You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Monika Wujec and Version 2 by Rita Xu.
Vaccinium uliginosum
L. (commonly known as bog bilberry) and
Vaccinium myrtillus
L. (commonly known as bilberry) are species of the genus
Vaccinium
(family
Ericaceae
). The red–purple–blue coloration of blueberries is attributed largely to the anthocyanins found in bilberries. Anthocyanins, known for their potent biological activity as antioxidants, have a significant involvement in the prophylaxis of cancer or other diseases, including those of metabolic origin.
- bog bilberry
- Vaccinium uliginosum
- Vaccinium myrtillus
1. Introduction
Vaccinium uliginosum L. (bog bilberry) and Vaccinium myrtillus L. (bilberry) are species of the genus Vaccinium (family Ericaceae). They are low-growing deciduous shrubs that produce dark purple fruits (berries) which are edible (Figure 1). Commonly called bilberries, their fruits are highly valued as a rich source of anthocyanins, which are naturally occurring compounds. In fresh berries, their content is about 0.5% [1][2][3][4][1,2,3,4]. In addition to fresh fruit, berries can also be consumed as frozen, dried, juices, jams, and food supplements [5]. It has recently become more popular to consume fermented products made from bilberries [6][7][6,7]. In vitro studies have shown that bilberry extracts have an impact on the effects of, among other things, anti-glycation and the scavenging of external radicals. Strong antioxidant properties were also found because of the occurrence of abundant bioactive substances, such as anthocyanins and flavanols [1][8][9][1,8,9]. Thanks to these properties, the supplementation of bilberries can have an impact on health in many cases of diseases. Its known pharmacological effects include vascular regulation, dysentery, antigens, diabetic retinopathy, and potential anti-cancer effects [10][11][12][13][14][10,11,12,13,14].

Figure 1. V. myrtillus and V. uliginosum in their natural habitat and the external appearance of their parts (leaves and fruit).
There are many studies on Vaccinium species, but so far there is no comparison of both species, V. uliginosum and V. myrtillus, especially in terms of their biological activity and possible use as functional food. The biological effect of the fruit extract of V. uliginosum is known primarily from both Chinese and European folk medicine.
2. Occurrence
Most V. myrtillus and V. uliginosum are mainly acquired from their native habitats [15][16][15,16]. These members of the Ericaceae family grow best in humid and moderate climates. Mountains and high mountains are the most common habitats in their southernmost distribution [17]. V. myrtillus is found in European mountains and forests, while V. uliginosum grows in areas of Asia, Europe, and North America [18]. V. uliginosum, V. myrtillus, and V. vitis-idaea are the species that grow on the Iberian Peninsula. Observations of V. uliginosum on Portugal’s mainland suggest fragmented populations and uncertain survival in the uppermost parts of Serra da Estrela. Serra da Estrela as well as Serra da Freita both have fragmented populations of V. myrtillus, but the latter is more plentiful in northern Portugal’s mountains [19]. Bilberry (Vaccinium myrtillus L.) is the most important economically wild berry in Northern Europe, and it is also extensively used in juice and food production. The bog bilberry is used to a lesser extent, but it is widespread in northern areas [20]. Compared to cultivated species, wild berries have a more complex chemical composition [18]. A very important aspect is also climate and weather conditions, which determine the content of the various bioactive substances (phenolic acids, anthocyanins, etc.) in