Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Mechanical
|
Chemistry, Applied
Phase-change materials (PCMs) are becoming more widely acknowledged as essential elements in thermal energy storage, greatly aiding the pursuit of lower building energy consumption and the achievement of net-zero energy goals. PCMs are frequently constrained by their subpar heat conductivity, despite their expanding importance.
- thermal performance
- NEPCMs
- energy storage
- NEnPCMs
- latent heat
1. Introduction
The encapsulation of phase-change materials (PCMs) is discussed, which has garnered significant attention due to its effectiveness in addressing leakage issues, enhancing structural stability, improving fracture resistance, and precisely controlling particle size [29]. On the other hand, numerous studies demonstrate that reducing capsule size contributes significantly to the enhancement of the PCM’s performance [30,31,32,33]. Indeed, nano capsules (NEPCM) are considered to be better than microcapsules (MePCM) for the following reasons [34]:
-
Applications related to heat transfer require the use of suspensions that are stable during pumping and flowing. Therefore, it is more interesting to use nanoencapsulated PCMs, which have higher suspension stability, super-high specific surface area, minimal pump breakage, and promising structures in terms of management and storage of energy.
-
PCMs can release or store heat by exchanging it with the surrounding environment. Indeed, these exchanges are more important and faster when the size of the PCM particles is reduced (high surface area to volume ratio).
-
Compared to the microencapsulation process, the nanoencapsulation of PCMs is expected to result in the fabrication of energy-storage systems with higher characteristics, and the generation of more heat transfer in the system.
2. Preparation of NEPCMs
The utilization, storage, and transportation of PCMs are easier when they are encapsulated. Therefore, many processes, such as polymerization and physicochemical or physicomechanical processes have been adopted [34,35,36,37] in order to produce nanoencapsulated PCMs. These techniques allow the fabrication of different NEPCMs with different shapes (regular or irregular), different numbers of cores within the capsules (single or several), and different numbers of walls [38]. As illustrated in Figure 2, if the particle size is in the range 1–1000 nm, we can say that we have a nanoencapsulated PCM (NEPCM) [32].
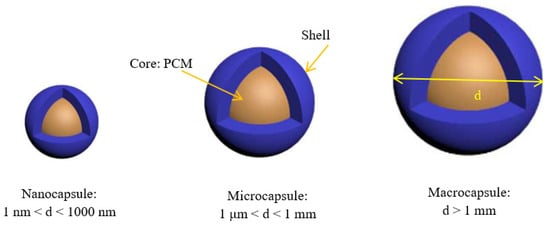
Figure 2. Classification of encapsulated phase-change materials as a function of their size.
Shell materials used for encapsulation should be more thermally stable than the core materials, with a melting point higher than that of the PCM, and should not be affected by the variation in volume and pressure caused by the melting/solidification cycles.
2.1. In-Situ Polymerization
In-situ polymerization involves the process whereby chemical reaction takes place between two immiscible liquids (water-soluble phase and oil-soluble phase) in a continuous phase, such as emulsion/mini-emulsion, suspension, and interfacial polycondensation [39].
Figure 3 shows the encapsulation process, using in-situ polymerization, of n-octadecane with a resorcinol-modified melamine–formaldehyde shell.
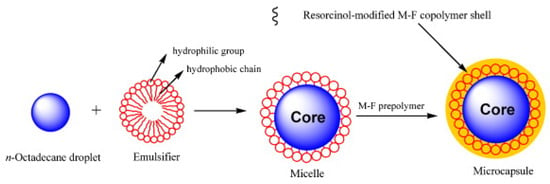
Figure 3. Illustration of the in-situ polymerization process for the formation of the encapsulated PCM, based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell [40].
Suspension Polymerization
A suspension polymerization-based process (Figure 4) can be used to prepare PCM micro and nano capsules with a polymer shell [41]. Indeed, suspension polymerization is a heterogeneous radical polymerization process, usually initiated by a monomer-soluble initiator, which is responsible for the generation of free radicals at the water–monomer droplet interface [42].
