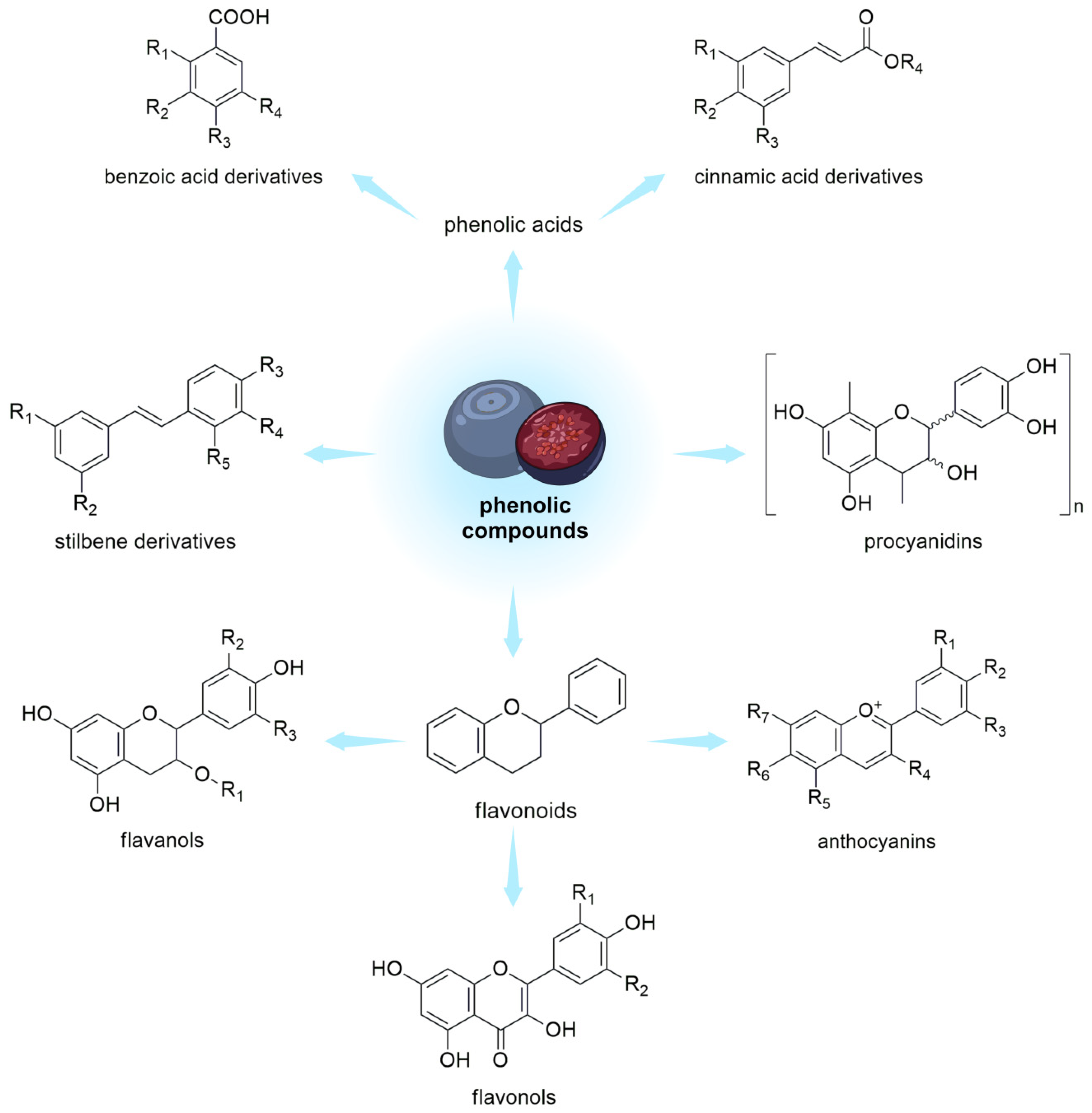
Figure 2. Phenolic compounds in V. uliginosum and V. myrtillus.
The content and availability of polyphenols in blueberries can be affected by various factors, including agricultural practices, storage, and processing technologies. Organic farming practices, which avoid synthetic pesticides and fertilizers, may promote higher polyphenol content in blueberries. This is because plants often produce more phytochemicals, including polyphenols, as a defense mechanism against pests and diseases. Harvesting techniques are very important too. Picking blueberries at the right ripeness can affect their polyphenol content. Polyphenol levels may increase as the berries ripen [39]. Using gentle harvesting methods to avoid damaging the berries can help preserve their polyphenol content. Proper temperature, pressure, and humidity control during storage are crucial to prevent polyphenol degradation [40]. Cold storage can help maintain polyphenol levels in fresh blueberries. Modified Atmosphere Packaging (MAP) involves adjusting the gas composition inside the packaging to extend the shelf life of blueberries while preserving their polyphenols [41]. Processing technology conditions are the most important factors influencing the content of polyphenols in products made from berries. Freeze drying is a method that can preserve the polyphenol content in blueberries by removing moisture without significant heat exposure, which can degrade polyphenols [42]. Drying blueberries at lower temperatures can help retain their polyphenol content compared to high-temperature drying methods. Processing blueberries into purees or juices can concentrate polyphenols. However, some heat exposure during processing may cause a slight reduction in polyphenol levels. Changes in the phenolic composition of berries may be related to various treatments, including ozone pretreatment using ultrasound [43] or using cold plasma [44].
Conventional methods for polyphenol extraction have limitations and drawbacks, which can include the use of harsh solvents, high energy consumption, and potential degradation of the polyphenols. These drawbacks have led to a growing demand for more sustainable and eco-friendly extraction techniques. To maximize the efficiency of polyphenol extraction while maintaining the total polyphenol content (TPC) and antioxidant capacity of the extract, it is essential to assess and compare different extraction conditions. Some novel technologies such as an ultrasound, microwave, cold plasma, pulsed electric field, and pressurized liquid were used as alternatives assisting the extraction process [45]. Factors such as temperature, pressure, and processing time can significantly influence the outcome.
It is important to note that while these technologies and practices can influence polyphenol content, the specific impact may vary depending on factors such as the blueberry variety and environmental conditions.
Polyphenol compounds in berries of Vaccinium spp. were determined by different methods (Table 1).
Table 1. Method of characterization of some polyphenol compounds in berries of Vaccinium genus.
