Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lioua Kolsi | -- | 2776 | 2023-11-29 12:38:49 | | | |
| 2 | Jessie Wu | + 2 word(s) | 2778 | 2023-11-30 02:24:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khlissa, F.; Mhadhbi, M.; Aich, W.; Hussein, A.K.; Alhadri, M.; Selimefendigil, F.; Öztop, H.F.; Kolsi, L. Nanoencapsulated Phase-Change Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/52183 (accessed on 07 March 2026).
Khlissa F, Mhadhbi M, Aich W, Hussein AK, Alhadri M, Selimefendigil F, et al. Nanoencapsulated Phase-Change Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/52183. Accessed March 07, 2026.
Khlissa, Faïçal, Mohsen Mhadhbi, Walid Aich, Ahmed Kadhim Hussein, Muapper Alhadri, Fatih Selimefendigil, Hakan F. Öztop, Lioua Kolsi. "Nanoencapsulated Phase-Change Materials" Encyclopedia, https://encyclopedia.pub/entry/52183 (accessed March 07, 2026).
Khlissa, F., Mhadhbi, M., Aich, W., Hussein, A.K., Alhadri, M., Selimefendigil, F., Öztop, H.F., & Kolsi, L. (2023, November 29). Nanoencapsulated Phase-Change Materials. In Encyclopedia. https://encyclopedia.pub/entry/52183
Khlissa, Faïçal, et al. "Nanoencapsulated Phase-Change Materials." Encyclopedia. Web. 29 November, 2023.
Copy Citation
Phase-change materials (PCMs) are becoming more widely acknowledged as essential elements in thermal energy storage, greatly aiding the pursuit of lower building energy consumption and the achievement of net-zero energy goals. PCMs are frequently constrained by their subpar heat conductivity, despite their expanding importance.
thermal performance
NEPCMs
energy storage
NEnPCMs
latent heat
1. Introduction
The encapsulation of phase-change materials (PCMs) is discussed, which has garnered significant attention due to its effectiveness in addressing leakage issues, enhancing structural stability, improving fracture resistance, and precisely controlling particle size [1]. On the other hand, numerous studies demonstrate that reducing capsule size contributes significantly to the enhancement of the PCM’s performance [2][3][4][5]. Indeed, nano capsules (NEPCM) are considered to be better than microcapsules (MePCM) for the following reasons [6]:
-
Applications related to heat transfer require the use of suspensions that are stable during pumping and flowing. Therefore, it is more interesting to use nanoencapsulated PCMs, which have higher suspension stability, super-high specific surface area, minimal pump breakage, and promising structures in terms of management and storage of energy.
-
PCMs can release or store heat by exchanging it with the surrounding environment. Indeed, these exchanges are more important and faster when the size of the PCM particles is reduced (high surface area to volume ratio).
-
Compared to the microencapsulation process, the nanoencapsulation of PCMs is expected to result in the fabrication of energy-storage systems with higher characteristics, and the generation of more heat transfer in the system.
2. Preparation of Nanoencapsulated Phase-Change Materials
The utilization, storage, and transportation of PCMs are easier when they are encapsulated. Therefore, many processes, such as polymerization and physicochemical or physicomechanical processes have been adopted [6][7][8][9] in order to produce nanoencapsulated PCMs. These techniques allow the fabrication of different NEPCMs with different shapes (regular or irregular), different numbers of cores within the capsules (single or several), and different numbers of walls [10]. As illustrated in Figure 1, if the particle size is in the range 1–1000 nm, we can say that researchers have a nanoencapsulated PCM (NEPCM) [4].
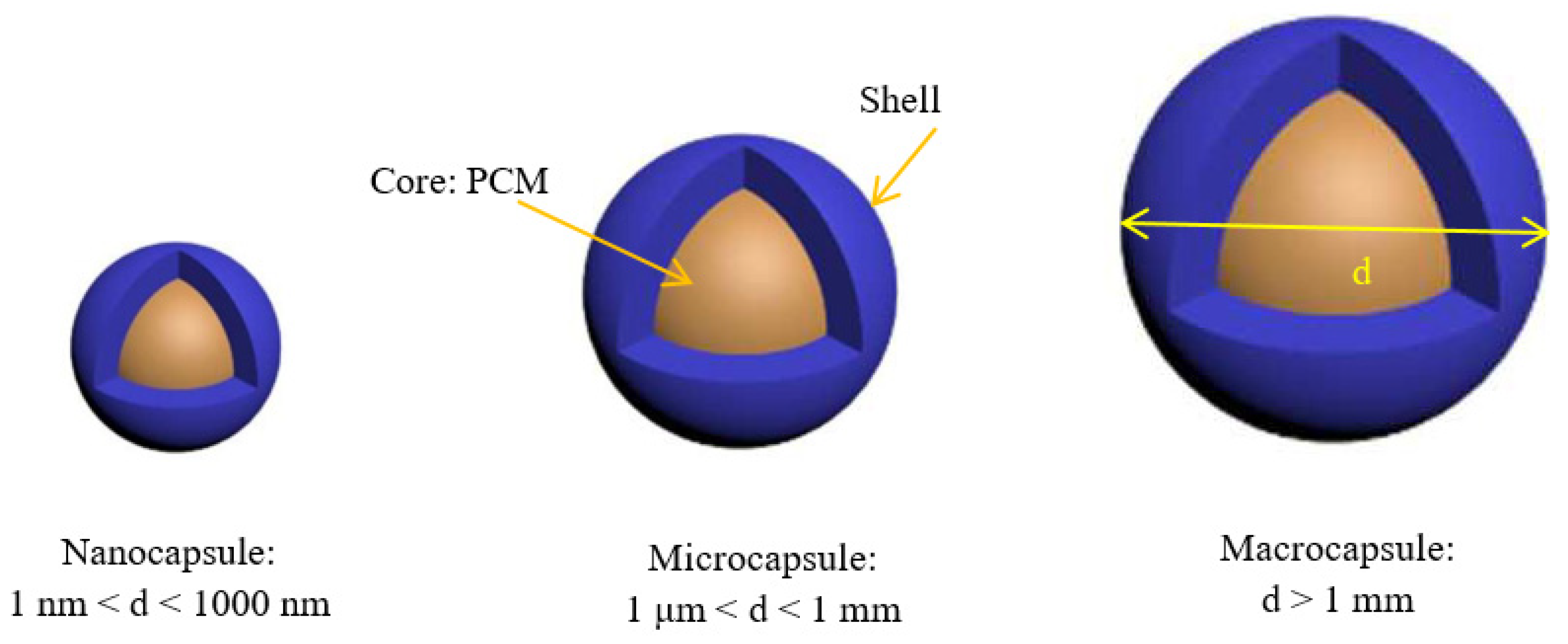
Figure 1. Classification of encapsulated phase-change materials as a function of their size.
Shell materials used for encapsulation should be more thermally stable than the core materials, with a melting point higher than that of the PCM, and should not be affected by the variation in volume and pressure caused by the melting/solidification cycles.
2.1. In-Situ Polymerization
In-situ polymerization involves the process whereby chemical reaction takes place between two immiscible liquids (water-soluble phase and oil-soluble phase) in a continuous phase, such as emulsion/mini-emulsion, suspension, and interfacial polycondensation [11].
Figure 2 shows the encapsulation process, using in-situ polymerization, of n-octadecane with a resorcinol-modified melamine–formaldehyde shell.