blueberries [21]. In turn, the qualitative–quantitative composition of phenolic compounds in bilberries depends on the plant parts used, growth stage, and genetic factors [22][23][22,23]. For this reason, buyers are interested in the origin of the berries, as those from specific areas or countries often have a higher price. As spectrophotometers are quick and easy to use, they are highly suitable for commercial purposes, especially for evaluating berry quality [24][25][26][24,25,26]. A study by Urbanaviciene and Dalia et al. determined the physicochemical properties, as well as the levels of total anthocyanins (TAC) and polyphenols (TPC) present in V. myrtillus populations, which occur in areas of Northern Europe (Lithuania, Latvia, Finland, and Norway), along with their ability to scavenge free radicals. In the investigation, V. myrtillus had pH values ranging from 2.94 to 3.47. Approximately 232.7 to 475.5 mg/100 g of fresh weight (FW) were obtained from the investigated V. myrtillus samples. The content of TPC was the highest in Norway and the lowest in Lithuania and varied between 452–902 mg/100 g FW. According to the study, the antioxidant capacity of V. myrtillus oscillated between 60.9 and 106.0 mol TE/g FW, with the lowest value in populations from Lithuania and the highest from Norway [27]. The main ingredients that make up more than 50% of the Lithuanian bilberry water extract are cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, delphinidin-3-O-galactoside, peonidin-3-O-glucoside, petunidin-3-O-glucoside, delphinidin glycosides, and cyanidin [28]. According to Szakiel et al., the content of triterpenoids in the leaves of V. myrtillus from wild habitats varies significantly depending on its location in Poland and Finland. Polish leaves were significantly richer in lupeol, and friedelin was only found on Finnish leaves, while taraxasterol was only found on leaves of plants from Poland. Polish leaves contained more than three times as much 2α-hydroxyursolic and 2α-hydroxyoleanolic acids as Finnish leaves, but they had similar levels of oleanolic and ursolic acids [29].3. V. uliginosum and V. myrtillus Composition
Blueberry composition depends on the genotype of the plant [30][31][32][30,31,32]. V. uliginosum berries contain many anthocyanins and flavonols. V. uliginosum has a characteristic profile of flavonols and anthocyanins compared to other berries of the Vaccinium family, which can be used to distinguish bog bilberry from V. myrtillus [33]. V. myrtillus seeds and oils contain natural antioxidants, anti-inflammatory, anti-atherosclerotic, and anticancer compounds, such as tocochromanols, carotenoids, flavonoids, phytosterols, and phenolic acids [34][35][34,35]. The caloric energy intake of fresh bilberries is approximately 45 kcal/100 g. They consist of water (84%), carbohydrates (9.6%), proteins (0.7%), fats (0.4%), and fibers (about 3.5%) [36]. This is compared to dry bilberry, which has 395 kcal/100 g and contains 94% carbohydrates, 3% proteins, and 1.5% fats [37]. The pH value of the bog bilberry’s berry (V. uliginosum L.) was relatively high (pH = 3.5), and their titratable acidity, in turn, was moderate (1 g of citric acid/100 g). The main identified soluble sugar was fructose (concentration of 2138 ± 149 mg/100 g FW), while glucose was the second in amount (concentration of 1664 ± 121 mg/100 g FW) [38].3.1. Polyphenols
Polyphenols are a group of naturally occurring compounds found in various plant foods, including berries from the Vaccinium genus (Figure 2).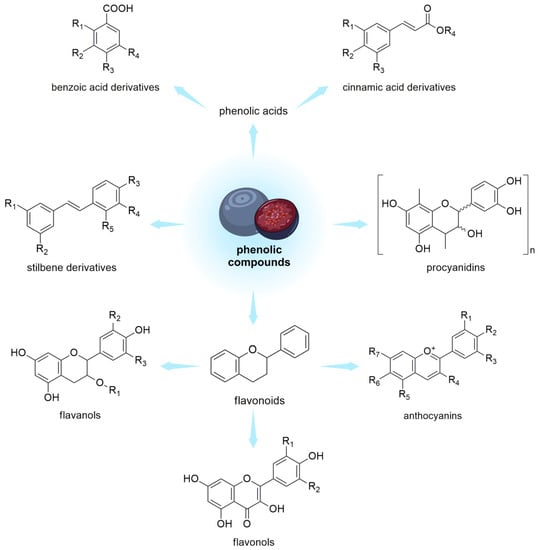
Figure 2. Phenolic compounds in V. uliginosum and V. myrtillus.
Table 1. Method of characterization of some polyphenol compounds in berries of Vaccinium genus.