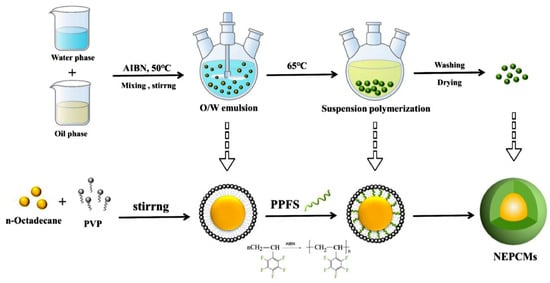
Figure 4. The suspension polymerization process used for the preparation of NEPCM [43].
Emulsion/Mini-Emulsion Polymerization
It was Harkins who first proposed a mechanism of emulsion polymerization [44]. An emulsion-polymerization system consists of a monomer that is slightly soluble in the dispersing medium (water), an emulsifier, an initiator, and a modifier, if necessary. Tiny amounts of monomers form droplets, which are suspended and stabilized by the association of the emulsifier molecules to form micelles surrounding the monomers [45]. The mini-emulsion method is the most employed method to synthesize encapsulated PCMs with nanometric sizes because of its efficiency and simplicity [32]. Emulsion polymerization was adopted by Zhang et al. [31] to fabricate a series of NEPCMs formed of paraffin wax (core) and melamine–formaldehyde (shell) with a regular shape and an average diameter of 260–450 nm (Figure 5). In their study, the morphology and the thermal properties of the prepared NEPCMs were studied. The findings demonstrated that the paraffin wax was successfully encapsulated in the melamine–formaldehyde without chemical contact, and the nanoencapsulated phase-change materials (NEPCMs) exhibited a regular spherical form with an average diameter of 260–450 nm. The amount of supplied core material had to be increased to increase the NEPCMs’ encapsulation effectiveness. The NEPCMs’ highest encapsulation efficiency reached up to about 75%. After 2000 thermal cycles, the NEPCMs could still operate with good thermal stability and dependability. Due to their exceptional encapsulation efficiency and thermal properties, the produced NEPCMs were able to be effectively used in latent-heat thermal energy storage and thermal management systems.

Figure 5. SEM micrographs of the NEPCMs prepared by Zhang et al. [31] for various Average Particle Size: (a): 449.6 nm, (b): 295.5 nm, (c): 261.3 nm, (d): 397.8 nm, (e): 352.3 nm, (f): 295.5 nm, (g): 339.4 nm.
Interfacial Polymerization
Interfacial polycondensation is an in-situ polymerization method which leads to the formation of a polymer shell at the interface between the two immiscible phases (water and oil) [46]. Park et al. [47] demonstrated that the incorporation of Fe3O4 nanoparticles in the polyurea shell (see Figure 6) was able to improve the thermal properties of NEPCM by increasing its thermal conductivity and reducing the supercooling of the paraffin (core PCM).
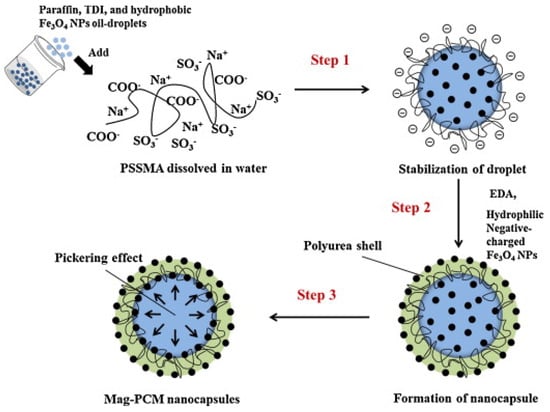
Figure 6. Interfacial polycondensation synthesis of Fe3O4 nanoparticle-embedded paraffin/polyurea NEPCM [47].