| Polyphenol Compounds | Method of Characterization | References |
|---|---|---|
| delphinidin-3-O-galactoside malvidin-3-O-galactoside malvidin-3-O-arabinoside delphinidin-3-O-arabinoside |
CIELAB HPLC-DAD |
[46] |
| delphinidin 3-glucoside cyanidin 3-glucoside petunidin 3-glucoside delphinidin 3-glucoside |
HPLC-DAD | [47] |
| chlorogenic acid quercetin-3-O-galactoside quercetin-3-O-glucuronide delphinidin-3-O-galactoside delphinidin-3-O-glucoside cyanidin-3-O-galactoside petunidin-3-O-glucoside |
HPLC-UV/DAD HPLC-ESI-MS MS |
[48] |
| delphinidin 3-O-glucoside malvidin 3-O-glucoside myricetin 3-O-hexoside quercetin 3-O-galactoside |
HPLC-DAD HPLC-ESI-MS |
[49] |
| cyanidin-3-O-glucoside cyanidin-3-O-rutinoside catechin quercetin-3-O-galactoside quercetin-3-O-arabinoside myricetin 3-O-hexose |
HPLC-FT-ICR MS/MS | [50] |
| gallic acid vanillic acid ferulic acid caffeic acid p-coumaric acid quercetin |
HPLC | [51] |
| (–)-epicatechin kaempferol derivative chlorogenic acid ellagic acid |
HPLC | [52] |
| glycosides of quercetin myricetin kaempferol isorhamnetin syringetin laricitrin |
HPLC–MS | [53] |
Quercetin, kaempferol, phenolic acid, and gentisic acid were the largest fraction of polyphenols identified in V. myrtillus extracts [54]. In one of the studies on V. uliginosum gaultherioides and V. myrtillus berries, differences in terms of relative percentages of total monomeric anthocyanins (TMA) concerning total soluble polyphenols (TSP) were shown, which was the predominant polyphenolic class in blueberry, but this was not observed in bog bilberry [55]. The bog bilberry juice was abundant in myricetin-3-O-galactoside and quercetin-3-O-galactoside [56]. The ferric reducing antioxidant power (FRAP) test yielded the highest antioxidant capacity values (117 μmol TE/g FW), followed by the oxygen radical absorbance capacity (ORAC) test (84 μmol TE/g FW) [38]. In a study by Wang Yu et al. in 10 different populations of V. uliginosum from the Changbai Mountains (China), the content of TF (total flavonoids), TA (total anthocyanins), and TP (total phenols) was assessed, and the spatial distribution and correlation between these components were examined. Fifteen anthocyanins were identified and described, and the amount of malvidin-glucoside, petunidin-glucoside, and delphinidin-glucoside was the highest in this phytochemical group. TF, TA, and TP values were the highest in the Dongfanghong forest farm (DFHI) and the Lanjia forest farm (LJII) populations, respectively. As compared to the other samples, the TF content of the DFHI-8 sample was higher, as was the TA content of the LJIII-1 and the TP content of the LJIII-4. At an altitude from 740 to 838 m, TA and TP content exhibited a positive correlation. In turn, at altitudes >838 m, their dependence showed negative values [57].
Antioxidant properties of juices of bog blueberry (Vaccinium uliginosum) were evaluated by ABTS scavenging capacity (RSC), FRAP, ORAC, TPC (total phenolic content), and TAC (total anthocyanin content) assays. The TPC values ranged from 0.85 to 2.81 mg gallic acid equivalent/mL; ORAC, FRAP, and RSC values were 4.21–45.68, 3.07–17.8, and 6.38–20.9 μmol Trolox equivalent/g, respectively. Bog blueberry had a very high TAC, 14.19 mg/100 mL. In the ABTS decolorization test, blueberry juices showed the highest RSC (20.9 μmol TE/g), FRAP (31.99 μmol Fe2+/g and 17.80 μmol TE/g), and ORAC (45.68 μmol TE/g). Bog bilberry, even though it contained moderate amounts of quantified compounds, showed a very high antioxidant capacity; it had a slightly different chromatographic profile. It was found that there was a moderate negative correlation between berry weight and both FRAP and ORAC assays. Berries with a larger mass probably accumulate more macronutrients, e.g., carbohydrates. The values obtained in the FRAP and ORAC assays also correlated with quinic and chlorogenic acid concentrations (p ≤ 0.01). According to the results of this study, new cultivars exhibiting higher antioxidant capacity can potentially be created through the use of the germplasm of half-highbush blueberry and V. uliginosum [58].
The team of Bayazid AB et al. conducted in vitro studies evaluating the antioxidant and anti-inflammatory properties of 70% ethanolic extracts of bilberry. Antioxidant activity was measured by total phenols, flavonoids, and ascorbic acid. Bilberry extract dose-dependently inhibited linoleic acid oxidation and showed free radical elimination activity. This extract reversed pro-inflammatory cytokines such as inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), tumor necrosis factor α (TNF-α), and interleukin-6 (IL-6) in LPS (lipopolysaccharide)-induced RAW 264.7 cells and suppressed NO (nitric oxide) generation. It was suggested that V. myrtillus blueberry extract is a natural preparation with strong antioxidant properties and acts as an anti-inflammatory agent due to its high concentration of anthocyanins [59].
3.1.1. Flavonols and Flavanols
Latti et al., in their studies, were the first to show the presence of kaempferol and isorhamnetin aglycones in V. uliginosum. In their study, about 1/4 of bog blueberry samples contained more flavonols than anthocyanins [33].
One study found that EC (epicatechin) and EGC (epigallocatechin) were the major flavanols in blueberry juice [56]. V. uliginosum also contains flavonols such as laricitrin, syringetin, myricetin, and quercetin. Based on the findings of myricetin and quercetin arabinosides, the minor laricitrin, isorhamnetin, and syringing pentosides were further named arabinosides. Both laricitrin and isorhamnetin were also detected in V. myrtillus [33]. Laricithrin, isorhamnetin, myricetin, kaempferol, syringetinhexosides, pentosides, and glucuronides, as well as glucuronide and pentosides QUE (quercetin), were identified in bilberry and bog bilberry in various amounts, and flavonol predominance in bog bilberry [55]. Figure 3 shows the chemical structures of flavonols and flavanols (and the sugars to which they are linked) that are contained in V. uliginosum and V. myrtillus.
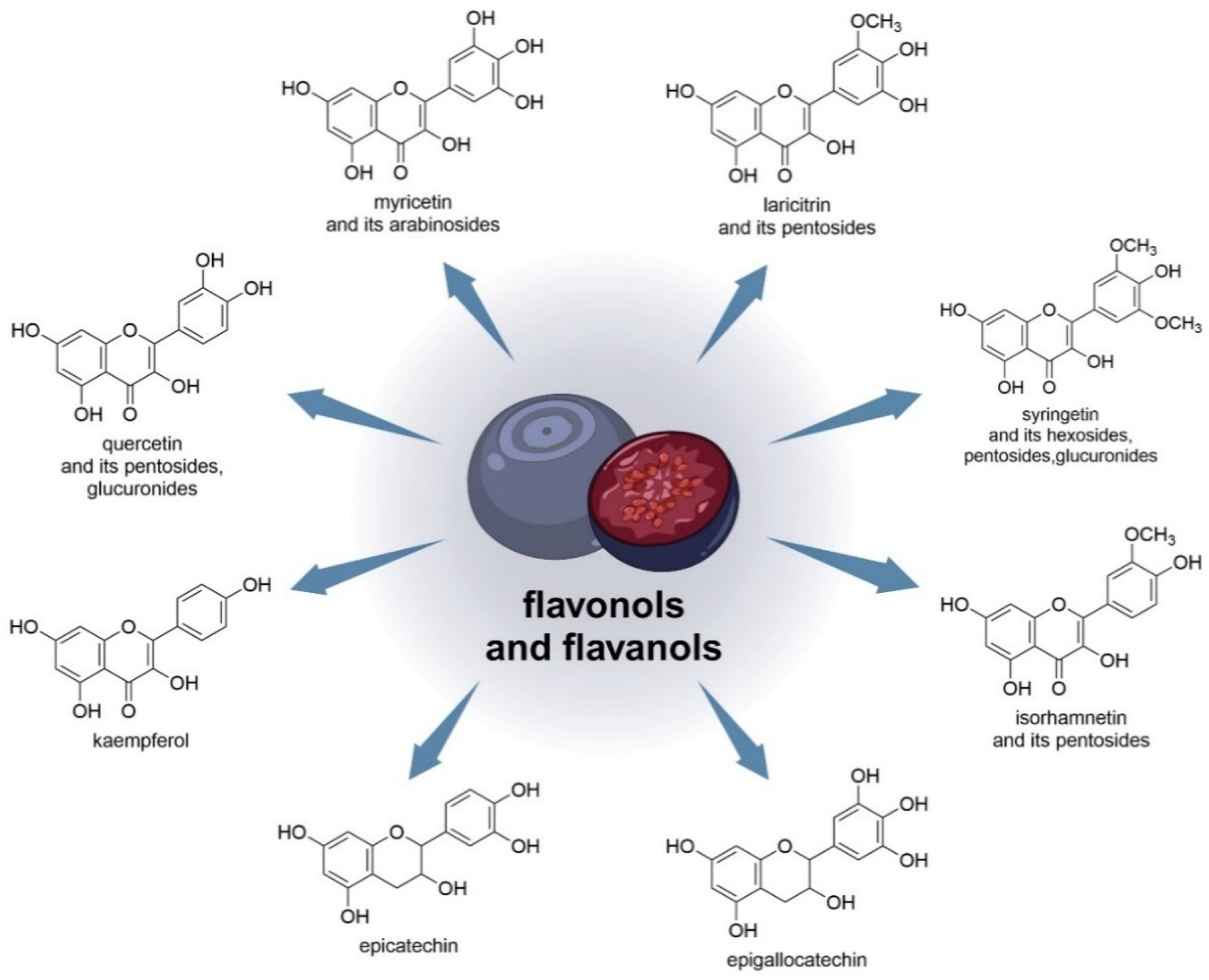
Figure 3. Chemical structures of flavonols and flavanols (and the sugars to which they are linked) that are contained in V. uliginosum and V. myrtillus.