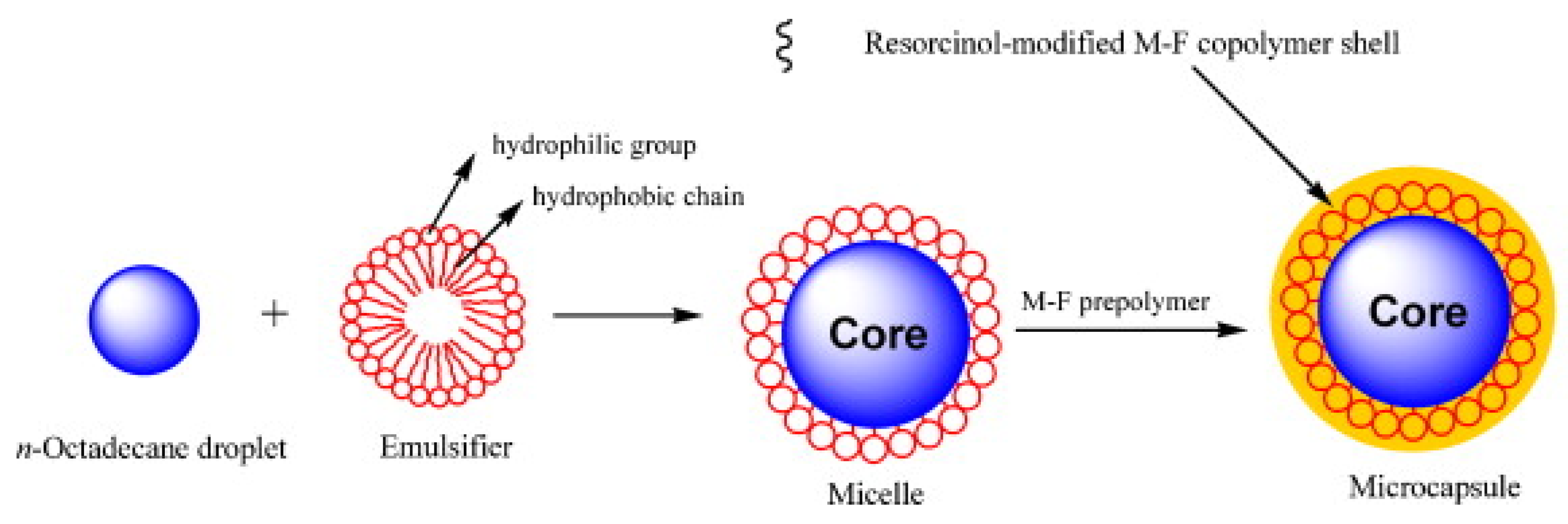
Figure 2. Illustration of the in-situ polymerization process for the formation of the encapsulated PCM, based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell [12].
Suspension Polymerization
A suspension polymerization-based process (Figure 3) can be used to prepare PCM micro and nano capsules with a polymer shell [13]. Indeed, suspension polymerization is a heterogeneous radical polymerization process, usually initiated by a monomer-soluble initiator, which is responsible for the generation of free radicals at the water–monomer droplet interface [14].
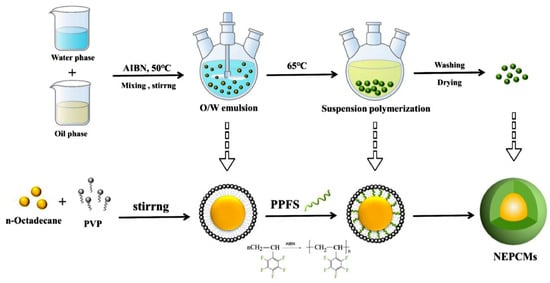
Figure 3. The suspension polymerization process used for the preparation of NEPCM [15].
Emulsion/Mini-Emulsion Polymerization
It was Harkins who first proposed a mechanism of emulsion polymerization [16]. An emulsion-polymerization system consists of a monomer that is slightly soluble in the dispersing medium (water), an emulsifier, an initiator, and a modifier, if necessary. Tiny amounts of monomers form droplets, which are suspended and stabilized by the association of the emulsifier molecules to form micelles surrounding the monomers [17]. The mini-emulsion method is the most employed method to synthesize encapsulated PCMs with nanometric sizes because of its efficiency and simplicity [4]. Emulsion polymerization was adopted by Zhang et al. [3] to fabricate a series of NEPCMs formed of paraffin wax (core) and melamine–formaldehyde (shell) with a regular shape and an average diameter of 260–450 nm (Figure 4). In their study, the morphology and the thermal properties of the prepared NEPCMs were studied. The findings demonstrated that the paraffin wax was successfully encapsulated in the melamine–formaldehyde without chemical contact, and the nanoencapsulated phase-change materials (NEPCMs) exhibited a regular spherical form with an average diameter of 260–450 nm. The amount of supplied core material had to be increased to increase the NEPCMs’ encapsulation effectiveness. The NEPCMs’ highest encapsulation efficiency reached up to about 75%. After 2000 thermal cycles, the NEPCMs could still operate with good thermal stability and dependability. Due to their exceptional encapsulation efficiency and thermal properties, the produced NEPCMs were able to be effectively used in latent-heat thermal energy storage and thermal management systems.

Figure 4. SEM micrographs of the NEPCMs prepared by Zhang et al. [3] for various Average Particle Size: (a): 449.6 nm, (b): 295.5 nm, (c): 261.3 nm, (d): 397.8 nm, (e): 352.3 nm, (f): 295.5 nm, (g): 339.4 nm.
Interfacial Polymerization
Interfacial polycondensation is an in-situ polymerization method which leads to the formation of a polymer shell at the interface between the two immiscible phases (water and oil) [18]. Park et al. [19] demonstrated that the incorporation of Fe3O4 nanoparticles in the polyurea shell (see Figure 5) was able to improve the thermal properties of NEPCM by increasing its thermal conductivity and reducing the supercooling of the paraffin (core PCM).