| Polyphenol Compounds | Method of Characterization | References |
|---|---|---|
| delphinidin-3-O-galactoside malvidin-3-O-galactoside malvidin-3-O-arabinoside delphinidin-3-O-arabinoside |
CIELAB HPLC-DAD |
[46] |
| delphinidin 3-glucoside cyanidin 3-glucoside petunidin 3-glucoside delphinidin 3-glucoside |
HPLC-DAD | [47] |
| chlorogenic acid quercetin-3-O-galactoside quercetin-3-O-glucuronide delphinidin-3-O-galactoside delphinidin-3-O-glucoside cyanidin-3-O-galactoside petunidin-3-O-glucoside |
HPLC-UV/DAD HPLC-ESI-MS MS |
[48] |
| delphinidin 3-O-glucoside malvidin 3-O-glucoside myricetin 3-O-hexoside quercetin 3-O-galactoside |
HPLC-DAD HPLC-ESI-MS |
[49] |
| cyanidin-3-O-glucoside cyanidin-3-O-rutinoside catechin quercetin-3-O-galactoside quercetin-3-O-arabinoside myricetin 3-O-hexose |
HPLC-FT-ICR MS/MS | [50] |
| gallic acid vanillic acid ferulic acid caffeic acid p-coumaric acid quercetin |
HPLC | [51] |
| (–)-epicatechin kaempferol derivative chlorogenic acid ellagic acid |
HPLC | [52] |
| glycosides of quercetin myricetin kaempferol isorhamnetin syringetin laricitrin |
HPLC–MS | [53] |
3.1.1. Flavonols and Flavanols
Latti et al., in their studies, were the first to show the presence of kaempferol and isorhamnetin aglycones in V. uliginosum. In their study, about 1/4 of bog blueberry samples contained more flavonols than anthocyanins [33]. One study found that EC (epicatechin) and EGC (epigallocatechin) were the major flavanols in blueberry juice [56]. V. uliginosum also contains flavonols such as laricitrin, syringetin, myricetin, and quercetin. Based on the findings of myricetin and quercetin arabinosides, the minor laricitrin, isorhamnetin, and syringing pentosides were further named arabinosides. Both laricitrin and isorhamnetin were also detected in V. myrtillus [33]. Laricithrin, isorhamnetin, myricetin, kaempferol, syringetinhexosides, pentosides, and glucuronides, as well as glucuronide and pentosides QUE (quercetin), were identified in bilberry and bog bilberry in various amounts, and flavonol predominance in bog bilberry [55]. Figure 3 shows the chemical structures of flavonols and flavanols (and the sugars to which they are linked) that are contained in V. uliginosum and V. myrtillus.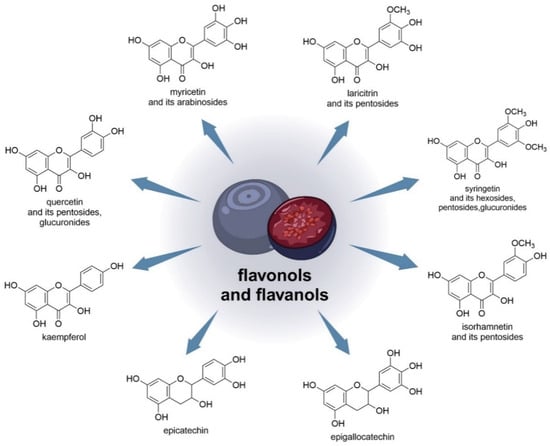
Figure 3. Chemical structures of flavonols and flavanols (and the sugars to which they are linked) that are contained in V. uliginosum and V. myrtillus.
3.1.2. Anthocyanins
Compared with some common edible berries, bog bilberries contain more complex anthocyanins [60]. It was found that the TAC in blueberries is about 6 g/kg of fruit [61]. Holkem et al. researched V. myrtillus extracts. It was proven that the best antiproliferative effect was shown by an anthocyanin-rich extract due to the abundance of bioactive substances occurring in it; for this extract, there was an elevation in the antioxidative effect after the introduction of bacteria [62]. In one study conducted on V. myrtillus juice, results indicated that blueberry juice and cyanidin increased mitochondrial activity and reduced intracellular reactive oxygen species (ROS) generation and hydrogen peroxide-induced lipid peroxidation. In addition, the juice caused an increase in the activity of antiradical enzymes—superoxide dismutase (SOD) and catalase (CAT) [63]. It has been proven that they have antioxidant, anti-cancer, and anti-inflammatory effects and that these compounds can alleviate chronic and acute colitis [64][65][64,65]. Bog bilberries were the subject of one study that identified five key anthocyanidins, among which malvidin 3-glucoside was the main compound. It was observed that the TAC fraction showed particularly high variability in antioxidant capacity, which was mainly influenced by the type of phenolic structure that was eluted by solid-phase extraction (SPE) [38]. V. uliginosum, in their structure, have an aglycone part and a glycosyl part. Malvidin, delphinidin, cyanidin, peonidin, and petunidin constitute the aglycone part (Figure 4), while arabinoside, glucoside, xyloside and galactoside belong to the glycosyl part of the compound. In the course of the research, it was observed that specific habitats conditioned noticeable differences in the quantitative composition of anthocyanidin glycosides [33]. Most anthocyanins in V. uliginosum derived from B-ring tri-substituted anthocyanidins (80 ± 3%); the most important was the malvidin-type (46 ± 6%), followed by cyanidin (21 ± 3%), delphinidin (13 ± 3%), petunidin (13 ± 0%), and peonidin (7 ± 1%) [38].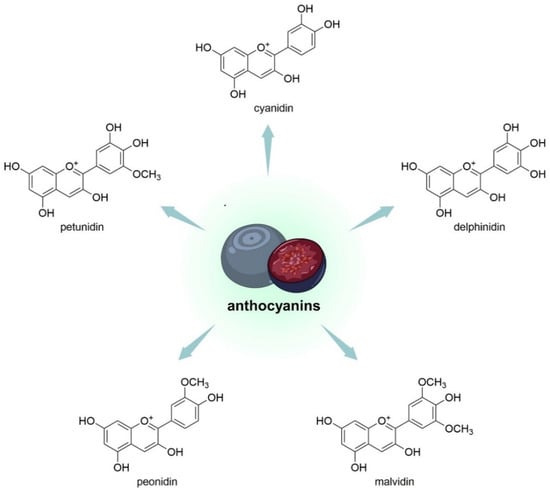
Figure 4. Chemical structures of aglycone parts of anthocyanins (so-called anthocyanidins) that are contained in V. uliginosum and V. myrtillus.