Due to the very high concentration of quercetin-3-galactoside, the prevalence of quercetin-3-rhamnoside in blueberries contrasts with QUE and its derivatives in V. uliginosum subsp. gaultheroides. Blueberries contained about ten times more QUE-3-RHA than bog blueberries [55].
3.1.2. Anthocyanins
Compared with some common edible berries, bog bilberries contain more complex anthocyanins [60]. It was found that the TAC in blueberries is about 6 g/kg of fruit [61]. Holkem et al. researched V. myrtillus extracts. It was proven that the best antiproliferative effect was shown by an anthocyanin-rich extract due to the abundance of bioactive substances occurring in it; for this extract, there was an elevation in the antioxidative effect after the introduction of bacteria [62]. In one study conducted on V. myrtillus juice, results indicated that blueberry juice and cyanidin increased mitochondrial activity and reduced intracellular reactive oxygen species (ROS) generation and hydrogen peroxide-induced lipid peroxidation. In addition, the juice caused an increase in the activity of antiradical enzymes—superoxide dismutase (SOD) and catalase (CAT) [63]. It has been proven that they have antioxidant, anti-cancer, and anti-inflammatory effects and that these compounds can alleviate chronic and acute colitis [64][65]. Bog bilberries were the subject of one study that identified five key anthocyanidins, among which malvidin 3-glucoside was the main compound. It was observed that the TAC fraction showed particularly high variability in antioxidant capacity, which was mainly influenced by the type of phenolic structure that was eluted by solid-phase extraction (SPE) [38].
V. uliginosum, in their structure, have an aglycone part and a glycosyl part. Malvidin, delphinidin, cyanidin, peonidin, and petunidin constitute the aglycone part (Figure 4), while arabinoside, glucoside, xyloside and galactoside belong to the glycosyl part of the compound. In the course of the research, it was observed that specific habitats conditioned noticeable differences in the quantitative composition of anthocyanidin glycosides [33]. Most anthocyanins in V. uliginosum derived from B-ring tri-substituted anthocyanidins (80 ± 3%); the most important was the malvidin-type (46 ± 6%), followed by cyanidin (21 ± 3%), delphinidin (13 ± 3%), petunidin (13 ± 0%), and peonidin (7 ± 1%) [38].
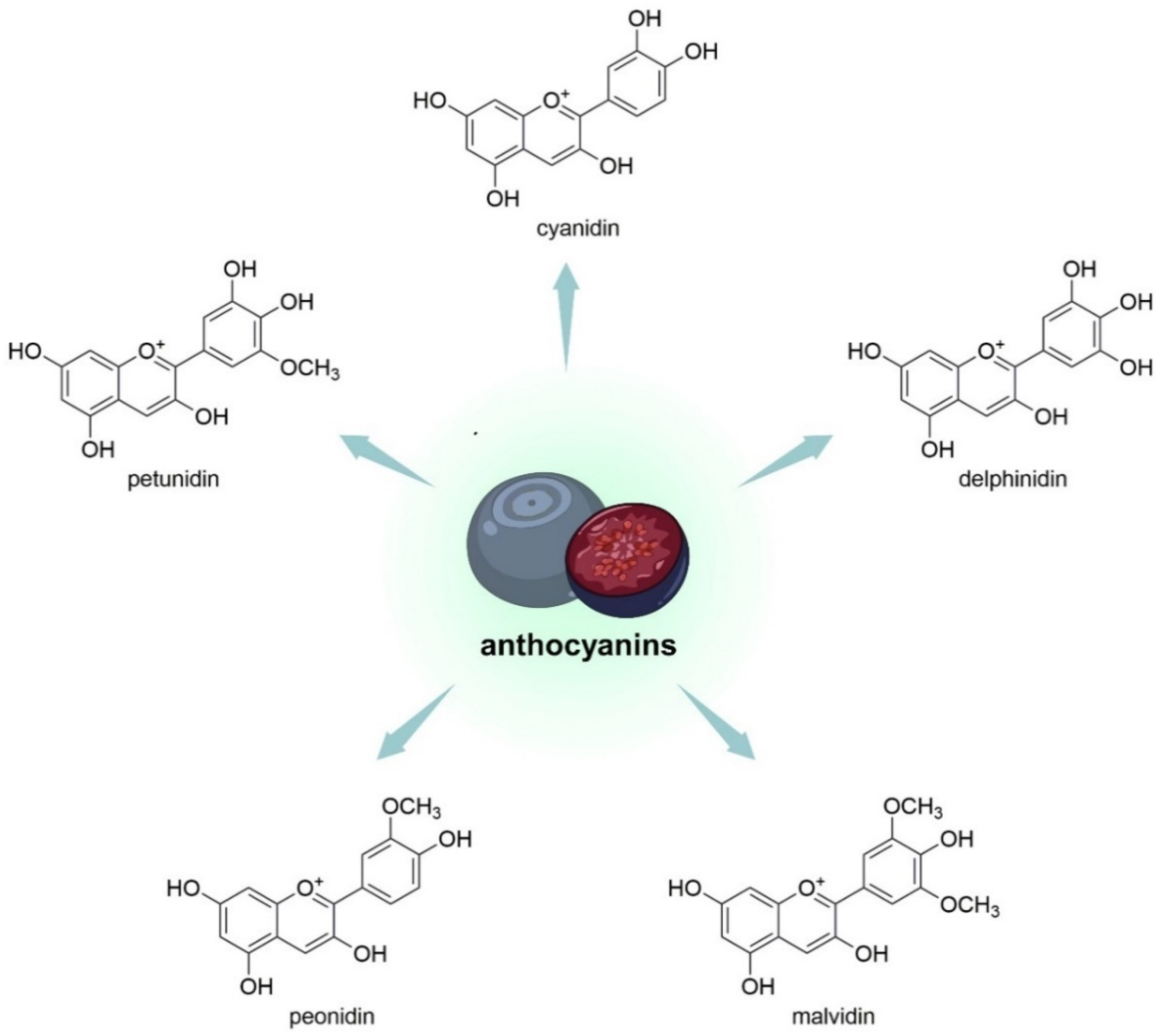
Figure 4. Chemical structures of aglycone parts of anthocyanins (so-called anthocyanidins) that are contained in V. uliginosum and V. myrtillus.
