Parkinson’s Disease (PD) is a neurodegenerative disease characterized by the progressive loss of dopaminergic neurons of the substantia nigra pars compacta with a reduction in dopamine concentration in the striatum. It is a substantial loss of dopaminergic neurons that is responsible for the classic triad of PD symptoms, i.e., resting tremor, muscular rigidity, and bradykinesia. Several current therapies for PD may only offer symptomatic relief and do not address the underlying neurodegeneration of PD. The recent developments in cellular reprogramming have enabled the development of previously unachievable cell therapies and patient-specific modeling of PD through Induced Pluripotent Stem Cells (iPSCs). iPSCs possess the inherent capacity for pluripotency, allowing for their directed differentiation into diverse cell lineages, such as dopaminergic neurons, thus offering a promising avenue for addressing the issue of neurodegeneration within the context of PD.
- Parkinson’s disease
- induced pluripotent stem cells
- dopaminergic neuron
- substantia nigra
- autologous transplantation
- allogeneic transplantation
1. Introduction
1.1. Overview of Parkinson’s Disease
1.2. Brief Overview of Current Treatment Options for PD
Existing strategies for managing PD are symptomatic and typically involve the replacement of DA neurotransmission by DA drugs, which relieve the patients of some of their motor symptoms [6]. Levodopa, also known as L-dopa, is a precursor of dopamine that crosses the blood–brain barrier and is converted into dopamine in the brain. This conversion helps to replenish the depleted dopamine levels in the brain, providing relief from motor symptoms such as rigidity, bradykinesia, and tremors. However, its long-term use may be associated with complications, such as motor fluctuations and dyskinesias, which may limit its effectiveness over time [7]. Alternatively, deep brain stimulation (DBS) is a surgical procedure that involves implanting electrodes into specific regions of the brain to deliver electrical impulses to modulate the activity of these brain regions [8].1.3. Levodopa
One of the most common pharmacological treatments for PD is Levodopa, a dopamine precursor in the form of a pill that has been widely used for over 40 years [10][9]. Levodopa is often combined with a dopa-decarboxylase inhibitor, inhibiting the synthesis of peripheral dopamine by aromatic amino-acid decarboxylase enzymes in the blood [10][9]. The benefits of pharmacological treatment fade with disease progression, and prolonged usage of these medications often results in side effects. After three to five years of treatment, motor fluctuations, loss of efficacy, and development of involuntary movements known as Levodopa-induced dyskinesia (LID) occur, significantly limiting the usefulness of L-DOPA as a therapeutic tool [11][10]. The administration of L-DOPA supplements helps alleviate motor symptoms but does not halt the progressive degeneration of DA neurons [12][11]. Approximately 49% of PD patients treated with levodopa developed dyskinesia after 5 years of treatment, and this percentage increases to 55% after 6 years of treatment [13][12].1.4. Deep Brain Stimulation
Alternatively, surgical strategies, such as deep brain stimulation (DBS), have been shown to alleviate PD motor symptoms and offer symptomatic relief that cannot be controlled with medications [14,15][13][14]. However, DBS is limited to early- to-mid PD stages and may lose effectiveness after a few years. Although DBS surgery improves motor symptoms and quality of life, some symptoms may remain resistant to stimulation. Furthermore, the implantable pulse generator’s limited lifespan may lead to a potential worsening of Parkinsonian symptoms or even life-threatening crises upon withdrawal from the symptomatic benefits [16][15].2. Cell Therapies for Parkinson’s Disease
Given the limitations of the current treatment, there is a growing focus on developing innovative therapies that target the root cause of PD. One promising avenue is cell therapy, which holds great potential in replenishing the lost dopaminergic neurons and restoring dopamine production in the brain [18][16]. Cell therapy involves the transplantation of healthy and functional cells, such as dopaminergic neurons, into the brain to replace the damaged neurons and potentially halt the neurodegenerative process [19][17]. This approach aims to provide a sustainable and long-lasting source of dopamine, offering a more comprehensive and targeted strategy to address the underlying cause of PD. Mesenchymal stem cells (MSCs): MSCs have limited differentiation potential, typically giving rise to specific cell types such as bone, cartilage, and fat cells [20][18] (Figure 1). MSCs are obtained from donor sources, introducing compatibility and immune response challenges. MSCs, as adult stem cells, exhibit self-renewal and multilineage differentiation capabilities and can be isolated from various tissues, such as the umbilical cord, endometrial polyps, menses blood, bone marrow, and adipose tissue, making them practical for experimental and potential clinical use [21][19]. Reports have raised concerns about the potential tumorigenicity and tumor support or suppression effects of MSCs, necessitating long-term safety assessments. Ongoing clinical trials and comprehensive studies are essential to establish the safety profile of MSC-based therapies in human patients [22][20].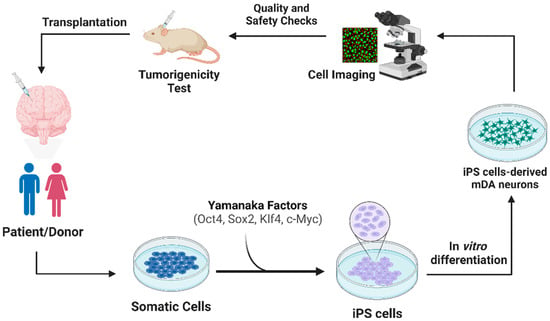