Nikpourian et al. [30] and Barlak et al. [48] used the interfacial polymerization method to encapsulate paraffin wax within a polyurethane shell. They obtained spherical nano capsules, with diameters between 25 and 185 nm and high thermal resistance, even after 100 melting/solidifying cycles. Solid fractions of the NEPCMs, prepared by Barlak et al. [48] were added to water and ethylene glycol to improve the thermal properties of the corresponding nanofluids with increasing temperature. The same synthesis method was adopted by Liang et al. [49] to encapsulate paraffin within a silica shell. The prepared nano capsules (169 to 563 nm) were thermally stable and had high heat-storage capability with the melting enthalpy and encapsulation ratios as high as 109.5 J g−1 and 51.5%, respectively.
2.2. Physicochemical Techniques
Coacervation
Coacervation is a physicochemical encapsulation technique which consists in the deposition of a single polyelectrolyte or a mixture of polyelectrolytes, initially in the solution around the core PCM material [32]. Figure 7 illustrates the flow diagram, which is an example of complex coacervation in which gum arabic encapsulates gelatin. This method has the advantage of allowing good control of NEPCM’s particle size, but it cannot prevent agglomeration and difficulty in scale-up.
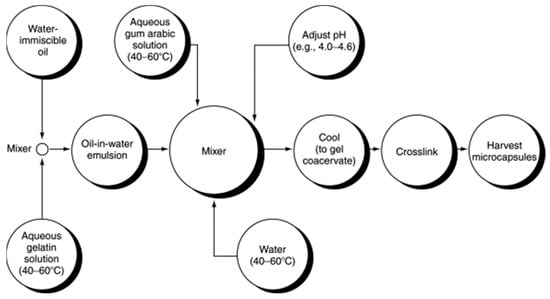
Figure 7. Flow diagram of a complex coacervation of gelatin with gum arabic [50].
Sol–Gel
Using the sol–gel method for the encapsulation of PCM-core materials with inorganic shell materials such as silica is a well-investigated method, since silica shell materials have better mechanical and thermal properties than polymer shell materials [41,51,52,53]. The sol–gel process refers traditionally to the hydrolysis and condensation of alkoxide-based precursors such as tetraethyl orthosilicate (Si(OEt)4, or TEOS) [54]. The sol–gel process can be described as illustrated in Figure 8.
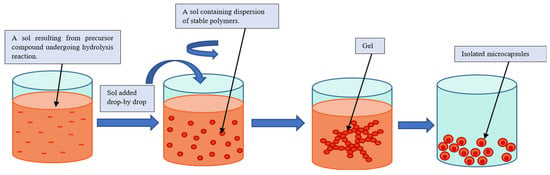
Figure 8. Schematic representation of a sol–gel process [29].
Supercritical CO2-Assisted Encapsulation
Supercritical carbon dioxide (scCO2) has attracted much interest as an alternative solvent for materials synthesis and processing because it is non-toxic, non-flammable, and naturally abundant [55]. Haldorai et al. [56] summarized the synthesis of polymer-inorganic filler nanocomposites in scCO2 and concluded that although there are three methods for the preparation of nanocomposites (blending, sol–gel and in-situ polymerization), scCO2 has been demonstrated (Figure 9) to be a viable alternative to the conventional solvents.
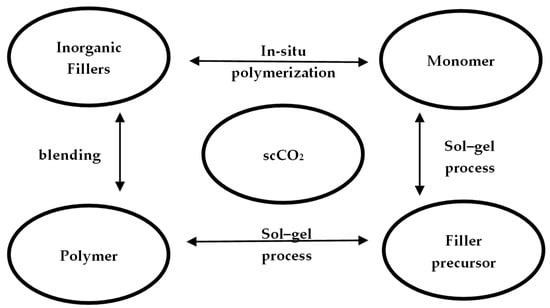
Figure 9. The three general approaches for preparation of polymer/inorganic filler nanocomposites in scCO2.
2.3. Physicomechanical Techniques
Spray-Drying Techniques
Spray-drying techniques (Figure 10) were first developed in the 1930s. The spray-drying process uses relatively less energy and is ideal for temperature-sensitive materials with significant encapsulation efficiency [29].