Table 2. The total content of anthocyanins and other polyphenolic compounds in dry and fresh weight of Polish V. myrtillus and V. uliginosum.
| Dry Samples | Fresh Samples | |||
|---|---|---|---|---|
| Total Anthocyanins Content (mg/g) |
Total Phenolics Content (mg/g) |
Total Anthocyanins Content (mg/g) |
Total Phenolics Content (mg/g) |
|
| V. myrtillus | 21.8 ± 0.1 | 26.6 ± 0.1 | 19.4 ± 0.1 | 23.7 ± 0.1 |
| V. uliginosum | 14.3 ± 0.3 | 21.1 ± 0.3 | 12.4 ± 0.2 | 18.2 ± 0.2 |
3.1.3. Proanthocyanidins
In the dry weight (DW) of V. uliginosum, the main monomers and dimers of proanthocyanidins, i.e., procyanidin B2 (Figure 5), EC, phlorizin, taxifolin, gallocatechin, and EGC, were determined using a validated quantitative method. In total, the total procyanidin content was 159.4 µg/g DW, and the main monomers and dimers were EC and procyanidin B2. The content of phlorizin was 2.942 µg/g DW, and that of taxifolin was 2.807 µg/g DW. In turn, gallocatechin and EGC were identified in the tested fruits only in trace amounts [73].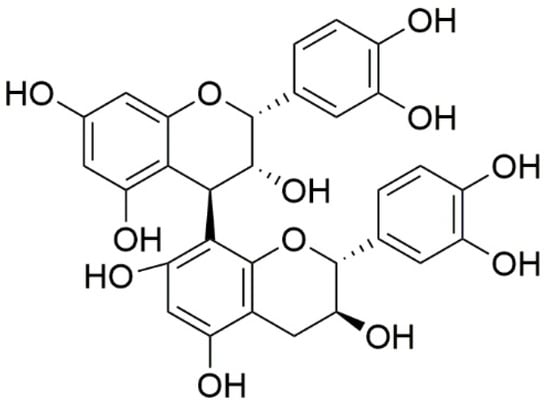
Figure 5. Chemical structures of procyanidin B2 contained in V. uliginosum.
3.1.4. Phenolic Acids
The antioxidant effects of V. myrtillus fruit were shown to depend on its phenolic content. Researchers found that even very low doses of the compound produced intracellular antioxidant activity [74]. Researchers have also proven that leaves contain more phenolic compounds compared to fruits [75]. It was determined that the main phenolic acids of bog bilberry juice are protocatechuic and chlorogenic acids [56]. The total content of phenolic acids in the dry matter of bilberries is approximately 2 mg/g [73]. Similar contents of flavonoids (EC and quercetin-3-glucoside) and p-coumaric acid were found in V. uliginosum and V. myrtillus. It was reported that V. uliginosum subsp. gaultherioides contains twenty-fold chlorogenic acid than V. myrtillus. Blueberries contained about ten times more cryptochlorogenic acid (Figure 5) than bog bilberries [55]. Ellagic, gallic, p-coumaric, ferulic, and syringic acids constitute a higher percentage of phenolic and hydroxycinnamic acids in V. myrtillus fruits. Moreover, the fruit of V. myrtillus also contains small quantities of vanillic acid, salicylic acid, and hydroxybenzoic acid [75]. In one study, the quantitative composition of eleven phenolic acids (Figure 6) and seventeen anthocyanin 3-glycosides in V. uliginosum was identified and determined. Caffeic acid (351 and 1076 μg/100 g in free and glycoside form, respectively) and syringic acid (in ester form 3524 μg/100 g FW) were the main phenolic acids of bog bilberry. It is also worth mentioning that the content of major phenolic acids in Vaccinium berries seems to suggest intra- and interspecies differences [38].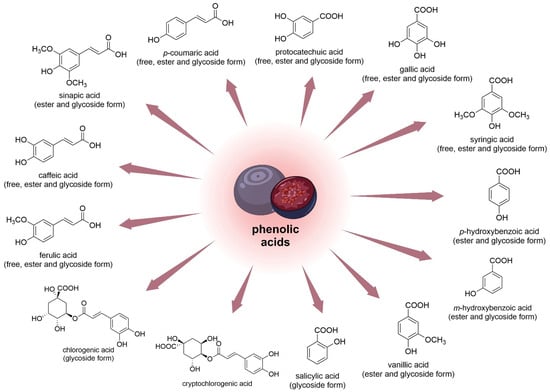
Figure 6. Chemical structures of phenolic acids (and their forms) that are contained in the fruits of V. uliginosum and V. myrtillus.