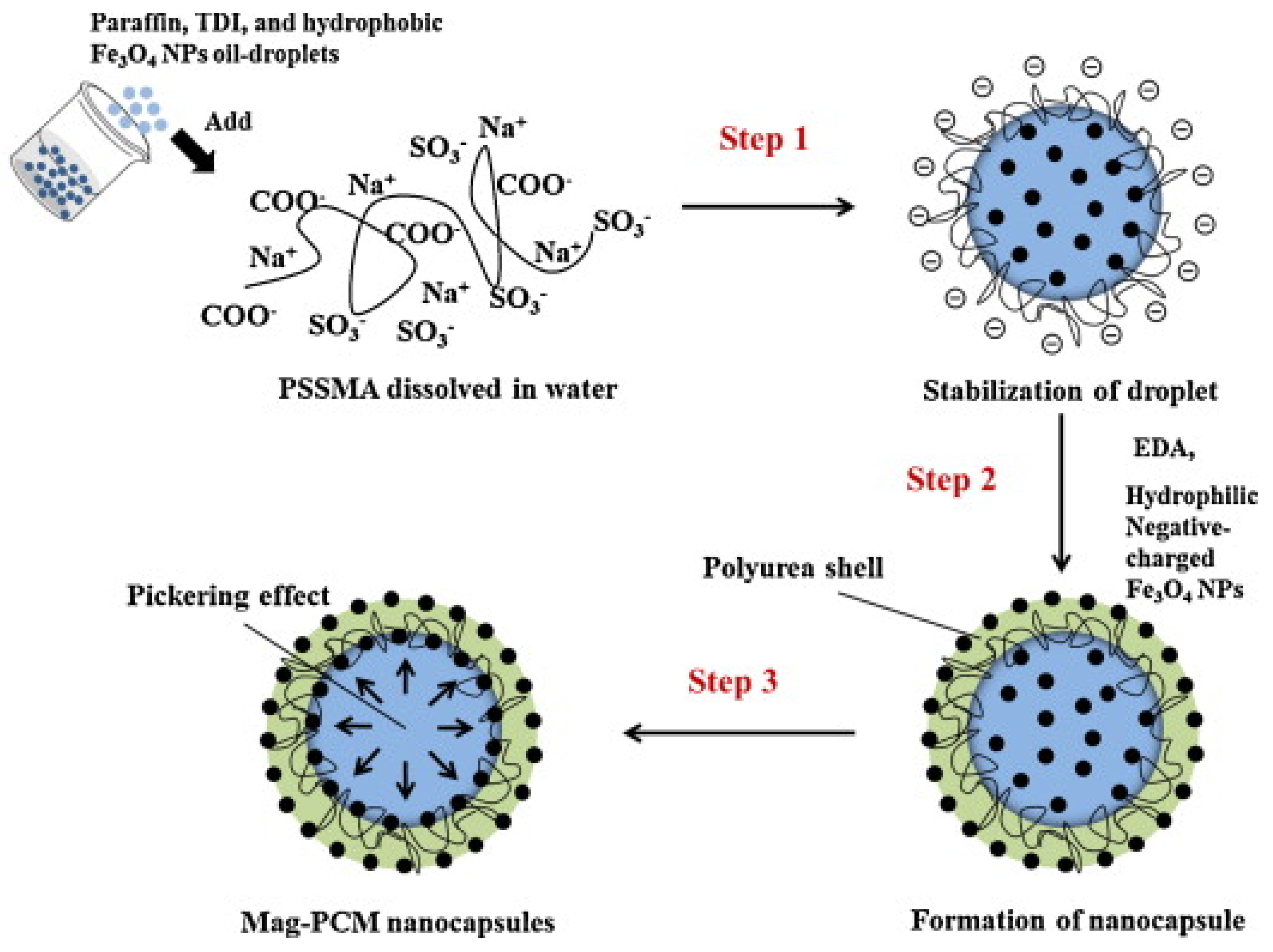
Figure 5. Interfacial polycondensation synthesis of Fe3O4 nanoparticle-embedded paraffin/polyurea NEPCM [19].
Nikpourian et al. [2] and Barlak et al. [20] used the interfacial polymerization method to encapsulate paraffin wax within a polyurethane shell. They obtained spherical nano capsules, with diameters between 25 and 185 nm and high thermal resistance, even after 100 melting/solidifying cycles. Solid fractions of the NEPCMs, prepared by Barlak et al. [20] were added to water and ethylene glycol to improve the thermal properties of the corresponding nanofluids with increasing temperature. The same synthesis method was adopted by Liang et al. [21] to encapsulate paraffin within a silica shell. The prepared nano capsules (169 to 563 nm) were thermally stable and had high heat-storage capability with the melting enthalpy and encapsulation ratios as high as 109.5 J g−1 and 51.5%, respectively.
2.2. Physicochemical Techniques
Coacervation
Coacervation is a physicochemical encapsulation technique which consists in the deposition of a single polyelectrolyte or a mixture of polyelectrolytes, initially in the solution around the core PCM material [4]. Figure 6 illustrates the flow diagram, which is an example of complex coacervation in which gum arabic encapsulates gelatin. This method has the advantage of allowing good control of NEPCM’s particle size, but it cannot prevent agglomeration and difficulty in scale-up.
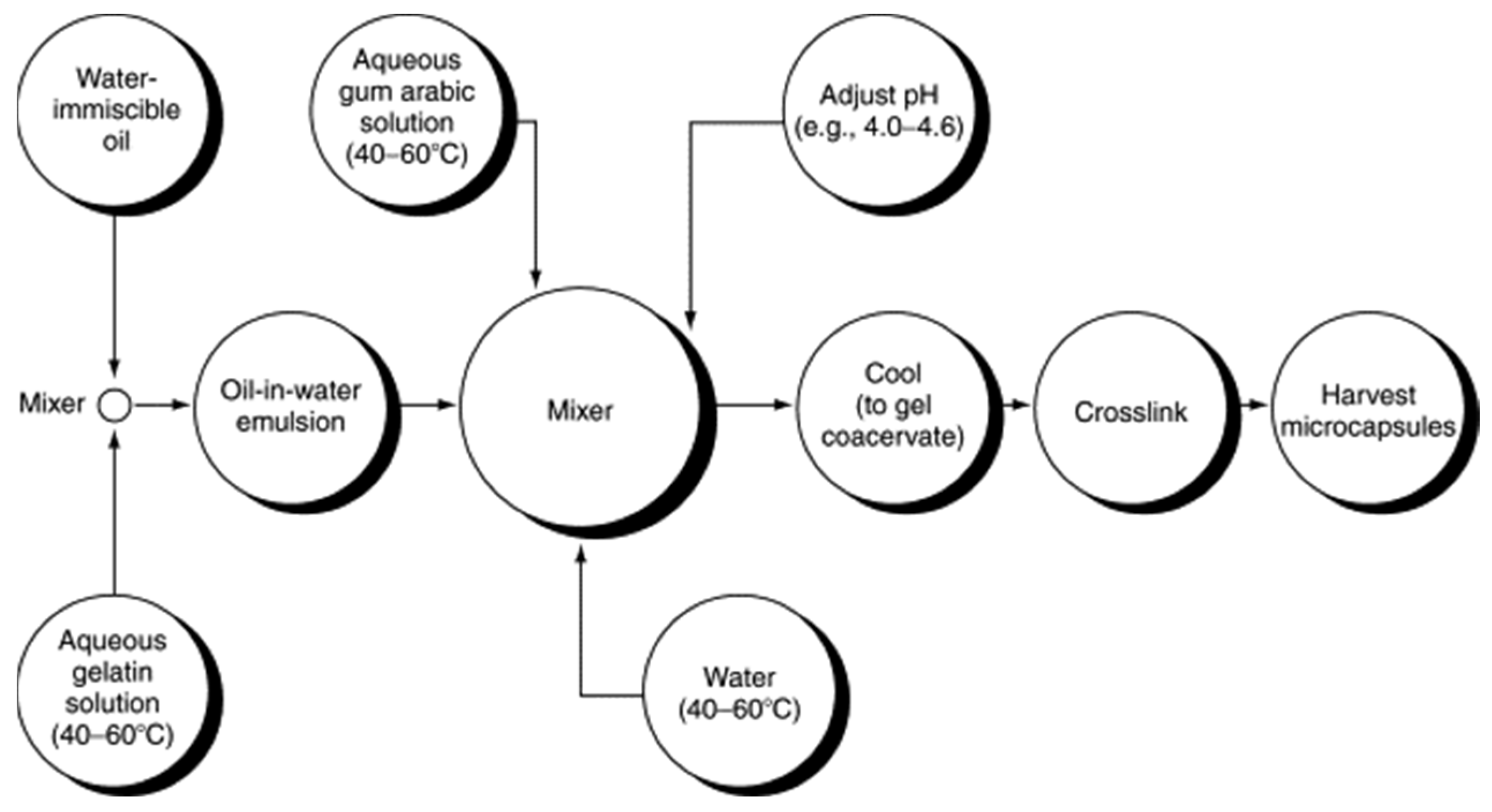
Figure 6. Flow diagram of a complex coacervation of gelatin with gum arabic [22].