V. myrtillus anthocyanin extracts contain at least 16 anthocyanin monomers [66]. In its extract composition were cyanidin-3-O-rutinoside, delphinidin-3-galactoside, delphinidin-3-glucoside, cyanidin-3-galactoside, and chlorogenic acid as the main native phenolic compounds. They were also contained in the extract in smaller amounts of petunidin-3-glucoside, malvidin-3-glucoside, and cyanidin-3-glucoside. Cyanidin-3-O-rutinoside was higher in V. uliginosum compared to V. myrtillus, accounting for 136.8909 mg/g ± 11.48 (36.63%) and 43.5743 mg/g ± 4.01 (26.40%) of total anthocyanins, respectively [50]. The comparative analysis shows that the two Vaccinium species have different quantitative compositions of the 15 tested anthocyanins, all at different concentrations (p < 0.001). It was found that in bilberry there is a predominance of all target anthocyanins, except for malvidin-3-glucoside (its concentration was 471 mg and 230 mg/100 d.w. for V. uliginosum subsp. gaultherioides and V. myrtillus, respectively). Malvidin derivatives represented a major percentage of the anthocyanins found in bog bilberry—approximately 50% of the total concentration of target anthocyanins. The other anthocyanins identified in V. uliginosum occurred as follows (from lowest to highest concentration): peonidin < cyanidin = petunidin < delphinidin. As with V. myrtillus, glycoside abundance was also different (70% of the total), with glucosides accounting for 70% of the total, while galactosides and arabinosides were found at very similar percentages (16% and 14%, respectively) [55].
It is known that polyphenols and anthocyanins have a strong impact on antioxidant activity—the higher their content, the more potent their free-radical-eliminating action [67][68][69][70][71]. The team of Kusznierewicz et al. analyzed the content of bioactive substances in samples of wild and bog bilberry from Poland. They determined the content of anthocyanins and polyphenols in dry and fresh samples (Table 2) [72].
Table 2. The total content of anthocyanins and other polyphenolic compounds in dry and fresh weight of Polish V. myrtillus and V. uliginosum.
| Dry Samples | Fresh Samples | |||
|---|---|---|---|---|
| Total Anthocyanins Content (mg/g) |
Total Phenolics Content (mg/g) |
Total Anthocyanins Content (mg/g) |
Total Phenolics Content (mg/g) |
|
| V. myrtillus | 21.8 ± 0.1 | 26.6 ± 0.1 | 19.4 ± 0.1 | 23.7 ± 0.1 |
| V. uliginosum | 14.3 ± 0.3 | 21.1 ± 0.3 | 12.4 ± 0.2 | 18.2 ± 0.2 |
The polyphenolic compounds had comparable contents. Furthermore, the antioxi-dant activity of V. myrtillus and V. uliginosum was also essentially similar. The obtained results suggested that both berries are a good dietary source of anthocyanins.
3.1.3. Proanthocyanidins
In the dry weight (DW) of V. uliginosum, the main monomers and dimers of proanthocyanidins, i.e., procyanidin B2 (Figure 5), EC, phlorizin, taxifolin, gallocatechin, and EGC, were determined using a validated quantitative method. In total, the total procyanidin content was 159.4 µg/g DW, and the main monomers and dimers were EC and procyanidin B2. The content of phlorizin was 2.942 µg/g DW, and that of taxifolin was 2.807 µg/g DW. In turn, gallocatechin and EGC were identified in the tested fruits only in trace amounts [73].
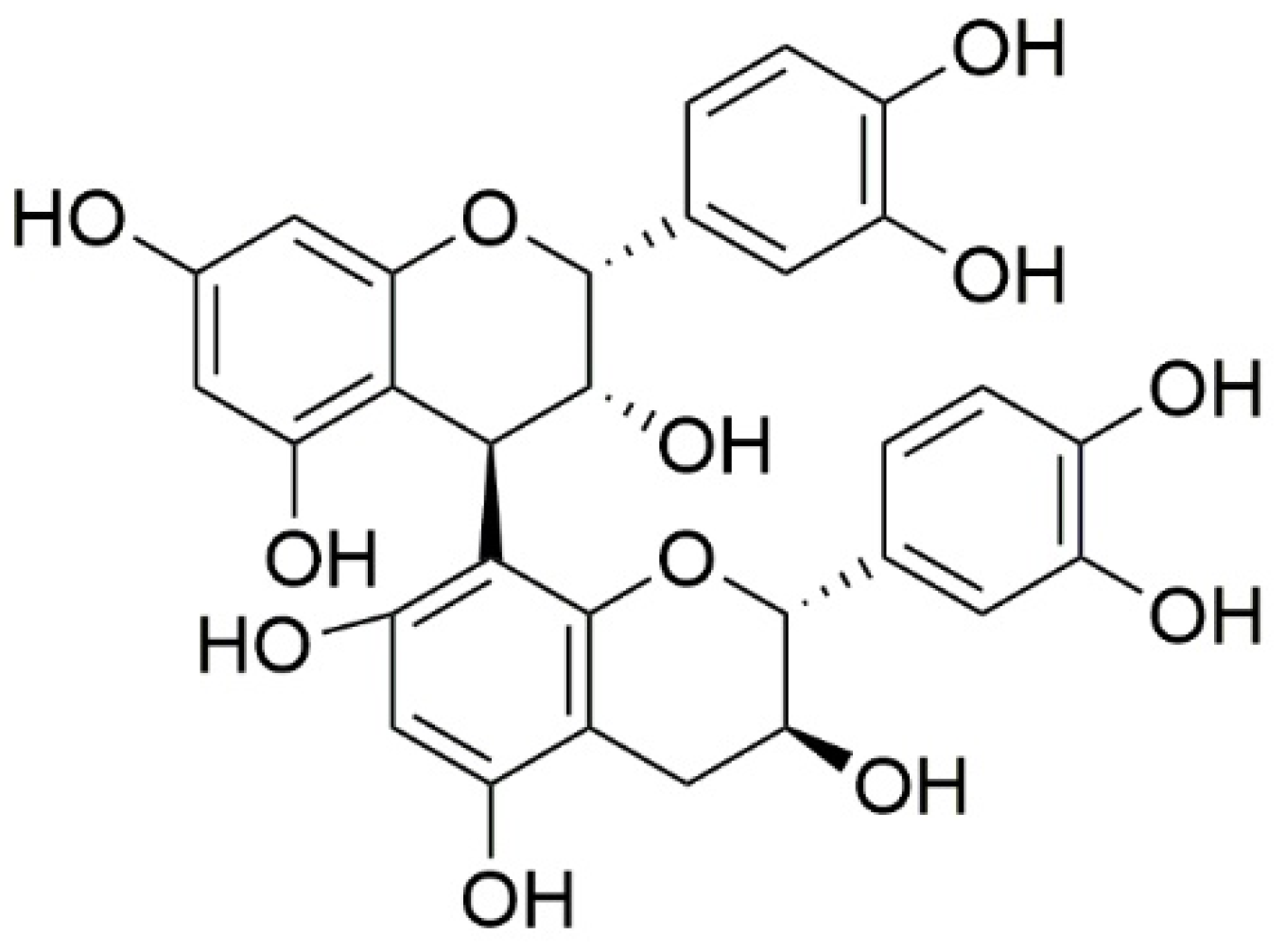
Figure 5. Chemical structures of procyanidin B2 contained in V. uliginosum.