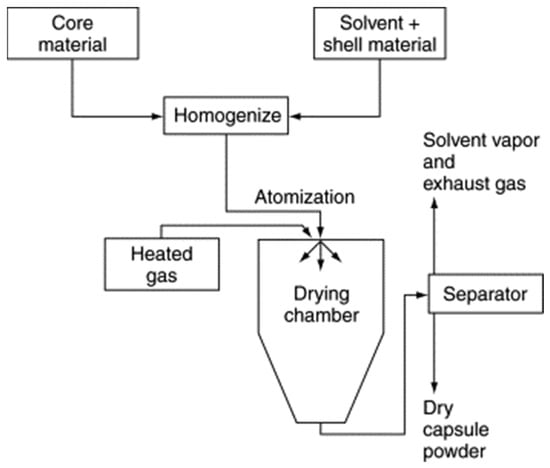
Figure 10. Flow diagram of a typical spray-drying encapsulation process [50].
The spray-drying encapsulation process usually gives polynuclear or matrix type microcapsules. However, the common problems of this rapid microencapsulation method remain its low coating ratio and the agglomeration of microcapsules [57]. The spray-drying method has mainly been employed to encapsulate PCM core materials with polymers [58,59,60].
Electrohydrodynamic Process
With the electrohydrodynamic process (encompassing electrospinning and electrospraying), it is possible to control the morphology of PCM capsules, and reduce their size to micro-, sub micro-, and nano-sized scales [61,62]. Figure 11 depicts the schematic diagram of the electrospraying method for producing silk fibroin nanoparticles. In electrospraying, the electric field forces the liquid pouring out of a capillary nozzle, which is kept at a high electric potential, to be scattered into microscopic droplets.
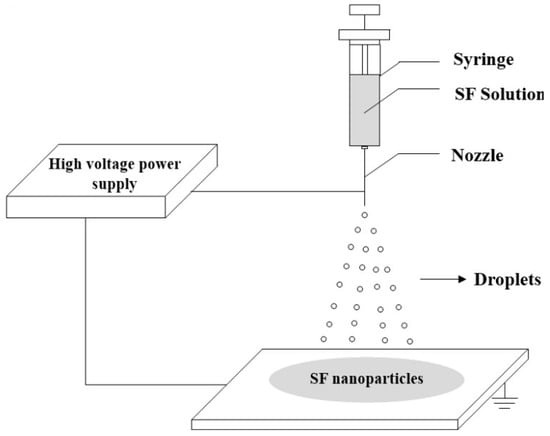
Figure 11. Preparation of silk fibroin (SF) nanoparticles via electrospraying [63].
This technique was used by Chalco-Sandoval et al. [64,65,66] to prepare encapsulated PCM in different polymers, in order to prepare new TES systems for smart food packaging. The prepared composite materials had good energy storage capacity, and were dedicated to thermal insulation applications.
3. Applications of NEPCMs
Currently, there is an increase in the usage of NEPCM materials in various fields of modern technology as well as in engineering applications, ranging from drug delivery to thermal energy storage in buildings [67,68]. In general, NEPCM materials offer the four following noteworthy and distinctive properties [69]:
-
Solid-to-liquid phase transition;
-
Large amount of energetic changes;
-
Stabilization of temperature;
-
Variation in thermal conduction during phase transition.
However, it remains very difficult to classify NEPCMs because of the variety and specificity of their potential applications, which are illustrated in Figure 12.
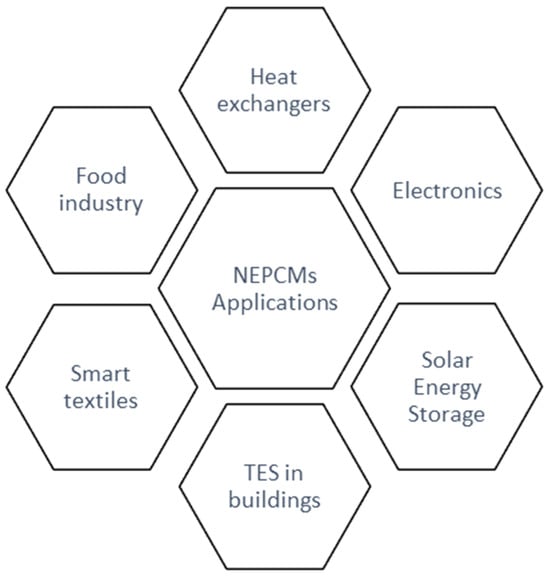
Figure 12. Main fields of NEPCMs applications.