Sol–Gel
Using the sol–gel method for the encapsulation of PCM-core materials with inorganic shell materials such as silica is a well-investigated method, since silica shell materials have better mechanical and thermal properties than polymer shell materials [13][23][24][25]. The sol–gel process refers traditionally to the hydrolysis and condensation of alkoxide-based precursors such as tetraethyl orthosilicate (Si(OEt)4, or TEOS) [26]. The sol–gel process can be described as illustrated in Figure 7.
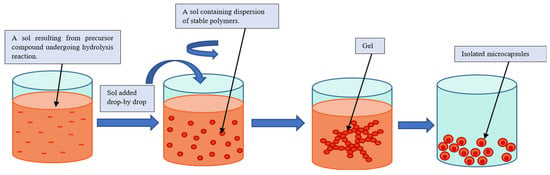
Figure 7. Schematic representation of a sol–gel process [1].
Supercritical CO2-Assisted Encapsulation
Supercritical carbon dioxide (scCO2) has attracted much interest as an alternative solvent for materials synthesis and processing because it is non-toxic, non-flammable, and naturally abundant [27]. Haldorai et al. [28] summarized the synthesis of polymer-inorganic filler nanocomposites in scCO2 and concluded that although there are three methods for the preparation of nanocomposites (blending, sol–gel and in-situ polymerization), scCO2 has been demonstrated (Figure 8) to be a viable alternative to the conventional solvents.
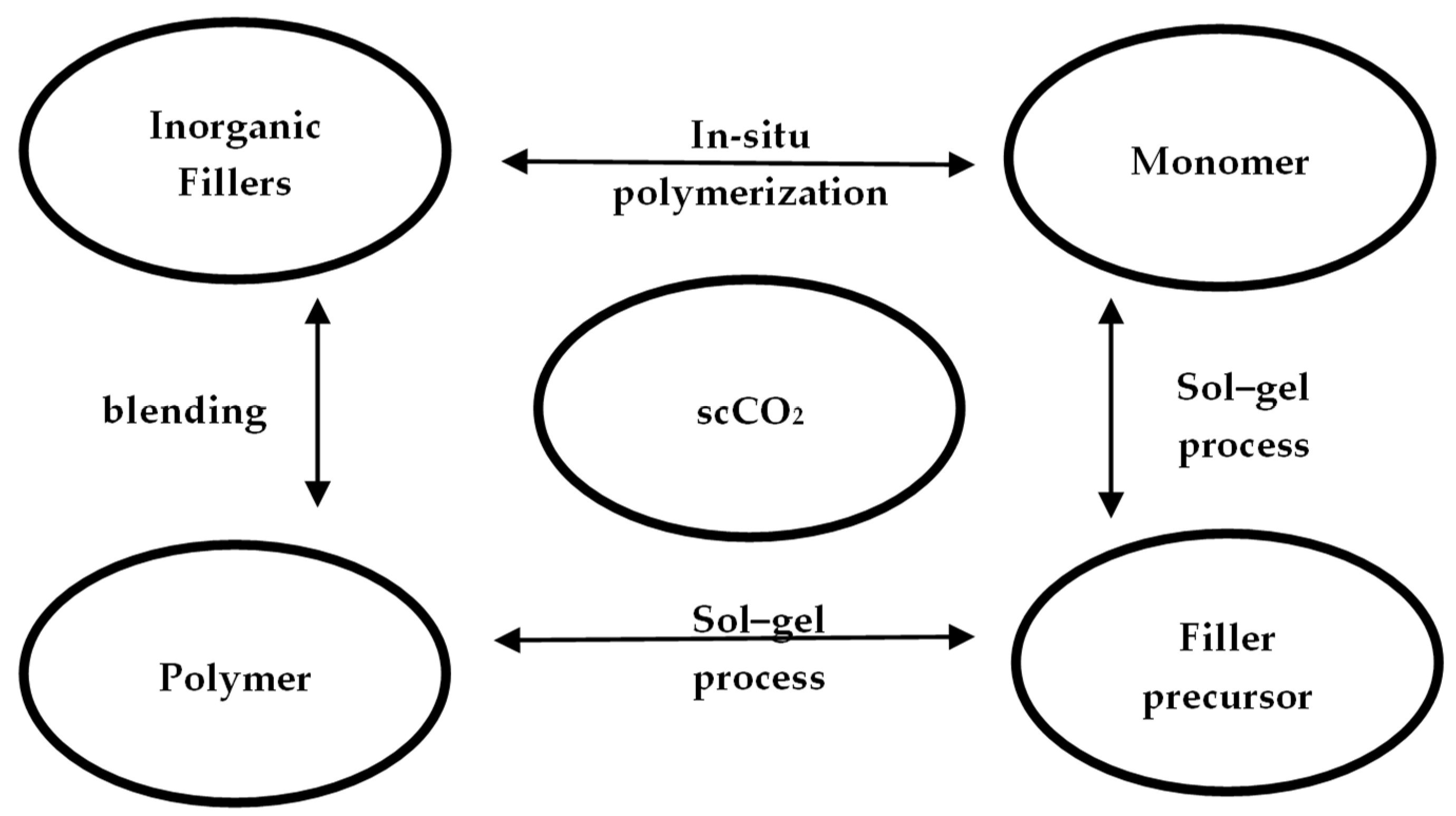
Figure 8. The three general approaches for preparation of polymer/inorganic filler nanocomposites in scCO2.
2.3. Physicomechanical Techniques
Spray-Drying Techniques
Spray-drying techniques (Figure 9) were first developed in the 1930s. The spray-drying process uses relatively less energy and is ideal for temperature-sensitive materials with significant encapsulation efficiency [1].