3.1.4. Phenolic Acids
The antioxidant effects of V. myrtillus fruit were shown to depend on its phenolic content. Researchers found that even very low doses of the compound produced intracellular antioxidant activity [74]. Researchers have also proven that leaves contain more phenolic compounds compared to fruits [75].
It was determined that the main phenolic acids of bog bilberry juice are protocatechuic and chlorogenic acids [56]. The total content of phenolic acids in the dry matter of bilberries is approximately 2 mg/g [73]. Similar contents of flavonoids (EC and quercetin-3-glucoside) and p-coumaric acid were found in V. uliginosum and V. myrtillus. It was reported that V. uliginosum subsp. gaultherioides contains twenty-fold chlorogenic acid than V. myrtillus. Blueberries contained about ten times more cryptochlorogenic acid (Figure 5) than bog bilberries [55]. Ellagic, gallic, p-coumaric, ferulic, and syringic acids constitute a higher percentage of phenolic and hydroxycinnamic acids in V. myrtillus fruits. Moreover, the fruit of V. myrtillus also contains small quantities of vanillic acid, salicylic acid, and hydroxybenzoic acid [75].
In one study, the quantitative composition of eleven phenolic acids (Figure 6) and seventeen anthocyanin 3-glycosides in V. uliginosum was identified and determined. Caffeic acid (351 and 1076 μg/100 g in free and glycoside form, respectively) and syringic acid (in ester form 3524 μg/100 g FW) were the main phenolic acids of bog bilberry. It is also worth mentioning that the content of major phenolic acids in Vaccinium berries seems to suggest intra- and interspecies differences [38].
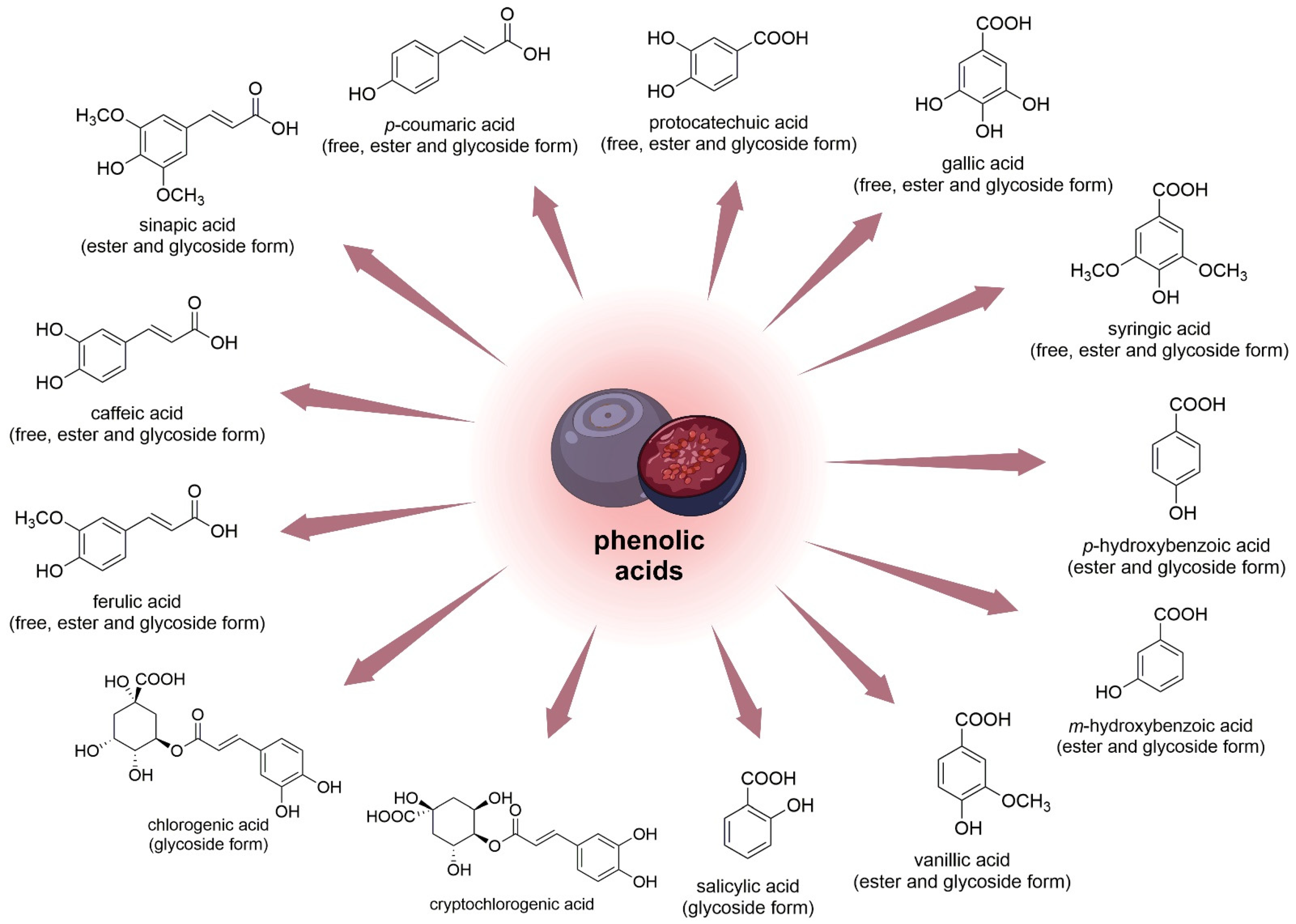
Figure 6. Chemical structures of phenolic acids (and their forms) that are contained in the fruits of V. uliginosum and V. myrtillus.
3.2. Other Organic Acids
Bilberry fruits also contain simple organic acids (citric/shikimic/malic/quinic acid; Figure 7) [75]. Among the main organic acids, in terms of concentration, in V. uliginosum are citric acid, malic acid, and ascorbic acid, with concentrations of 172 ± 11. 21 ± 4, and 12 ± 1 mg/100 g FW, respectively [34].