3.1. Thermal Management of Electronic Devices
As the miniaturization of electronic devices increases, the problems of heat dissipation become more important. More efficient thermal management often leads to increased reliability and lifespan of devices. Indeed, the heat loads may be dissipated through a microchannel heat sink (MCHS) [70], which often uses liquid coolants (Figure 13) able to store considerable amounts of heat and with suitable thermophysical properties. A uniformly fixed temperature was applied to the model’s bottom surface in order to take into account the source of heat generation. The cover plate was totally insulated, thus there was no heat transfer through it. All the setups under study had the same fluid inlet temperature (296.15 K), which was purposefully chosen to be below the PCM particles’ melting point to ensure that the PCM particles entering the fluid were all in the solid state. The edges of computational domain borders were ignored in calculations and were thought to be symmetric. Two specialized 50- and 70-mm inlet and outlet blocks were considered to enable a fully developed flow along the channels, which had a 100 mm length. According to the scenarios examined, the configuration using nanofluid/NEPCM slurry coolants with 0.04/0.2 volumetric concentrations in upper/lower layers, respectively, were able to be used to achieve the system’s optimum flow and cooling performance. By increasing the NEPCM concentration to 0.3, the system’s cooling performance was improved, but the system’s overall efficiency decreased significantly. Nanofluid and NEPCMs are coolants of great interest in thermal management systems for high-tech cooling applications in microelectronics. Therefore, paraffins are frequently used in systems where the temperature must be kept below 40–45 °C, to melt, absorb, and dissipate the released heat [71,72,73].
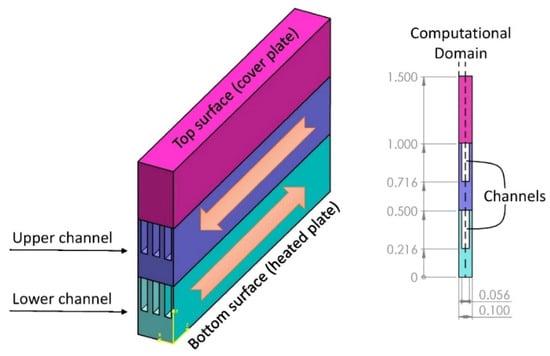
Figure 13. Microelectronics cooling using PCM in MCHS [74].
Ho et al. [75] mention that the presence of working fluid (water/NEPCM particles) improves heat dissipation and the index of performance of the microchannel walls up to 70% and 45%, respectively. Krishna et al. [76] confirmed that the use of NEPCM formed using a mixture Al2O3 nanoparticles and tricosane as the energy storage medium for electronic cooling purposes contributed to the reduction of the evaporator temperature of the heat pipes by 25.75% and therefore was able to save approximately 53% of the fan power.
3.2. Food Industry
To preserve the cold chain, extend food products’ shelf-life and reduce microbial activity, some food products have to be marketed, distributed, and sold under freezing, chilled, or refrigerated conditions. NEPCMs have been used in the food industry for applications such as heat processing, chilled storage, and packaging [77,78,79]. Chalco-Sandoval et al. [65] synthesized a smart food package by incorporating a phase-change material formed of a commercial blend of paraffin with a transition temperature of 5 °C into the packaging structures (polystyrene film). The measured latent heat of the prepared smart package was about 88–119 J/g. McCann et al. [80] used a coaxial electrospinning-based method to fabricate phase-change nanofibers formed of long-chain hydrocarbon cores and composite sheaths. The large heat of fusion of the long-chain hydrocarbons endows the fabricated phase-change nanofibers with the ability to absorb, hold, and release large amounts of thermal energy over a certain range of temperature.
3.3. Thermal Storage in Buildings
According to a report by the international energy agency (IEA), in 2019, the global shares of final energy consumption and CO2 emissions by buildings and the construction industry were equal, 35% and 38%, respectively, with growing need for space heating and cooling [1]. A recent review article classified thermal energy storage (TES) applications in buildings using PCMs in two classes: active and passive systems.