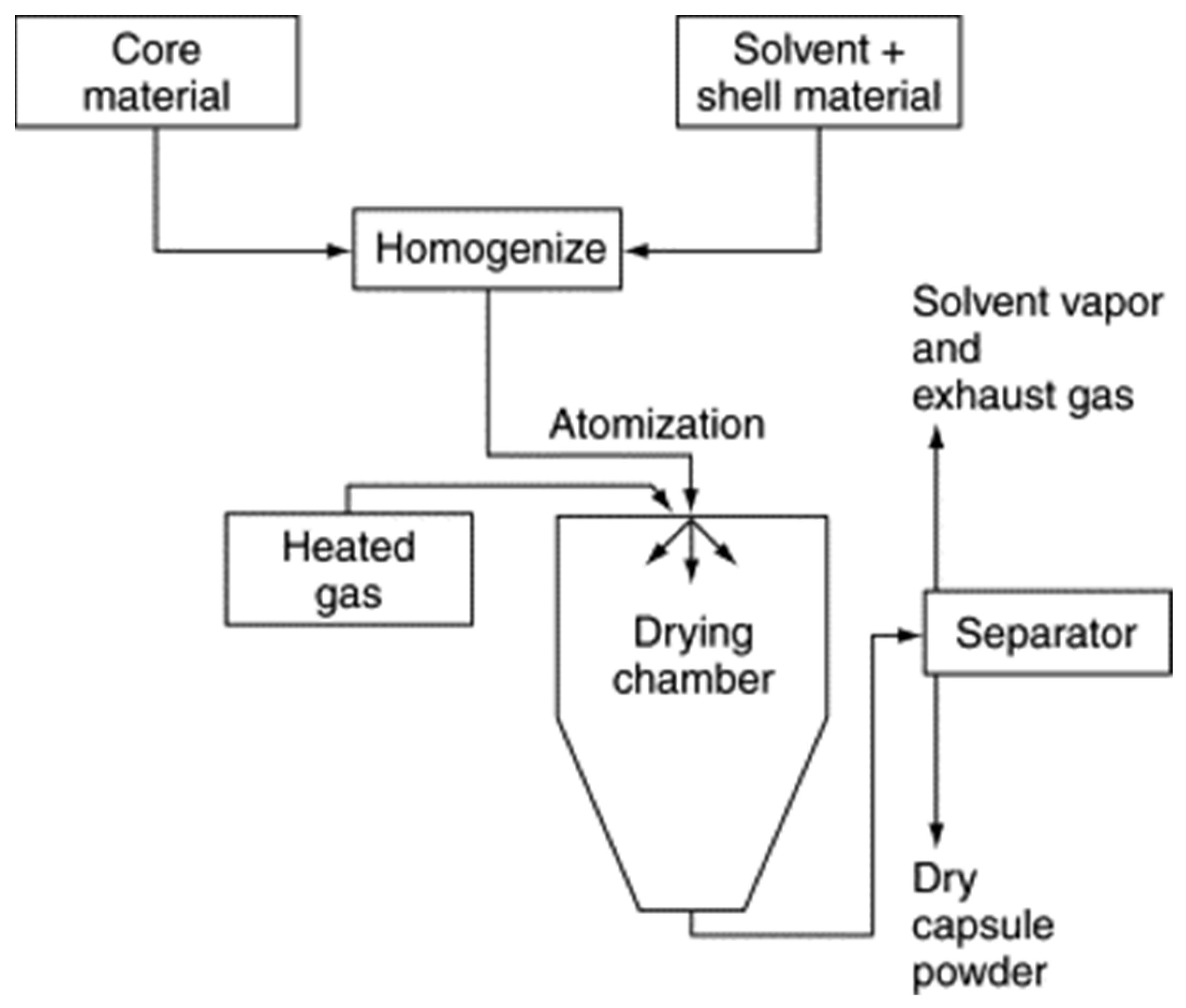
Figure 9. Flow diagram of a typical spray-drying encapsulation process [22].
The spray-drying encapsulation process usually gives polynuclear or matrix type microcapsules. However, the common problems of this rapid microencapsulation method remain its low coating ratio and the agglomeration of microcapsules [29]. The spray-drying method has mainly been employed to encapsulate PCM core materials with polymers [30][31][32].
Electrohydrodynamic Process
With the electrohydrodynamic process (encompassing electrospinning and electrospraying), it is possible to control the morphology of PCM capsules, and reduce their size to micro-, sub micro-, and nano-sized scales [33][34]. Figure 10 depicts the schematic diagram of the electrospraying method for producing silk fibroin nanoparticles. In electrospraying, the electric field forces the liquid pouring out of a capillary nozzle, which is kept at a high electric potential, to be scattered into microscopic droplets.
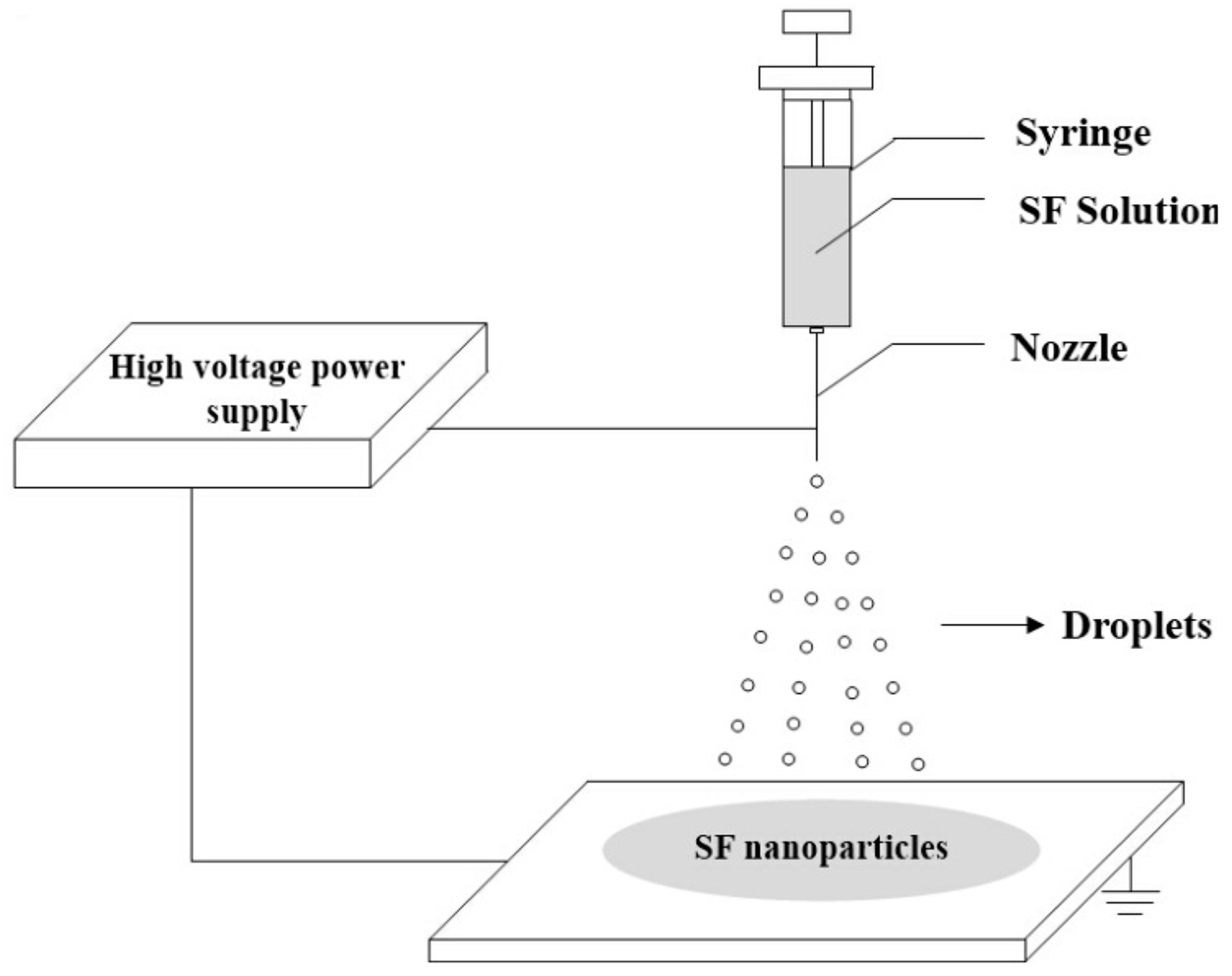
Figure 10. Preparation of silk fibroin (SF) nanoparticles via electrospraying [35].
This technique was used by Chalco-Sandoval et al. [36][37][38] to prepare encapsulated PCM in different polymers, in order to prepare new TES systems for smart food packaging. The prepared composite materials had good energy storage capacity, and were dedicated to thermal insulation applications.
3. Applications of NEPCMs
Currently, there is an increase in the usage of NEPCM materials in various fields of modern technology as well as in engineering applications, ranging from drug delivery to thermal energy storage in buildings [39][40]. In general, NEPCM materials offer the four following noteworthy and distinctive properties [41]:
-
Solid-to-liquid phase transition;
-
Large amount of energetic changes;
-
Stabilization of temperature;
-
Variation in thermal conduction during phase transition.
However, it remains very difficult to classify NEPCMs because of the variety and specificity of their potential applications, which are illustrated in Figure 11.

Figure 11. Main fields of NEPCMs applications.