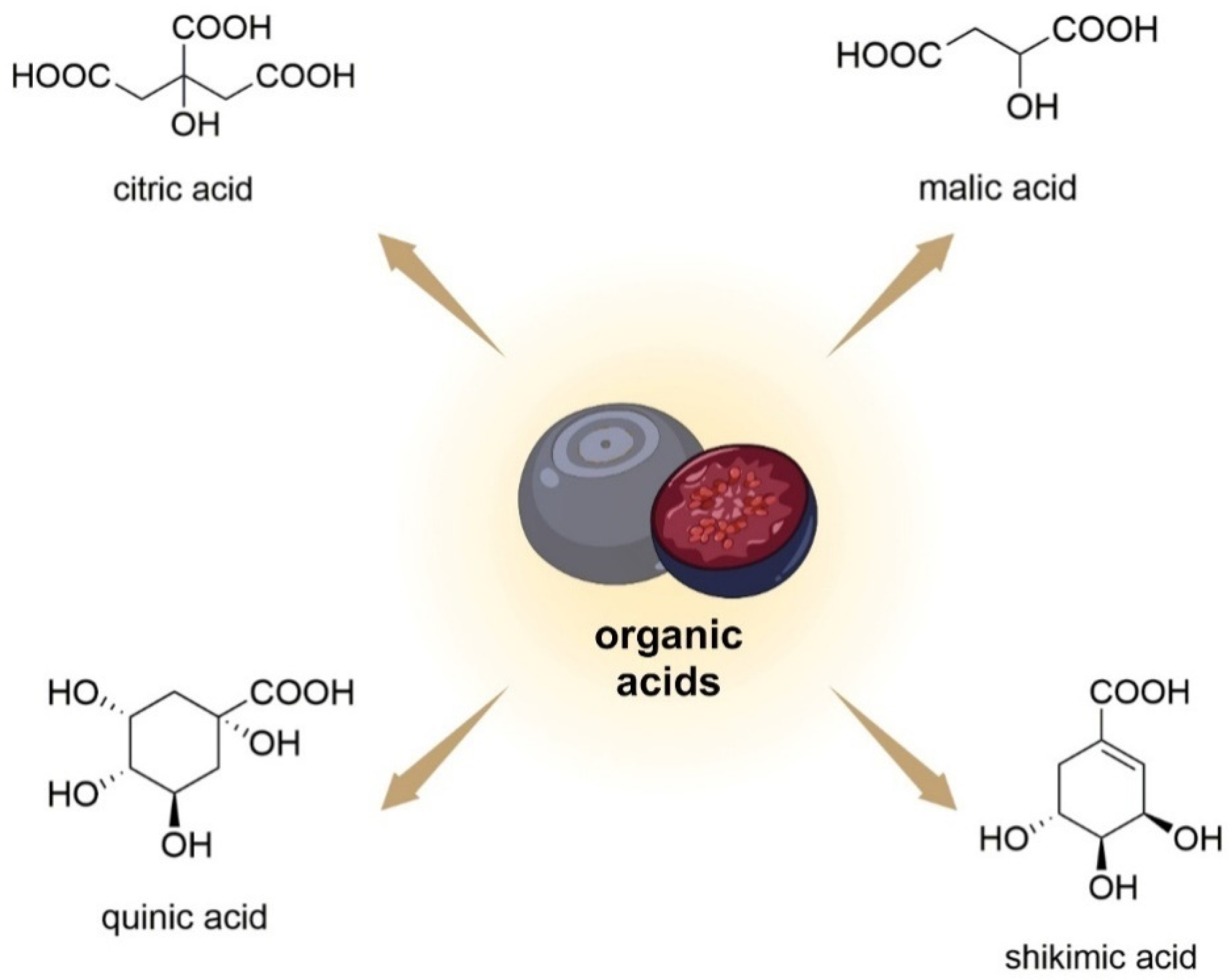
Figure 7. Chemical structures of organic acids that are contained in V. uliginosum and V. myrtillus fruits.
3.3. PUFAs (Polyunsaturated Fatty Acids)
PUFAs (polyunsaturated fatty acids) are a group of exogenous fatty acids that have to be supplemented through food. This is because the human organism lacks the enzymes needed to form double bonds in the chain of fatty acids outside C-9; thus, they cannot be synthesized in our body. Fatty acids n-3 and n-6 are part of phospholipids, which are important building components of cell membranes. Importantly, the proportion of these acids in tissues depends on their dietary intake. In addition to the above, they are also essential compounds during the synthesis of many biologically active molecules, for example, prostaglandins [34].
One study evaluated the chemical properties of cold-extracted native oils from V. myrtillus seeds to identify the qualitative composition of the fatty acids they contain and their positional distribution. It has been proven that seeds of V. myrtillus are abundant in PUFAs. The analysis conducted in this study showed a high α-linolenic acid (n-3) content in V. myrtillus oil, which was 28.99%. Additionally, oleic acid was detected as the predominant one in bilberry—21.02%. A very important and particularly desirable aspect of human nutrition is that vegetable oils in people’s diets should be characterized by a low n-6/n-3 acid ratio. V. myrtillus oils have been shown to have an n-3/n-6 ratio of 1–2, indicating that they may be beneficial in people after heart attacks and cardiac surgery [34]. Figure 8 contains the chemical formulas of the fatty acids detected in V. myrtillus seed oil.
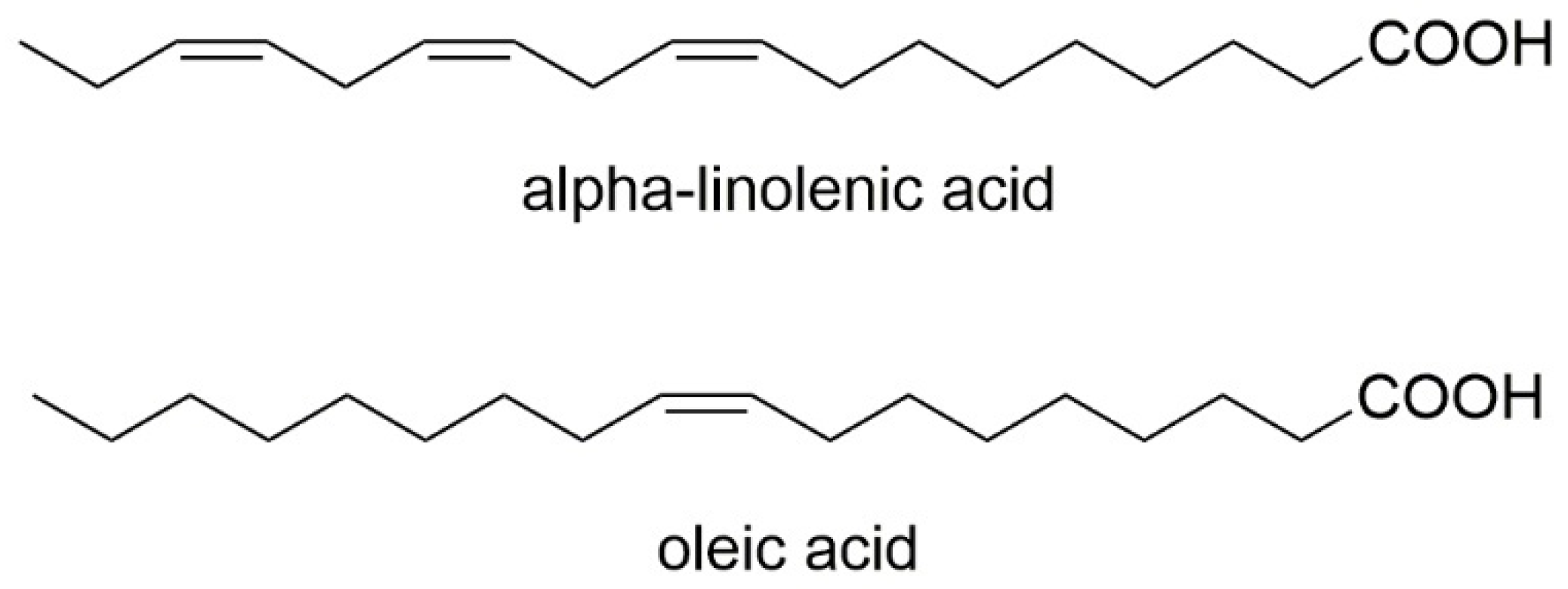
Figure 8. Chemical structures of fatty acids that are contained in V. myrtillus seed oil.
3.4. α-Tocopherol
Bederska-Łojewska D. et al. found that V. myrtillus seed oils have higher levels of α-tocopherol (Figure 9) than commercial tocopherol-rich oils (made from soybean and corn), and 4.84 mg of vitamin E were found per 100 g of blueberry [34].