2.1. Transplantation Approach of iPSCs
2.2. Methods to Increasing Survival of Transplanted Neurons
The cell transplantation of iPSC-derived dopaminergic neurons alone is not a complete therapeutic approach due to the persisting neurotoxic microenvironment caused by α-syn aggregation. α-Syn, a protein found in Lewy bodies, has been closely associated with neurodegeneration, particularly in dopaminergic neurons [34][29]. The aggregates disrupt the functionality of neurons, impairing their ability to communicate effectively and ultimately triggering a cascade of neurotoxic events [35][30]. The selective vulnerability of these neurons to α-syn-induced damage was first observed in studies involving the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which resulted in the death of substantia nigra dopaminergic neurons [36][31]. The detrimental effects of α-syn aggregates extend beyond their presence in Lewy bodies. These aggregates may enter mitochondria and disrupt energy production, leading to mitochondrial dysfunction and increased oxidative stress [37][32].2.3. Nanobodies
The ability of transplanted cells to be resilient against the impact of misfolded proteins originating from the host is necessary for long-term survival [39][33]. α-syn aggregation needs to be targeted because it may be released into extracellular spaces and taken up by adjacent cells, where they induce further misfolding and aggregation of protein [40][34]. Nanobodies are single-domain antibodies derived from camelid species that have emerged as a promising therapeutic approach for targeting α-syn aggregation and enhancing the survival of transplanted iPSC-derived dopaminergic neurons [41][35]. Antibodies 9E4, 1H7, and 5C1, which are nanobodies, were evaluated in a mouse model of PD to assess their impact on α-syn aggregation and motor function. Stereological analysis revealed a significant reduction in α-syn immunoreactivity in the neuropil of animals treated with these nanobodies compared to the control group. Additionally, immunohistochemical analysis with the SYN105 antibody demonstrated a significant reduction in abnormal α-syn aggregates in both the temporal cortex and striatum of the treated mice.2.4. LAG3-Related Pathway
Lymphatic activation gene 3 (LAG3) is a receptor that facilitate the pathogenic α-syn and tau cell-to-cell transmission [43,44,45][36][37][38]. Genetic depletion of LAG3 and anti-LAG3 antibodies can significantly block pathogenic α-syn aggregation, inflammation, neurotoxicity in vitro and in vivo as well as other prion-like seeds [46,47][39][40]. Moreover, biochemical and behavioral deficits associated with PD pathology are also mitigated, indicating the potential of LAG3-targeted therapies in preventing pathology propagation, preserving dopaminergic function and improving motor outcomes. By transplanting LAG3 deficient neurons or using LAG3 antibodies, the internalization of α-syn by transplanted neurons may be decreased, thereby likely leading to their reduced vulnerability to α-syn-induced neurotoxicity [47][40]. This attenuation of α-syn transmission to the transplanted cells not only enhances their survival but also contributes to the overall preservation of the neuronal network and motor function in PD [46][39].2.5. Exosomes
Exosomes are nano-sized vesicles released by various cell types, including neurons, astrocytes, and microglia, in the brain. These extracellular vesicles have garnered interest due to their ability to shuttle genetic material and proteins between cells, thereby influencing gene expression and protein activity in recipient cells [48][41]. Exosomes isolated from the blood in an NHP model were loaded with a saturated dopamine solution, serving as potential carriers for targeted therapy in PD. The interaction between transferrin and the transferrin receptor allowed the dopamine-loaded exosomes to efficiently cross the blood–brain barrier, resulting in enhanced therapeutic efficacy and reduced toxicity compared to systemic intravenous delivery of free dopamine [49][42]. Exosomes have shown promise in targeting neuroinflammation, a critical