3.1. Thermal Management of Electronic Devices
As the miniaturization of electronic devices increases, the problems of heat dissipation become more important. More efficient thermal management often leads to increased reliability and lifespan of devices. Indeed, the heat loads may be dissipated through a microchannel heat sink (MCHS) [42], which often uses liquid coolants (Figure 12) able to store considerable amounts of heat and with suitable thermophysical properties. A uniformly fixed temperature was applied to the model’s bottom surface in order to take into account the source of heat generation. The cover plate was totally insulated, thus there was no heat transfer through it. All the setups under study had the same fluid inlet temperature (296.15 K), which was purposefully chosen to be below the PCM particles’ melting point to ensure that the PCM particles entering the fluid were all in the solid state. The edges of computational domain borders were ignored in calculations and were thought to be symmetric. Two specialized 50- and 70-mm inlet and outlet blocks were considered to enable a fully developed flow along the channels, which had a 100 mm length. According to the scenarios examined, the configuration using nanofluid/NEPCM slurry coolants with 0.04/0.2 volumetric concentrations in upper/lower layers, respectively, were able to be used to achieve the system’s optimum flow and cooling performance. By increasing the NEPCM concentration to 0.3, the system’s cooling performance was improved, but the system’s overall efficiency decreased significantly. Nanofluid and NEPCMs are coolants of great interest in thermal management systems for high-tech cooling applications in microelectronics. Therefore, paraffins are frequently used in systems where the temperature must be kept below 40–45 °C, to melt, absorb, and dissipate the released heat [43][44][45].
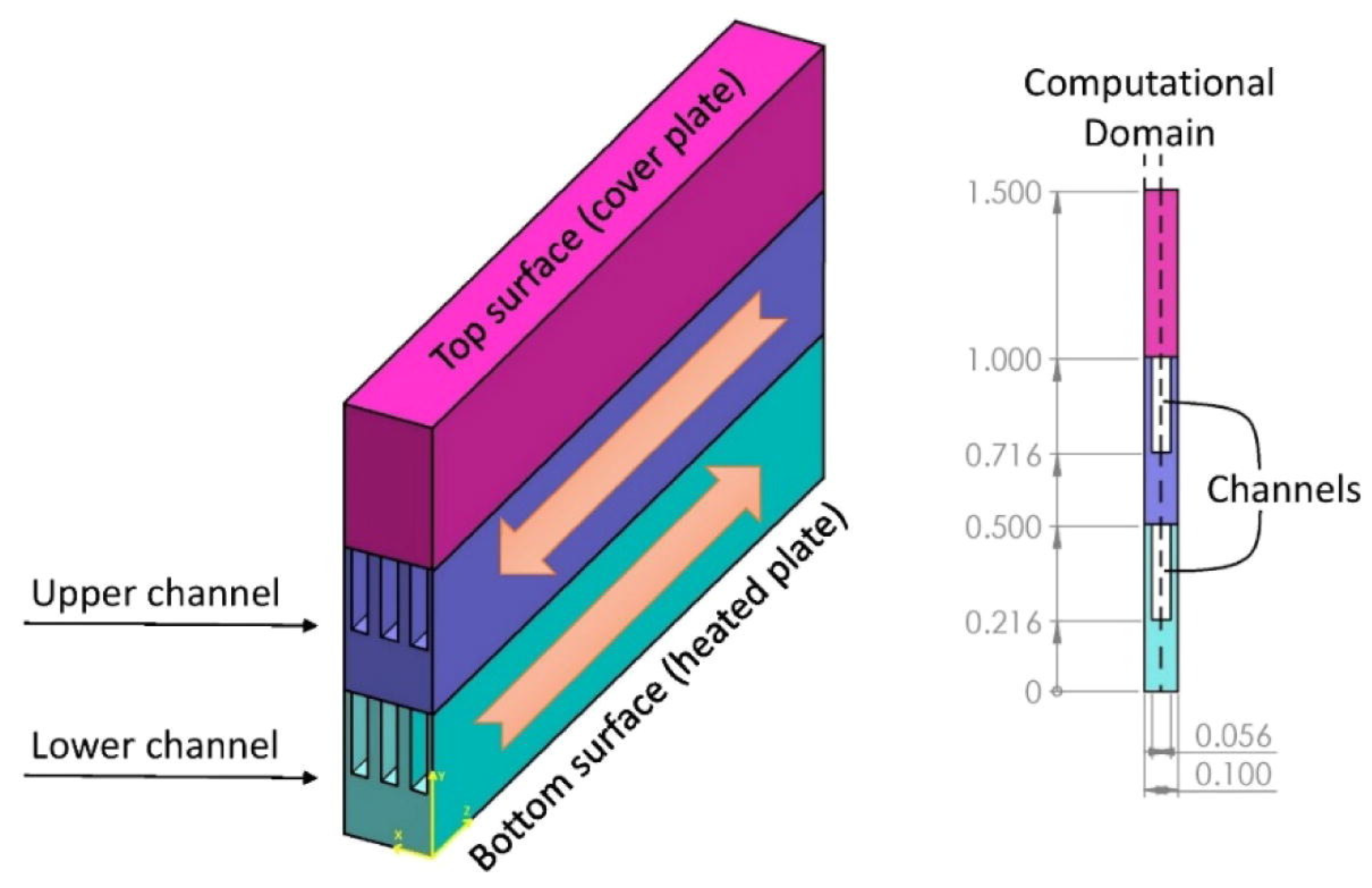
Figure 12. Microelectronics cooling using PCM in MCHS [46].
Ho et al. [47] mention that the presence of working fluid (water/NEPCM particles) improves heat dissipation and the index of performance of the microchannel walls up to 70% and 45%, respectively. Krishna et al. [48] confirmed that the use of NEPCM formed using a mixture Al2O3 nanoparticles and tricosane as the energy storage medium for electronic cooling purposes contributed to the reduction of the evaporator temperature of the heat pipes by 25.75% and therefore was able to save approximately 53% of the fan power.
3.2. Food Industry
To preserve the cold chain, extend food products’ shelf-life and reduce microbial activity, some food products have to be marketed, distributed, and sold under freezing, chilled, or refrigerated conditions. NEPCMs have been used in the food industry for applications such as heat processing, chilled storage, and packaging [49][50][51]. Chalco-Sandoval et al. [37] synthesized a smart food package by incorporating a phase-change material formed of a commercial blend of paraffin with a transition temperature of 5 °C into the packaging structures (polystyrene film). The measured latent heat of the prepared smart package was about 88–119 J/g. McCann et al. [52] used a coaxial electrospinning-based method to fabricate phase-change nanofibers formed of long-chain hydrocarbon cores and composite sheaths. The large heat of fusion of the long-chain hydrocarbons endows the fabricated phase-change nanofibers with the ability to absorb, hold, and release large amounts of thermal energy over a certain range of temperature.
3.3. Thermal Storage in Buildings
According to a report by the international energy agency (IEA), in 2019, the global shares of final energy consumption and CO2 emissions by buildings and the construction industry were equal, 35% and 38%, respectively, with growing need for space heating and cooling [53]. A recent review article classified thermal energy storage (TES) applications in buildings using PCMs in two classes: active and passive systems.
-
Active TES systems: heat transfer is generated by forced convection and, in some cases, also by mass transfer such as with a heat exchanger [54][55]. Active PCM-based systems require mechanical equipment or an additional power source for their operation, such as electricity for pumps or fans. These systems are best suited to situations where greater heat-transfer performance or better application control is required.