-
Active TES systems: heat transfer is generated by forced convection and, in some cases, also by mass transfer such as with a heat exchanger [81,82]. Active PCM-based systems require mechanical equipment or an additional power source for their operation, such as electricity for pumps or fans. These systems are best suited to situations where greater heat-transfer performance or better application control is required.
-
Passive TES systems: the employed PCMs exploit naturally available energy sources (for example, solar power or wind) as well as the architecture of building components to minimize energy requirements [83]. Passive systems reduce the use of mechanical heating or cooling systems. There is no need for additional energy input as heat is charged or discharged when the temperature of the environment rises or falls beyond the phase-change temperature of the PCM. These PCMs can be used in building ceilings; floors; walls; cooling, heating, and hot water systems; etc. [84,85].
3.4. Solar Energy Storage
Solar energy is the most abundant source of energy on the earth. Indeed, average annual solar energy received represents 6000 times the current annual world energy consumption [86]. It is therefore essential to fully benefit from this inexhaustible source of renewable energy and find scientific and technological solutions to overcome the characteristic drawbacks of solar energy, which are:
-
It is intermittent (day/night);
-
It is random (thunderstorms and cloud passages);
-
It is diluted and shifted in relation to daily or seasonal energy demands.
One of the most interesting solutions proposed to remediate these inconveniences is the use of PCMs to store the excess of thermal energy given off by the sun using latent heat storage (LHS), then releasing it during peak hours to meet demand. Some studies have proved that the use of nanoparticles raises the heat transfer rate [87,88]. Xu and Zhou [89] synthesized NEPCMs with a size of 600–900 nm which had Cu and Cu2O as a shell and paraffin as the core. They reported excellent LHS capacity and thermal conductivity, with about 127.55 J g−1 and 0.92 Wm−1K−1, respectively. Hammou and Lacroix [90] have proposed hybrid thermal–electrical energy storage. Their results showed that energy consumption was considerably reduced.
3.5. Heat Exchangers
The main selection characteristics of heat exchangers are their capacity of storing heat energy and the rates at which they release and absorb this heat. To improve these characteristics, PCMs can be incorporated into heat exchangers. They contribute to decreasing the pipeline’s size, the heat exchanger’s size, and the transport energy consumption [33,71]. As shown in Section 3, it has been proved that the addition of nanoparticles to PCM leads to the enhancement of the stored thermal energy [91].
3.6. Smarts: Textiles, Clothes, and Footwear
The need for humans to have thermal comfort has pushed them to develop “smart” textiles, which can acclimatize automatically to environmental changes. Textiles containing PCMs are “smart” materials, since they react immediately to changes in environmental temperatures, and the temperatures in different areas of the body, by absorbing heat and storing it in the liquified PCM when a rise in temperature occurs. However, when the temperature falls again, the PCM releases the stored heat energy and solidifies again [92]. Fiber technology, coating, lamination, and micro and nano encapsulation methods have been found suitable for incorporating PCMs in textiles. When choosing the PCM type, importance should be given to the quantity of PCM to be used, its latent heat, its melting temperature, its crystallization temperature, and the area covered by the PCM on the human body [93,94,95]. Karthikeyan et al. [96] used the pad-dry-cure method to apply coated nano capsules (average size of 260 nm) over cotton fabric. These thermally stable nano capsules have a latent heat of fusion of 74.2 J g−1 and a melting temperature of 64.30 °C. For cotton fabric with a 20 wt% and a 40 wt% coating of nano capsules, the heat storage capacities were 1.52 and 1.91 J g−1, respectively. The tensile strength, water absorption, and abrasion resistance of the coated cotton fabric were improved. In the early 1980s, NASA aimed to reduce the influence of variations in extreme temperature encountered by astronauts during space missions by encapsulating PCMs in the textile fibers of the space suits [97,98].
This entry is adapted from the peer-reviewed paper 10.3390/pr11113219
This entry is offline, you can click here to edit this entry!