aspect of PD pathology. In a study, exosomes loaded with catalase, an antioxidant enzyme, were investigated for their potential to alleviate neural inflammation and enhance neuronal survival in a PD mouse model. To evaluate the therapeutic effects, C57BL/6 mice were stereotactically injected with 6-OHDA into the substantia nigra pars compacta to induce PD-like pathology. The mice were then intranasally injected with exosomes containing catalase activity. Two exoCAT formulations were evaluated: catalase-loaded exosomes obtained by sonication and permeabilization with saponin. Control groups included PD mice injected with phosphate-buffered saline (PBS) and healthy mice injected with PBS or empty exosomes. Exosomes containing modified α-syn siRNAs reduced the amount of α-syn mRNA transcription and translation in the brain of transgenic mice [51][43].3. Animal and Human Models
3.1. Nonhuman Primates (NHPs)
iPSCs have been proven to potentially impact many areas of medicine and therapeutics, highlighting the potential of the instrument in various fields of biomedicine. iPSCs have become a powerful tool in basic, as well as translational, and clinical research because of their ability to be maintained indefinitely while preserving the host’s genetic makeup. This method was tested on a nonhuman primates (NHPs) model in which autologous DA cells were introduced into the brain of a cynomolgus monkey PD model without immunosuppression; three PD monkeys that had received no grafts served as controls. The PD monkey that had received autologous grafts experienced behavioral improvement compared with that of controls [53][44]. In addition, emerging regenerative medicine therapies are being developed using neurons derived from autologous stem cells, enabling the development of patient-specific treatments [54][45]. To enhance preclinical studies and improve predictive validity for clinical use, it is essential to utilize animal models that closely mimic PD as observed in patients [57][46]. NHPs have been extensively employed in research and nonclinical development over the past decades due to their genetic, anatomical, physiological, and immunological similarities to humans [58][47]. A study found the protocol of NCAM(+)/CD29(low) sorting to result in enriching ventral midbrain dopaminergic neurons from the pluripotent stem cell-derived neural cell populations. Further, these neurons also exhibited increased expression of FOXA2, LMX1A, TH, GIRK2, PITX3, EN1, and NURR1 mRNA. These neurons were also found to bear the potential to restore motor function among the 6-hydroxydopamine lesioned rats 16 weeks after transplantation [59][48].3.2. N-of-1 Report
In relation to the causes of PD, cell transplantation in patients with PD replaces the lost dopaminergic neurons of the substantia nigra pars compacta. Through this transplantation, the loss of dopaminergic neurons, responsible for the symptomatic motor deficits of PD, may be restored. Through an N-of-1 report held by Mass Brigham General Hospital, patient-derived midbrain dopaminergic progenitor cells were implanted into the putamen (left hemisphere followed by the right hemisphere, six months apart) of a patient with PD without the need for immunosuppression. Clinical measures of PD symptoms stabilized or improved at 18 to 24 months after implantation [29][25].4. Summary
Through clinical applications, the autologous transplantation approach utilizes iPSCs derived from patients with PD to generate dopaminergic neurons. These dopaminergic neurons may be successfully transplanted into the adult rodent striatum without signs of neurodegeneration [60][49]. In preclinical studies, autologous transplantation of iPSC-derived dopaminergic neurons has been suggested to be a promise. In a non-human primate (NHP) model, iPSC-derived dopaminergic neurons were transplanted, leading to functional recovery and the survival of approximately 20,000 tyrosine hydroxylase (TH)-positive neurons in the graft [60][49]. The feasibility of iPSC-based transplantation in restoring dopaminergic function was demonstrated through this approach. Allogeneic transplantation of iPSC-derived dopaminergic neurons has also been explored as an alternative approach. In a rat model, the transplantation of PD patient iPSC (PDiPS) cell-derived dopaminergic neurons demonstrated a significant reduction in contralateral rotations and improved motor function [61][50]. To assess the long-term effects of iPSC-derived dopaminergic neurons, studies have investigated the survival and integration of the transplanted cells. Autologous transplantation in NHP models revealed robust survival of TH-positive neurons for up to 1.5 years, accompanied by behavioral improvements and increased binding sites of the dopamine transporter [60][49]. These findings support