-
Passive TES systems: the employed PCMs exploit naturally available energy sources (for example, solar power or wind) as well as the architecture of building components to minimize energy requirements [56]. Passive systems reduce the use of mechanical heating or cooling systems. There is no need for additional energy input as heat is charged or discharged when the temperature of the environment rises or falls beyond the phase-change temperature of the PCM. These PCMs can be used in building ceilings; floors; walls; cooling, heating, and hot water systems; etc. [57][58].
3.4. Solar Energy Storage
Solar energy is the most abundant source of energy on the earth. Indeed, average annual solar energy received represents 6000 times the current annual world energy consumption [59]. It is therefore essential to fully benefit from this inexhaustible source of renewable energy and find scientific and technological solutions to overcome the characteristic drawbacks of solar energy, which are:
-
It is intermittent (day/night);
-
It is random (thunderstorms and cloud passages);
-
It is diluted and shifted in relation to daily or seasonal energy demands.
One of the most interesting solutions proposed to remediate these inconveniences is the use of PCMs to store the excess of thermal energy given off by the sun using latent heat storage (LHS), then releasing it during peak hours to meet demand. Some studies have proved that the use of nanoparticles raises the heat transfer rate [60][61]. Xu and Zhou [62] synthesized NEPCMs with a size of 600–900 nm which had Cu and Cu2O as a shell and paraffin as the core. They reported excellent LHS capacity and thermal conductivity, with about 127.55 J g−1 and 0.92 Wm−1K−1, respectively. Hammou and Lacroix [63] have proposed hybrid thermal–electrical energy storage. Their results showed that energy consumption was considerably reduced.
3.5. Heat Exchangers
The main selection characteristics of heat exchangers are their capacity of storing heat energy and the rates at which they release and absorb this heat. To improve these characteristics, PCMs can be incorporated into heat exchangers. They contribute to decreasing the pipeline’s size, the heat exchanger’s size, and the transport energy consumption [5][43]. As shown in Section 3, it has been proved that the addition of nanoparticles to PCM leads to the enhancement of the stored thermal energy [64].
3.6. Smarts: Textiles, Clothes, and Footwear
The need for humans to have thermal comfort has pushed them to develop “smart” textiles, which can acclimatize automatically to environmental changes. Textiles containing PCMs are “smart” materials, since they react immediately to changes in environmental temperatures, and the temperatures in different areas of the body, by absorbing heat and storing it in the liquified PCM when a rise in temperature occurs. However, when the temperature falls again, the PCM releases the stored heat energy and solidifies again [65]. Fiber technology, coating, lamination, and micro and nano encapsulation methods have been found suitable for incorporating PCMs in textiles. When choosing the PCM type, importance should be given to the quantity of PCM to be used, its latent heat, its melting temperature, its crystallization temperature, and the area covered by the PCM on the human body [66][67][68]. Karthikeyan et al. [69] used the pad-dry-cure method to apply coated nano capsules (average size of 260 nm) over cotton fabric. These thermally stable nano capsules have a latent heat of fusion of 74.2 J g−1 and a melting temperature of 64.30 °C. For cotton fabric with a 20 wt% and a 40 wt% coating of nano capsules, the heat storage capacities were 1.52 and 1.91 J g−1, respectively. The tensile strength, water absorption, and abrasion resistance of the coated cotton fabric were improved. In the early 1980s, NASA aimed to reduce the influence of variations in extreme temperature encountered by astronauts during space missions by encapsulating PCMs in the textile fibers of the space suits [70][71].
References
- Sivanathan, A.; Dou, Q.; Wang, Y.; Li, Y.; Corker, J.; Zhou, Y.; Fan, M. Phase change materials for building construction: An overview of nano-/micro-encapsulation. Nanotechnol. Rev. 2020, 9, 896–921.
- Nikpourian, H.; Bahramian, A.R.; Abdollahi, M. On the thermal performance of a novel PCM nanocapsule: The effect of core/shell. Renew. Energy 2020, 151, 322–331.
- Zhang, N.; Yuan, Y. Synthesis and thermal properties of nanoencapsulation of paraffin as phase change material for latent heat thermal energy storage. Energy Built Environ. 2020, 1, 410–416.
- Shchukina, E.; Graham, M.; Zheng, Z.; Shchukin, D. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175.
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826.
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for energy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226.
- Jelle, B.; Kalnæs, S. Phase change materials for application in energy-efficient buildings. Cost-Eff. Energy Effic. Build. Retrofit. 2017, 57–118.
- Wen, R.; Zhang, X.; Huang, Y.; Yin, Z.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X. Preparation and properties of fatty acid eutectics/expanded perlite and expanded vermiculite shape-stabilized materials for thermal energy storage in buildings. Energy Build. 2017, 139, 197–204.
- Chen, D.-Z.; Qin, S.-Y.; Tsui, G.C.; Tang, C.-Y.; Ouyang, X.; Liu, J.-h.; Tang, G.N.; Zuo, J.D. Fabrication, morphology and thermal properties of octadecylamine-grafted graphene oxide-modified phase-change microcapsules for thermal energy storage. Compos. Part B Eng. 2019, 157, 239–247.
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873.
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391.
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138.
- Jamekhorshid, A.; Sadrameli, S.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542.
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol. Energy Mater. Sol. Cells 2012, 98, 283–293.
- Zhang, K.; Wang, J.; Xu, L.; Xie, H.; Guo, Z. Preparation and thermal characterization of n-octadecane/pentafluorostyrene nanocapsules for phase-change energy storage. J. Energy Storage 2021, 35, 102327.
- Harkins, W.D. A general theory of emulsion polymerization. J. Am. Chem. Soc. 1947, 69, 428–444.
- Jensen, A.T.; Neto, W.S.; Ferreira, G.R.; Glenn, A.F.; Gambetta, R.; Gonçalves, S.B.; Valadares, L.F.; Machado, F. 8—Synthesis of Polymer/Inorganic Hybrids through Heterophase Polymerizations. In Recent Developments in Polymer Macro, Micro and Nano Blends; Visakh, P.M., Markovic, G., Pasquini, D., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 207–235.
- Cho, J.-S.; Kwon, A.; Cho, C.-G. Microencapsulation of octadecane as a phase-change material by interfacial polymerization in an emulsion system. Colloid Polym. Sci. 2002, 280, 260–266.
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51.
- Barlak, S.; Sara, O.N.; Karaipekli, A.; Yapıcı, S. Thermal conductivity and viscosity of nanofluids having nanoencapsulated phase change material. Nanoscale Microscale Thermophys. Eng. 2016, 20, 85–96.
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692.
- Thies, C. Microcapsules, Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: New York, NY, USA, 2003; pp. 3892–3903. ISBN 9780122270550.
- Yuan, H.; Bai, H.; Lu, X.; Zhao, X.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Effect of alkaline pH on formation of lauric acid/SiO2 nanocapsules via sol-gel process for solar energy storage. Sol. Energy 2019, 185, 374–386.
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255.
- Shi, J.; Wu, X.; Fu, X.; Sun, R. Synthesis and thermal properties of a novel nanoencapsulated phase change material with PMMA and SiO2 as hybrid shell materials. Thermochim. Acta 2015, 617, 90–94.
- Livage, J.; Sanchez, C.; Henry, M.; Doeuff, S. The chemistry of the sol-gel process. Solid State Ionics 1989, 32–33, 633–638.
- Jessop, P.G.; Leitner, W. Chemical Synthesis Using Supercritical Fluids; Wiley Online Library: Hoboken, NJ, USA, 1999.