3.2. Other Organic Acids
Bilberry fruits also contain simple organic acids (citric/shikimic/malic/quinic acid; Figure 7) [75]. Among the main organic acids, in terms of concentration, in V. uliginosum are citric acid, malic acid, and ascorbic acid, with concentrations of 172 ± 11. 21 ± 4, and 12 ± 1 mg/100 g FW, respectively [34].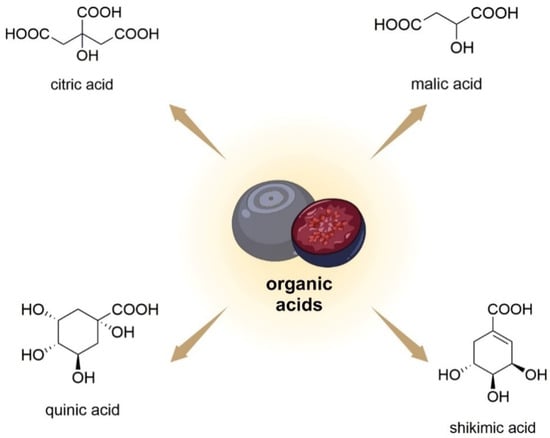
Figure 7. Chemical structures of organic acids that are contained in V. uliginosum and V. myrtillus fruits.
3.3. PUFAs (Polyunsaturated Fatty Acids)
PUFAs (polyunsaturated fatty acids) are a group of exogenous fatty acids that have to be supplemented through food. This is because the human organism lacks the enzymes needed to form double bonds in the chain of fatty acids outside C-9; thus, they cannot be synthesized in our body. Fatty acids n-3 and n-6 are part of phospholipids, which are important building components of cell membranes. Importantly, the proportion of these acids in tissues depends on their dietary intake. In addition to the above, they are also essential compounds during the synthesis of many biologically active molecules, for example, prostaglandins [34]. One study evaluated the chemical properties of cold-extracted native oils from V. myrtillus seeds to identify the qualitative composition of the fatty acids they contain and their positional distribution. It has been proven that seeds of V. myrtillus are abundant in PUFAs. The analysis conducted in this study showed a high α-linolenic acid (n-3) content in V. myrtillus oil, which was 28.99%. Additionally, oleic acid was detected as the predominant one in bilberry—21.02%. A very important and particularly desirable aspect of human nutrition is that vegetable oils in people’s diets should be characterized by a low n-6/n-3 acid ratio. V. myrtillus oils have been shown to have an n-3/n-6 ratio of 1–2, indicating that they may be beneficial in people after heart attacks and cardiac surgery [34]. Figure 8 contains the chemical formulas of the fatty acids detected in V. myrtillus seed oil.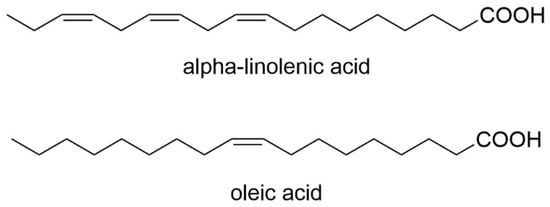
Figure 8. Chemical structures of fatty acids that are contained in V. myrtillus seed oil.
3.4. α-Tocopherol
Bederska-Łojewska D. et al. found that V. myrtillus seed oils have higher levels of α-tocopherol (Figure 9) than commercial tocopherol-rich oils (made from soybean and corn), and 4.84 mg of vitamin E were found per 100 g of blueberry [34].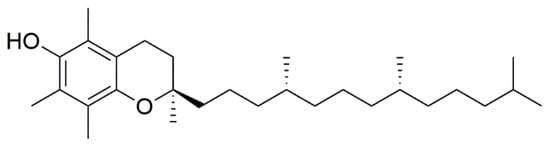
Figure 9. Chemical structure of α-tocopherol.
