
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaobo Mao | -- | 2866 | 2023-11-28 15:38:22 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 2867 | 2023-12-04 02:23:02 | | |
Video Upload Options
Parkinson’s Disease (PD) is a neurodegenerative disease characterized by the progressive loss of dopaminergic neurons of the substantia nigra pars compacta with a reduction in dopamine concentration in the striatum. It is a substantial loss of dopaminergic neurons that is responsible for the classic triad of PD symptoms, i.e., resting tremor, muscular rigidity, and bradykinesia. Several therapies for PD may only offer symptomatic relief and do not address the underlying neurodegeneration of PD. The developments in cellular reprogramming have enabled the development of previously unachievable cell therapies and patient-specific modeling of PD through Induced Pluripotent Stem Cells (iPSCs). iPSCs possess the inherent capacity for pluripotency, allowing for their directed differentiation into diverse cell lineages, such as dopaminergic neurons, thus offering a promising avenue for addressing the issue of neurodegeneration within the context of PD.
1. Introduction
1.1. Overview of Parkinson’s Disease
1.2. Brief Overview of Current Treatment Options for PD
1.3. Levodopa
1.4. Deep Brain Stimulation
2. Cell Therapies for Parkinson’s Disease
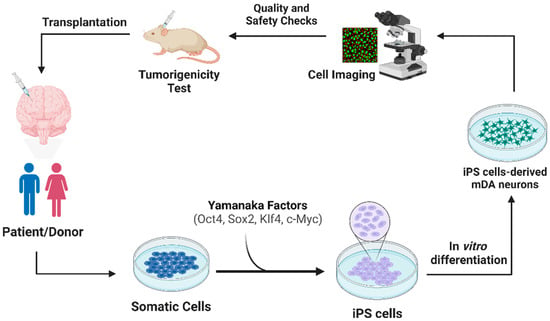
2.1. Transplantation Approach of iPSCs
2.2. Methods to Increasing Survival of Transplanted Neurons
2.3. Nanobodies
2.4. LAG3-Related Pathway
2.5. Exosomes
3. Animal and Human Models
3.1. Nonhuman Primates (NHPs)
3.2. N-of-1 Report
4. Summary
References
- Kieburtz, K.; Olanow, C.W. Translational experimental therapeutics: The translation of laboratory-based discovery into disease-related therapy. Mt. Sinai J. Med. 2007, 74, 7–14.
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32.
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018.
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621.
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532.
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018.
- Stacy, M.; Galbreath, A. Optimizing long-term therapy for Parkinson disease: Levodopa, dopamine agonists, and treatment-associated dyskinesia. Clin. Neuropharmacol. 2008, 31, 51–56.
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160.
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s disease: Current status and new developments. J. Park. Dis. 2013, 3, 255–269.
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinsons Dis. 2016, 2016, 9832839.
- Tao, Y.; Vermilyea, S.C.; Zammit, M.; Lu, J.; Olsen, M.; Metzger, J.M.; Yao, L.; Chen, Y.; Phillips, S.; Holden, J.E.; et al. Autologous transplant therapy alleviates motor and depressive behaviors in parkinsonian monkeys. Nat. Med. 2021, 27, 632–639.
- Pandey, S.; Srivanitchapoom, P. Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management. Ann. Indian Acad Neurol. 2017, 20, 190–198.
- Siegfried, J.; Lippitz, B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: A new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 1994, 35, 1126–1129, discussion 1129–1130.
- Limousin, P.; Pollak, P.; Benazzouz, A.; Hoffmann, D.; Le Bas, J.F.; Perret, J.E.; Benabid, A.L.; Broussolle, E. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995, 345, 91–95.
- Rossi, M.; Bruno, V.; Arena, J.; Cammarota, A.; Merello, M. Challenges in PD Patient Management After DBS: A Pragmatic Review. Mov. Disord. Clin. Pract. 2018, 5, 246–254.
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556.
- Wu, Y.-P.; Chen, W.-S.; Teng, C.; Zhang, N. Stem cells for the treatment of neurodegenerative diseases. Molecules 2010, 15, 6743–6758.
- Lee, S.H. The advantages and limitations of mesenchymal stem cells in clinical application for treating human diseases. Osteoporos Sarcopenia 2018, 4, 150.
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14.
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759.
- Thomson, J.A.; Itskovitz-Eldor, J.; Shaprio, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147.
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45.
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103, quiz 104–105.
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932.
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004.
- Krolewski, R. Personal Communication; Shastry, S., Ed.; APA Style: New York, NY, USA, 2021.
- Fan, Y.W.; Ng, S.-Y. Replacing what’s lost: A new era of stem cell therapy for Parkinson’s disease. Transl. Neurodegener. 2020, 9, 2.