- Haldorai, Y.; Shim, J.-J.; Lim, K.T. Synthesis of polymer–inorganic filler nanocomposites in supercritical CO2. J. Supercrit. Fluids 2012, 71, 45–63.
- Ghosh, S.K. Functional coatings and microencapsulation: A general perspective. In Functional Coatings: By Polymer Microencapsulation; Wiley: Hoboken, NJ, USA, 2006; pp. 1–28.
- Fei, B.; Lu, H.; Qi, K.; Shi, H.; Liu, T.; Li, X.; Xin, J.H. Multi-functional microcapsules produced by aerosol reaction. J. Aerosol Sci. 2008, 39, 1089–1098.
- Hawlader, M.; Uddin, M.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202.
- Borreguero, A.; Valverde, J.; Rodríguez, J.; Barber, A.; Cubillo, J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm® RT27 obtained by spray drying. Chem. Eng. J. 2011, 166, 384–390.
- Perez-Masia, R.; Lopez-Rubio, A.; Fabra, M.J.; Lagaron, J.M. Biodegradable polyester-based heat management materials of interest in refrigeration and smart packaging coatings. J. Appl. Polym. Sci. 2013, 130, 3251–3262.
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity-A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345.
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903.
- Chalco-Sandoval, W.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of solvents for the encapsulation of a phase change material in polymeric matrices by electro-hydrodynamic processing of interest in temperature buffering food applications. Eur. Polym. J. 2015, 72, 23–33.
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Development of polystyrene-based films with temperature buffering capacity for smart food packaging. J. Food Eng. 2015, 164, 55–62.
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of phase change materials to develop electrospun coatings of interest in food packaging applications. J. Food Eng. 2017, 192, 122–128.
- Zhang, Z.; Wang, Y.; Xu, S.; Yu, Y.; Hussain, A.; Shen, Y.; Guo, S. Photothermal gold nanocages filled with temperature sensitive tetradecanol and encapsulated with glutathione responsive polycurcumin for controlled DOX delivery to maximize anti-MDR tumor effects. J. Mater. Chem. B 2017, 5, 5464–5472.
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123.
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424.
- Tuckerman, D.; Pease, R. IIIB-8 implications of high performance heat sinking for electron devices. IEEE Trans. Electron Devices 1981, 28, 1230–1231.
- Fleischer, A.S. Thermal Energy Storage Using Phase Change Materials: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2015.
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221.
- Mudawar, I. Assessment of high-heat-flux thermal management schemes. IEEE Trans. Compon. Packag. Technol. 2001, 24, 122–141.
- Rajabifar, B. Enhancement of the performance of a double layered microchannel heatsink using PCM slurry and nanofluid coolants. Int. J. Heat Mass Transf. 2015, 88, 627–635.
- Ho, C.J.; Liu, Y.-C.; Ghalambaz, M.; Yan, W.-M. Forced convection heat transfer of Nano-Encapsulated Phase Change Material (NEPCM) suspension in a mini-channel heatsink. Int. J. Heat Mass Transf. 2020, 155, 119858.
- Krishna, J.; Kishore, P.; Solomon, A.B. Experimental Study of Thermal Energy Storage Characteristics Using Heat Pipe with Nano-Enhanced Phase Change Materials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012058.
- Lu, W.; Tassou, S. Characterization and experimental investigation of phase change materials for chilled food refrigerated cabinet applications. Appl. Energy 2013, 112, 1376–1382.
- Oró, E.; Miro, L.; Farid, M.M.; Cabeza, L.F. Thermal analysis of a low temperature storage unit using phase change materials without refrigeration system. Int. J. Refrig. 2012, 35, 1709–1714.
- Oró Prim, E.; Miró, L.; Barreneche Güerisoli, C.; Martorell, I.; Farid, M.M.; Cabeza, L.F. Corrosion of metal and polymer containers for use in PCM cold storage. Appl. Energy 2013, 109, 449–453.
- McCann, J.T.; Marquez, M.; Xia, Y. Melt coaxial electrospinning: A versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano Lett. 2006, 6, 2868–2872.
- Souayfane, F.; Fardoun, F.; Biwole, P.-H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431.
- Patil, N.D.; Karale, S. Design and Analysis of Phase Change Material based thermal energy storage for active building cooling: A Review. Int. J. Eng. Sci. Technol. 2012, 4, 2502.
- Jelle, B.P.; Hynd, A.; Gustavsen, A.; Arasteh, D.; Goudey, H.; Hart, R. Fenestration of today and tomorrow: A state-of-the-art review and future research opportunities. Sol. Energy Mater. Sol. Cells 2012, 96, 1–28.
- Chan, H.-Y.; Riffat, S.B.; Zhu, J. Review of passive solar heating and cooling technologies. Renew. Sustain. Energy Rev. 2010, 14, 781–789.
- Haillot, D.; Goetz, V.; Py, X.; Benabdelkarim, M. High performance storage composite for the enhancement of solar domestic hot water systems: Part 1: Storage material investigation. Sol. Energy 2011, 85, 1021–1027.
- Kalaiselvam, S.; Parameshwaran, R.; Harikrishnan, S. Analytical and experimental investigations of nanoparticles embedded phase change materials for cooling application in modern buildings. Renew. Energy 2012, 39, 375–387.
- Reddy, V.S.; Kaushik, S.; Ranjan, K.; Tyagi, S. State-of-the-art of solar thermal power plants—A review. Renew. Sustain. Energy Rev. 2013, 27, 258–273.
- Shamshirgaran, S.R.; Assadi, M.K.; Sharma, K.V. Application of nanomaterials in solar thermal energy storage. Heat Mass Transf. 2018, 54, 1555–1577.
- Kibria, M.; Anisur, M.; Mahfuz, M.; Saidur, R.; Metselaar, I. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89.
- Xu, B.; Zhou, J.; Ni, Z.; Zhang, C.; Lu, C. Synthesis of novel microencapsulated phase change materials with copper and copper oxide for solar energy storage and photo-thermal conversion. Sol. Energy Mater. Sol. Cells 2018, 179, 87–94.
- Hammou, Z.A.; Lacroix, M. A hybrid thermal energy storage system for managing simultaneously solar and electric energy. Energy Convers. Manag. 2006, 47, 273–288.
- Solé, C.; Medrano, M.; Castell, A.; Nogués, M.; Mehling, H.; Cabeza, L. Energetic and exergetic analysis of a domestic water tank with phase change material. Int. J. Energy Res. 2008, 32, 204–214.
- Rupp, J. Interactive textiles regulate body temperature. Int. Text. Bull. 1999, 45, 58–59.
- Wang, Y.; Cui, Y.; Shao, Z.; Gao, W.; Fan, W.; Liu, T.; Bai, H. Multifunctional polyimide aerogel textile inspired by polar bear hair for thermoregulation in extreme environments. Chem. Eng. J. 2020, 390, 124623.
- Pan, N.; Sun, G. Functional Textiles for Improved Performance, Protection and Health; Elsevier: Amsterdam, The Netherlands, 2011.
- Cabeza, L.; Martorell, I.; Miró, L.; Fernández, A.; Barreneche, C. Introduction to Thermal Energy Storage (TES) Systems. In Advances in Thermal Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–28.
- Karthikeyan, M.; Ramachandran, T.; Shanmugasundaram, O. Synthesis, characterization, and development of thermally enhanced cotton fabric using nanoencapsulated phase change materials containing paraffin wax. J. Text. Inst. 2014, 105, 1279–1286.
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550.
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62.
More
Information
Subjects:
Engineering, Mechanical; Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
30 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