the long-term viability and functional benefits of iPSC-derived dopaminergic neurons. Furthermore, iPSC-derived dopaminergic neurons demonstrated safety and regenerative abilities, as autologous transplantation demonstrated remarkable reinnervation of the denervated putamen without the need for immunosuppression. Notably, no graft overgrowth, tumor formation, or inflammatory reactions were observed [56,60][49][51]. Collectively, these studies propose the use of iPSC-derived dopaminergic neurons for transplantation as a potential therapeutic approach for PD. The transplantation of iPSCs into dopaminergic neurons demonstrates their feasibility and the absence of neurodegeneration upon transplantation into the adult rodent striatum [60][49]. Animal studies, including NHP and rat models, have provided evidence of improvement and an increase in dopaminergic neurons following both autologous and allogeneic transplantation of iPSC-derived dopaminergic neurons [56,61][50][51]. These studies also highlight the survival of transplanted cells, long-term behavioral improvements (including a 146% increase in daytime activity counts), and functional effects mediated by iPSC-derived dopaminergic neurons. Additionally, the safety and regenerative abilities of iPSC-derived dopaminergic neurons have been demonstrated, with no observed complications such as graft overgrowth, tumor formation, or inflammatory reactions. These findings support the potential of iPSC-based treatments for PD, underscoring their therapeutic potential in restoring dopamine levels and alleviating motor symptoms associated with the disease.References
- Kieburtz, K.; Olanow, C.W. Translational experimental therapeutics: The translation of laboratory-based discovery into disease-related therapy. Mt. Sinai J. Med. 2007, 74, 7–14.
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32.
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018.
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621.
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532.
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018.
- Stacy, M.; Galbreath, A. Optimizing long-term therapy for Parkinson disease: Levodopa, dopamine agonists, and treatment-associated dyskinesia. Clin. Neuropharmacol. 2008, 31, 51–56.
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160.
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s disease: Current status and new developments. J. Park. Dis. 2013, 3, 255–269.
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinsons Dis. 2016, 2016, 9832839.
- Tao, Y.; Vermilyea, S.C.; Zammit, M.; Lu, J.; Olsen, M.; Metzger, J.M.; Yao, L.; Chen, Y.; Phillips, S.; Holden, J.E.; et al. Autologous transplant therapy alleviates motor and depressive behaviors in parkinsonian monkeys. Nat. Med. 2021, 27, 632–639.
- Pandey, S.; Srivanitchapoom, P. Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management. Ann. Indian Acad Neurol. 2017, 20, 190–198.
- Siegfried, J.; Lippitz, B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: A new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 1994, 35, 1126–1129, discussion 1129–1130.
- Limousin, P.; Pollak, P.; Benazzouz, A.; Hoffmann, D.; Le Bas, J.F.; Perret, J.E.; Benabid, A.L.; Broussolle, E. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995, 345, 91–95.
- Rossi, M.; Bruno, V.; Arena, J.; Cammarota, A.; Merello, M. Challenges in PD Patient Management After DBS: A Pragmatic Review. Mov. Disord. Clin. Pract. 2018, 5, 246–254.
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556.
- Wu, Y.-P.; Chen, W.-S.; Teng, C.; Zhang, N. Stem cells for the treatment of neurodegenerative diseases. Molecules 2010, 15, 6743–6758.
- Lee, S.H. The advantages and limitations of mesenchymal stem cells in clinical application for treating human diseases. Osteoporos Sarcopenia 2018, 4, 150.
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14.
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759.
- Thomson, J.A.; Itskovitz-Eldor, J.; Shaprio, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147.
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45.
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103, quiz 104–105.
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932.
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004.
- Krolewski, R. Personal Communication; Shastry, S., Ed.; APA Style: New York, NY, USA, 2021.
- Fan, Y.W.; Ng, S.-Y. Replacing what’s lost: A new era of stem cell therapy for Parkinson’s disease. Transl. Neurodegener. 2020, 9, 2.
- Ricciardi, L.; Petrucci, S.; Guidubaldi, A.; Ialongo, T.; Serra, L.; Ferraris, A.; Spanò, B.; Bozzali, M.; Valente, E.M.; Bentivoglio, A.R. Phenotypic variability of PINK1 expression: 12 Years’ clinical follow-up of two Italian families. Mov. Disord. 2014, 29, 1561–1566.