- Ricciardi, L.; Petrucci, S.; Guidubaldi, A.; Ialongo, T.; Serra, L.; Ferraris, A.; Spanò, B.; Bozzali, M.; Valente, E.M.; Bentivoglio, A.R. Phenotypic variability of PINK1 expression: 12 Years’ clinical follow-up of two Italian families. Mov. Disord. 2014, 29, 1561–1566.
- Deyell, J.S.; Sriparna, M.; Ying, M.; Mao, X. The Interplay between alpha-Synuclein and Microglia in alpha-Synucleinopathies. Int. J. Mol. Sci. 2023, 24, 2477.
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980.
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299.
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159.
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71.
- Butler, Y.R.; Liu, Y.; Kumbhar, R.; Zhao, P.; Gadhave, K.; Wang, N.; Li, Y.; Mao, X.; Wang, W. alpha-Synuclein fibril-specific nanobody reduces prion-like alpha-synuclein spreading in mice. Nat. Commun. 2022, 13, 4060.
- Zhang, S.; Liu, Y.Q.; Jia, C.; Lim, Y.J.; Feng, G.; Xu, E.; Long, H.; Kimura, Y.; Tao, Y.; Zhao, C.; et al. Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2011196118.
- Mao, X.; Gu, H.; Kim, D.; Kimura, Y.; Wang, Y.; Xu, E.; Wang, H.; Chen, C.; Zhang, S.; Jia, C.; et al. Aplp1 and the Aplp1-Lag3 Complex facilitates transmission of pathologic α-synuclein. bioRxiv 2021.
- Chen, C.; Kumbhar, R.; Wang, H.; Yang, X.; Gadhave, K.; Rastegar, C.; Kimura, Y.; Behensky, A.; Katakam, S.; Jeong, D.; et al. Pathological Tau transmission initiated by binding lymphocyte-activation gene 3. bioRxiv 2023.
- Gu, H.; Yang, X.; Mao, X.; Xu, E.; Qi, C.; Wang, H.; Brahmachari, S.; York, B.; Sriparna, M.; Li, A.; et al. Lymphocyte Activation Gene 3 (Lag3) Contributes to α-Synucleinopathy in α-Synuclein Transgenic Mice. Front. Cell Neurosci. 2021, 15, 656426.
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374.
- Chistiakov, D.A.; Chistiakov, A.A. alpha-Synuclein-carrying extracellular vesicles in Parkinson’s disease: Deadly transmitters. Acta Neurol. Belg. 2017, 117, 43–51.
- Pinnell, J.R.; Cui, M.; Tieu, K. Exosomes in Parkinson disease. J. Neurochem. 2021, 157, 413–428.
- Cooper, J.M.; Wiklander, P.B.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485.
- Wang, S.; Zou, C.; Fu, L.; Wang, B.; An, J.; Song, G.; Wu, J.; Tang, X.; Li, M.; Zhang, J.; et al. Autologous iPSC-derived dopamine neuron transplantation in a nonhuman primate Parkinson’s disease model. Cell Discov. 2015, 1, 15012.
- Harris, J.P.; Burrell, J.C.; Struzyna, L.A.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; Duda, J.E.; Cullen, D.K. Emerging regenerative medicine and tissue engineering strategies for Parkinson’s disease. NPJ Park. Dis. 2020, 6, 4.
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402.
- Cauvin, A.J.; Peters, C.; Brennan, F. Advantages and limitations of commonly used nonhuman primate species in research and development of biopharmaceuticals. In The nonhuman Primate in Nonclinical Drug Development and Safety Assessment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 379–395.
- Sundberg, M.; Bogetofte, H.; Lawson, T.; Jansson, J.; Smith, G.; Astradsson, A.; Moore, M.; Osborn, T.; Cooper, O.; Spealman, R.; et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 2013, 31, 1548–1562.
- Osborn, T.; Dinesh, D.; Moskites, A.; MacBain, Z.; Moore, M.; Brekk, O. Pre-clinical studies toward autologous midbrain dopamine cell therapy for Parkinson’s disease. Stem J. 2020, in press.
- Hargus, G.; Cooper, O.; Deleidi, M.; Levy, A.; Lee, K.; Marlow, E.; Yow, A.; Soldner, F.; Hockemeyer, D.; Hallett, P.J.; et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA 2010, 107, 15921–15926.
- Hallett, P.J.; Deleidi, M.; Astradsson, A.; Smith, G.A.; Cooper, O.; Osborn, T.M.; Sundberg, M.; Moore, M.A.; Perez-Torres, E.; Brownell, A.L.; et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 2015, 16, 269–274.