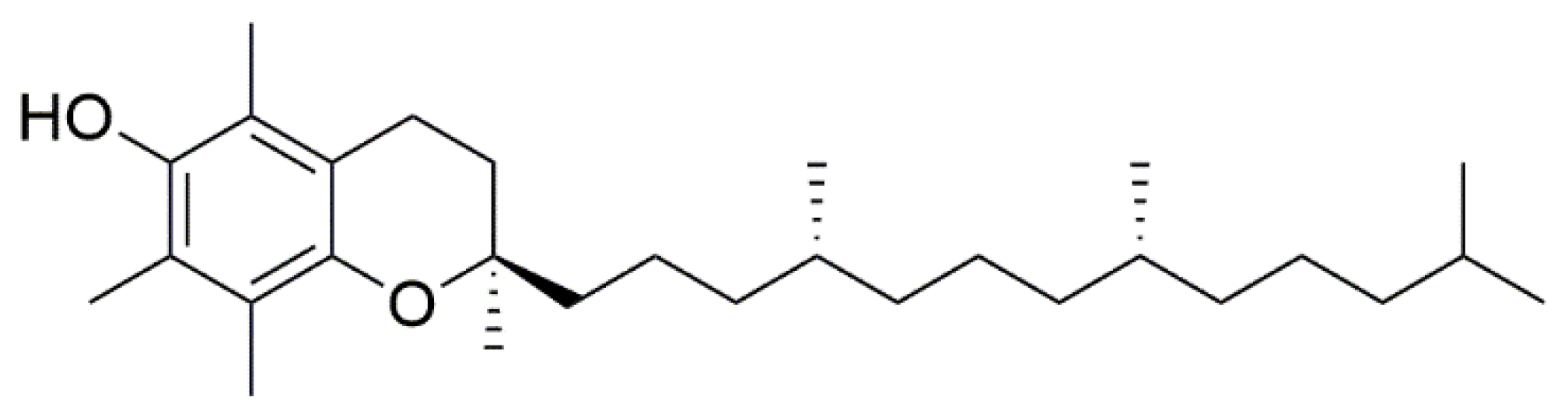
Figure 9. Chemical structure of α-tocopherol.
References
- Han, E.-K.; Kwon, H.-S.; Shin, S.-G.; Choi, Y.-H.; Kang, I.-J.; Chung, C.-K. Biological Effect of Vaccinium uliginosum L. on STZ-Induced Diabetes and Lipid Metabolism in Rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1727–1733.
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Stability and Antiglycoxidant Potential of Bilberry Anthocyanins in Simulated Gastrointestinal Tract Model. Foods 2020, 9, 1695.
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, S.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and Proapoptotic Effects of Anthocyanins from Bilberry Extract in Rats Exposed to Hepatotoxic Effects of Carbon Tetrachloride. Life Sci. 2016, 157, 168–177.
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the Developmental and Environmental Regulation of Secondary Metabolism in Vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655.
- Benzie, I.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011.
- Liu, S.; Laaksonen, O.; Yang, W.; Zhang, B.; Yang, B. Pyranoanthocyanins in Bilberry (Vaccinium myrtillus L.) Wines Fermented with Schizosaccharomyces Pombe and Their Evolution during Aging. Food Chem. 2020, 305, 125438.
- Behrends, A.; Weber, F. Influence of Different Fermentation Strategies on the Phenolic Profile of Bilberry Wine (Vaccinium myrtillus L.). J. Agric. Food Chem. 2017, 65, 7483–7490.
- Chen, L.; Zhang, X.; Wang, Q.; Li, W.; Liu, L. Effect of Vaccinium myrtillus Extract Supplement on Advanced Glycation End-Products: A Pilot Study (P06-098-19). Curr. Dev. Nutr. 2019, 3, 616.
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Screening and Characterization of Antiglycoxidant Anthocyanins from Vaccinium myrtillus Fruit Using DPPH and Methylglyoxal Pre-Column HPLC Assays. Antioxidants 2020, 9, 512.
- Maulik, M.; Mitra, S.; Sweeney, M.; Lu, B.; Taylor, B.E.; Bult-Ito, A. Complex Interaction of Dietary Fat and Alaskan Bog Blueberry Supplementation Influences Manganese Mediated Neurotoxicity and Behavioral Impairments. J. Funct. Foods 2019, 53, 306–317.
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75.
- Chan, S.W.; Tomlinson, B. Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk. Molecules 2020, 25, 1653.
- Bujor, O.C.; Tanase, C.; Popa, M.E. Phenolic Antioxidants in Aerial Parts of Wild Vaccinium Species: Towards Pharmaceutical and Biological Properties. Antioxidants 2019, 8, 649.
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928.
- Szakiel, A.; Pa̧czkowski, C.; Huttunen, S. Triterpenoid Content of Berries and Leaves of Bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849.
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and Other Non-Polar Compounds in Leaves of Wild and Cultivated Vaccinium Species. Plants 2021, 10, 94.
- Anadon-Rosell, A.; Palacio, S.; Nogués, S.; Ninot, J.M. Vaccinium myrtillus Stands Show Similar Structure and Functioning under Different Scenarios of Coexistence at the Pyrenean Treeline. Plant Ecol. 2016, 217, 1115–1128.
- Ştefanescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046.
- Gonçalves, A.C.; Sánchez-Juanes, F.; Meirinho, S.; Silva, L.R.; Alves, G.; Flores-Félix, J.D. Insight into the Taxonomic and Functional Diversity of Bacterial Communities Inhabiting Blueberries in Portugal. Microorganisms 2022, 10, 2193.
- Trivedi, P.; Karppinen, K.; Klavins, L.; Kviesis, J.; Sundqvist, P.; Nguyen, N.; Heinonen, E.; Klavins, M.; Jaakola, L.; Väänänen, J.; et al. Compositional and Morphological Analyses of Wax in Northern Wild Berry Species. Food Chem. 2019, 295, 441–448.
- Diaconeasa, Z. Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma. Molecules 2018, 23, 2534.
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68.
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium myrtillus L.) Growing at Different Locations. J. Sci. Food Agric. 2015, 95, 776–785.
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706.
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Technol. 2015, 52, 5898.
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115.
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588.
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and Characteristics of Alginate and Anthocyanin Complexes. Int. J. Biol. Macromol. 2020, 164, 726–734.
- Szakiel, A.; Pa̧czkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the Triterpenoid Content of Berries and Leaves of Lingonberry Vaccinium vitis-Idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002.
- Mi, J.C.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-Performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782.
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004.
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599.
- Lätti, A.K.; Jaakola, L.; Riihinen, K.R.; Kainulainen, P.S. Anthocyanin and Flavonol Variation in Bog Bilberries (Vaccinium uliginosum L.) in Finland. J. Agric. Food Chem. 2010, 58, 427–433.
- Bederska-Łojewska, D.; Pieszka, M.; Marzec, A.; Rudzińska, M.; Grygier, A.; Siger, A.; Cieślik-Boczula, K.; Orczewska-Dudek, S.; Migdał, W. Physicochemical Properties, Fatty Acid Composition, Volatile Compounds of Blueberries, Cranberries, Raspberries, and Cuckooflower Seeds Obtained Using Sonication Method. Molecules 2021, 26, 7446.
- Alves, E.; Simoes, A.; Domingues, M.R. Fruit Seeds and Their Oils as Promising Sources of Value-Added Lipids from Agro-Industrial Byproducts: Oil Content, Lipid Composition, Lipid Analysis, Biological Activity and Potential Biotechnological Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 1305–1339.
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663.
- Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065.
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosín-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog Bilberry Phenolics, Antioxidant Capacity and Nutrient Profile. Food Chem. 2016, 201, 339–349.
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant Capacity, Vitamin C, Phenolics, and Anthocyanins after Fresh Storage of Small Fruits. J. Agric. Food Chem. 1999, 47, 4638–4644.