- Deyell, J.S.; Sriparna, M.; Ying, M.; Mao, X. The Interplay between alpha-Synuclein and Microglia in alpha-Synucleinopathies. Int. J. Mol. Sci. 2023, 24, 2477.
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980.
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299.
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159.
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71.
- Butler, Y.R.; Liu, Y.; Kumbhar, R.; Zhao, P.; Gadhave, K.; Wang, N.; Li, Y.; Mao, X.; Wang, W. alpha-Synuclein fibril-specific nanobody reduces prion-like alpha-synuclein spreading in mice. Nat. Commun. 2022, 13, 4060.
- Zhang, S.; Liu, Y.Q.; Jia, C.; Lim, Y.J.; Feng, G.; Xu, E.; Long, H.; Kimura, Y.; Tao, Y.; Zhao, C.; et al. Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2011196118.
- Mao, X.; Gu, H.; Kim, D.; Kimura, Y.; Wang, Y.; Xu, E.; Wang, H.; Chen, C.; Zhang, S.; Jia, C.; et al. Aplp1 and the Aplp1-Lag3 Complex facilitates transmission of pathologic α-synuclein. bioRxiv 2021.
- Chen, C.; Kumbhar, R.; Wang, H.; Yang, X.; Gadhave, K.; Rastegar, C.; Kimura, Y.; Behensky, A.; Katakam, S.; Jeong, D.; et al. Pathological Tau transmission initiated by binding lymphocyte-activation gene 3. bioRxiv 2023.
- Gu, H.; Yang, X.; Mao, X.; Xu, E.; Qi, C.; Wang, H.; Brahmachari, S.; York, B.; Sriparna, M.; Li, A.; et al. Lymphocyte Activation Gene 3 (Lag3) Contributes to α-Synucleinopathy in α-Synuclein Transgenic Mice. Front. Cell Neurosci. 2021, 15, 656426.
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374.
- Chistiakov, D.A.; Chistiakov, A.A. alpha-Synuclein-carrying extracellular vesicles in Parkinson’s disease: Deadly transmitters. Acta Neurol. Belg. 2017, 117, 43–51.
- Pinnell, J.R.; Cui, M.; Tieu, K. Exosomes in Parkinson disease. J. Neurochem. 2021, 157, 413–428.
- Cooper, J.M.; Wiklander, P.B.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485.
- Wang, S.; Zou, C.; Fu, L.; Wang, B.; An, J.; Song, G.; Wu, J.; Tang, X.; Li, M.; Zhang, J.; et al. Autologous iPSC-derived dopamine neuron transplantation in a nonhuman primate Parkinson’s disease model. Cell Discov. 2015, 1, 15012.
- Harris, J.P.; Burrell, J.C.; Struzyna, L.A.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; Duda, J.E.; Cullen, D.K. Emerging regenerative medicine and tissue engineering strategies for Parkinson’s disease. NPJ Park. Dis. 2020, 6, 4.
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402.
- Cauvin, A.J.; Peters, C.; Brennan, F. Advantages and limitations of commonly used nonhuman primate species in research and development of biopharmaceuticals. In The nonhuman Primate in Nonclinical Drug Development and Safety Assessment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 379–395.
- Sundberg, M.; Bogetofte, H.; Lawson, T.; Jansson, J.; Smith, G.; Astradsson, A.; Moore, M.; Osborn, T.; Cooper, O.; Spealman, R.; et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 2013, 31, 1548–1562.
- Osborn, T.; Dinesh, D.; Moskites, A.; MacBain, Z.; Moore, M.; Brekk, O. Pre-clinical studies toward autologous midbrain dopamine cell therapy for Parkinson’s disease. Stem J. 2020, in press.
- Hargus, G.; Cooper, O.; Deleidi, M.; Levy, A.; Lee, K.; Marlow, E.; Yow, A.; Soldner, F.; Hockemeyer, D.; Hallett, P.J.; et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA 2010, 107, 15921–15926.
- Hallett, P.J.; Deleidi, M.; Astradsson, A.; Smith, G.A.; Cooper, O.; Osborn, T.M.; Sundberg, M.; Moore, M.A.; Perez-Torres, E.; Brownell, A.L.; et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 2015, 16, 269–274.