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of High Hydrostatic Pressure and Thermal Processing on Anthocyanin Content, Polyphenol Oxidase and β-Glucosidase Activities, Color, and Antioxidant Activities of Blueberry (Vaccinium spp.) Puree. Food Chem. 2021, 342, 128564.
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of Modified Atmosphere Packaging (MAP) and Gaseous Ozone Pre-Packaging Treatment on the Physico-Chemical, Microbiological and Sensory Quality of Small Berry Fruit. Food Packag. Shelf Life 2020, 26, 100573.
- Muñoz-Fariña, O.; López-Casanova, V.; García-Figueroa, O.; Roman-Benn, A.; Ah-Hen, K.; Bastias-Montes, J.M.; Quevedo-León, R.; Ravanal-Espinosa, M.C. Bioaccessibility of Phenolic Compounds in Fresh and Dehydrated Blueberries (Vaccinium corymbosum L.). Food Chem. Adv. 2023, 2, 100171.
- Maryam, A.; Anwar, R.; Malik, A.U.; Raheem, M.I.U.; Khan, A.S.; Hasan, M.U.; Hussain, Z.; Siddique, Z. Combined Aqueous Ozone and Ultrasound Application Inhibits Microbial Spoilage, Reduces Pesticide Residues and Maintains Storage Quality of Strawberry Fruits. J. Food Meas. Charact. 2021, 15, 1437–1451.
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of Cold Plasma on Blueberry Juice Quality. Food Chem. 2019, 290, 79–86.
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923.
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123.
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and in Vitro Availability of Four Different Berries. J. Chem. 2016, 2016, 5194901.
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite Profiling of Polyphenols in Vaccinium Berries and Determination of Their Chemopreventive Properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267.
- Wang, L.J.; Su, S.; Wu, J.; Du, H.; Li, S.S.; Huo, J.W.; Zhang, Y.; Wang, L.S. Variation of Anthocyanins and Flavonols in Vaccinium uliginosum Berry in Lesser Khingan Mountains and Its Antioxidant Activity. Food Chem. 2014, 160, 357–364.
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of Anthocyanins from Four Kinds of Berries and Their Inhibition Activity to α-Glycosidase and Protein Tyrosine Phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221.
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous Determination of Flavonols and Phenolic Acids by HPLC-CoulArray in Berries Common in the Nordic Diet. LWT 2016, 74, 128–134.
- Levaj, B.; Dragović-Uzelac, V.; Delonga, K.; Kovačević Ganić, K.; Banović, M.; Bursać Kovačević, D. Polyphenols and Volatiles in Fruits of Two Sour Cherry Cultivars, Some Berry Fruits and Their Jams. Food Technol. Biotechnol. 2010, 48, 538–547.
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn Identification and Quantification of Flavonol Glycosides in 28 Wild and Cultivated Berry Species. Food Chem. 2012, 135, 2138–2146.
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126.
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. Subsp. Gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184.
- Wei, M.; Wang, S.; Gu, P.; Ouyang, X.; Liu, S.; Li, Y.; Zhang, B.; Zhu, B. Comparison of Physicochemical Indexes, Amino Acids, Phenolic Compounds and Volatile Compounds in Bog Bilberry Juice Fermented by Lactobacillus plantarum under Different PH Conditions. J. Food Sci. Technol. 2018, 55, 2240.
- Wang, Y.; Liu, X.; Chen, J.Z.; Tian, X.; Zheng, Y.H.; Hao, J.; Xue, Y.J.; Ding, S.Y.; Zong, C.W. The Variation of Total Flavonoids, Anthocyanins and Total Phenols in Vaccinium uliginosum Fruits in Changbai Mountain of China Is Closely Related to Spatial Distribution. J. Berry Res. 2022, 12, 463–481.
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Česoniene, L.; Daubaras, R. Antioxidant Properties, Phenolic Composition and Potentiometric Sensor Array Evaluation of Commercial and New Blueberry (Vaccinium corymbosum) and Bog Blueberry (Vaccinium uliginosum) Genotypes. Food Chem. 2015, 188, 583–590.
- Bayazid, A.B.; Chun, E.M.; Al Mijan, M.; Park, S.H.; Moon, S.K.; Lim, B.O. Anthocyanins Profiling of Bilberry (Vaccinium myrtillus L.) Extract That Elucidates Antioxidant and Anti-Inflammatory Effects. Food Agric. Immunol. 2021, 32, 713–726.
- Jin, Y.; Zhang, Y.; Liu, D.; Liu, D.; Zhang, C.; Qi, H.; Gu, H.; Yang, L.; Zhou, Z. Efficient Homogenization-Ultrasound-Assisted Extraction of Anthocyanins and Flavonols from Bog Bilberry (Vaccinium uliginosum L.) Marc with Carnosic Acid as an Antioxidant Additive. Molecules 2019, 24, 2537.
- Seeram, N.P. Berry Fruits: Compositional Elements, Biochemical Activities, and the Impact of Their Intake on Human Health, Performance, and Disease. J. Agric. Food Chem. 2008, 56, 627–629.
- Holkem, A.T.; Robichaud, V.; Favaro-Trindade, C.S.; Lacroix, M. Chemopreventive Properties of Extracts Obtained from Blueberry (Vaccinium myrtillus L.) and Jabuticaba (Myrciaria cauliflora Berg.) in Combination with Probiotics. Nutr. Cancer 2021, 73, 671–685.
- Cásedas, G.; González-Burgos, E.; Smith, C.; López, V.; Gómez-Serranillos, M.P. Regulation of Redox Status in Neuronal SH-SY5Y Cells by Blueberry (Vaccinium myrtillus L.) Juice, Cranberry (Vaccinium macrocarpon A.) Juice and Cyanidin. Food Chem. Toxicol. 2018, 118, 572–580.
- Sharma, A.; Lee, H.J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583.
- Piberger, H.; Oehme, A.; Hofmann, C.; Dreiseitel, A.; Sand, P.G.; Obermeier, F.; Schoelmerich, J.; Schreier, P.; Krammer, G.; Rogler, G. Bilberries and Their Anthocyanins Ameliorate Experimental Colitis. Mol. Nutr. Food Res. 2011, 55, 1724–1729.
- Pan, F.; Liu, Y.; Liu, J.; Wang, E. Stability of Blueberry Anthocyanin, Anthocyanidin and Pyranoanthocyanidin Pigments and Their Inhibitory Effects and Mechanisms in Human Cervical Cancer HeLa Cells. RSC Adv. 2019, 9, 10842.
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition 2005, 21, 207–213.
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693.
- Wang, S.Y.; Lin, H.S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146.
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438.
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, Phenolics, and Antioxidant Capacity in Diverse Small Fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525.
- Kusznierewicz, B.; Piekarska, A.; Mrugalska, B.; Konieczka, P.; Namieśnik, J.; Bartoszek, A. Phenolic Composition and Antioxidant Properties of Polish Blue-Berried Honeysuckle Genotypes by HPLC-DAD-MS, HPLC Postcolumn Derivatization with ABTS or FC, and TLC with DPPH Visualization. J. Agric. Food Chem. 2012, 60, 1755–1763.
- Wang, C.; Zhang, M.; Wu, L.; Wang, F.; Li, L.; Zhang, S.; Sun, B. Qualitative and Quantitative Analysis of Phenolic Compounds in Blueberries and Protective Effects on Hydrogen Peroxide-Induced Cell Injury. J. Sep. Sci. 2021, 44, 2837–2855.
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and Blueberry Anthocyanins Act as Powerful Intracellular Antioxidants in Mammalian Cells. Food Chem. 2012, 134, 1878–1884.
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 909914.
More
Information
Subjects:
Nursing
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




