
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yulia Merkher | -- | 3006 | 2023-11-28 07:34:42 | | | |
| 2 | Jessie Wu | Meta information modification | 3006 | 2023-11-28 09:19:48 | | |
Video Upload Options
Flaxseed has been recognized as a valuable source of nutrients and bioactive compounds, including proteins that possess various health benefits. In recent years, studies have shown that flaxseed proteins, including albumins, globulins, glutelin, and prolamins, possess anti-cancer properties. These properties are attributed to their ability to inhibit cancer cell proliferation, induce apoptosis, and interfere with cancer cell signaling pathways, ultimately leading to the inhibition of metastasis.
1. Flaxseed Proteins
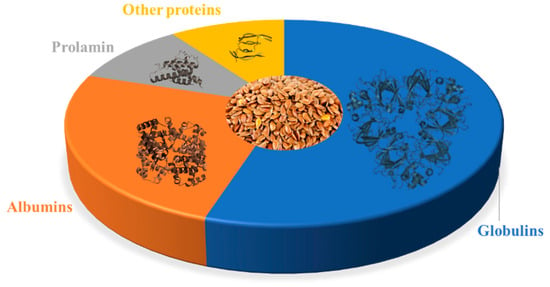
| Proteome Component |
Structure | Percentage of Total Protein | Potential Health Benefits |
|---|---|---|---|
| 11S Globulin |  |
43–48% | Contains anti-cancer peptides [19][20][21]; may have anti-inflammatory and antioxidant effects [22][23] |
| 2S Albumin |  |
16–18% | Contains peptides with potential anti-cancer and anti-inflammatory effects [21][24][25]; may have cholesterol-lowering effects [26] |
| 7S Vicilin-Like Globulin |  |
11–13% | May have antioxidant and anti-inflammatory effects [23][27]; contains peptides with potential anti-cancer effects [28] |
| Prolamin |  |
4–6% | Contains peptides with potential anti-cancer effects [29][30] |
| Glutelin-Like Protein |  |
2–3% | May have antioxidant and anti-inflammatory effects [31][32] |
| Other Proteins |  |
10–15% | May include lignans, which have antioxidant and anti-inflammatory and thus potential anti-cancer effects [33][34] |
2. Albumins of Flaxseed
| Action | Mode of Action | Evidence |
|---|---|---|
| Inhibits cancer proliferation and induce apoptosis | Interaction with the αvβ3 integrin via the FAK/ERK/NF-κB signaling pathway; activation of caspase-3 and cleavage of PARP [52][53] | Inhibited the proliferation and the tumorsphere-forming capacity of colon cancer HCT-116 cells [54]. Increased the amount of colon cancer cells KM12L4 undergoing apoptosis twofold [55]. Reduced tumor growth in NSCLC and melanoma xenografts [51]. |
| Inhibits cancer cell invasion and migration | Interaction with cellular receptors (integrins and EGFR); disrupts the activity of key signaling proteins (MMP-2/-9) via the FAK/Akt/ERK and NF-κB signaling pathways [56] | Caused a reduction in the migration (scratch assay) of HCT-116 and KM12L4 colon cancer cells [53]. Inhibited the migration and invasion activity in MDA-MB-231 and MCF-7 breast cancer cell lines [56]. Inhibited the invasion of melanoma A375 and B16-F10 cells to matrigel [50]. |
| Reduces inflammation and oxidation | Reduction in the production of certain inflammatory markers (ROS, TNF-α and IL-6) [57][58] | Mouse macrophage cells (LPS-stimulated RAW 264.7) reduced the production of certain inflammatory markers (ROS, TNF-α and IL-6) [59]. |
| Reduces cancer cells colonization | Reduction in phosphorylation of the intracellular kinases FAK and AKT; reduction in histone acetylation of lysine residues in H3 and H4 histones [50] | Reduced pulmonary colonization after injection of highly metastatic B16-F10 melanoma cells [50][51]. Inhibited formation of liver metastasis in murine model [53]. |
| Arrest cell cycle | Arrests the cell cycle at the G2/M and G1/S phase; altered the expression of the G1 specific cyclin-dependent kinase complex components, increased levels of p27Kip1, reduced levels of phosphorylated Akt [55][60] | Inhibited cell cycle progression at the G1/S phase for NSCLC H661 cells [60]. Lunasin treatment of MDA-MB-231 breast cancer cells resulted in a notable increase in RB1 level, which lead to arrest of G1 phase [61] |
3. Globulins of Flaxseed
| Action | Mode of Action | Evidence |
|---|---|---|
| Inhibits cancer cell growth and proliferation | Lectin was responsible for the antiproliferative activity of the MPI-h [74]. Acid fraction of glycinin, composed of low molecular weight peptides, was able to inhibit cancer cells growth [75]. | Inhibited the proliferation of UMR106 rat osteosarcoma-derived cells [74] and inhibited the growth of cervical cancer HeLa cells in a dose-dependent manner [75]. |
| Antioxidant effect | Small (~1 kDa) peptides generated from 11S globulin inhibit the formation of hydroxyl radicals by reaction of H2O2 and Co+2 and decreasing ROS [20] | Demonstrated peroxyl radical scavenging activity dependent on the structure of peptides in human adenocarcinoma cell line, Caco-2 TC7 [20] |
| Induces cancer cell death (apoptosis) | Increased the expression levels of apoptosis-related spot-like protein (ASC), caspase-1, the cleaved gasdermin D, and interleukin-1β [76]. Flaxseed orbitides influence mitochondrial- and death receptor-mediated intrinsic and extrinsic pathways [77]. | Glutelin extracts induced the apoptosis of cervical cancer HeLa cells [78]. Human gastric SGC-7901 cell apoptosis was induced by linusorb B3 flaxseed orbitides [77] |
| Arresting cell cycle | Linoorbitides arrested the cell at the G1 phase by downregulation of CDK2/4 and overexpression of p21WAF1/CIP1 and p27KIP1 genes [79]. | Cyclolinopeptides/linusorbs are capable of arresting cell cycle, and thus reduce metastasis spreading in human gastric SGC-7901 cells [79]. |
| Cytotoxic effect on cancer cells | Linoorbitides have strong cytotoxic effect probably due to its binding abilities to human serum albumin [11]. | Linusorb B3 is cytotoxic to human melanoma cells A375 and breast cancer cells Sk-Br-3 and MCF7 at high concentration [80]. |
4. The Combined Anti-Cancer Action of Flaxseed Proteins
5. Flaxseed Proteome Effect on Anti-Cancer Radiotherapy
6. Conclusions
Nutrition plays a crucial role in the overall well-being of cancer patients and can significantly contribute to reducing complications during treatment [168,169]. In recent years, there has been a growing focus on plant-based nutrition as a means to protect against a range of leading causes of death, including various types of cancer such as breast, prostate, colorectal, and gastrointestinal cancers. Plant-based diets comprising whole foods have demonstrated substantial protective effects against these cancers and other chronic diseases. These diets can serve as disease-modifying agents, enhancing the management and treatment of these conditions. Consequently, interventions involving nutrition in the prevention of diverse cancers offer a notable complement to existing medical therapies.
Addressing the current gaps in knowledge about flaxseed proteins is crucial for advancing both cancer treatment and overall health. Firstly, the limited representation of flaxseed proteins in existing databases underscores the need for a more comprehensive inclusion of this vital information. Additionally, the absence of established sequences for flaxseed proteins poses a challenge in understanding their biological activities. Furthermore, the scarcity of research on the properties of flaxseed for cancer treatment emphasizes the importance of exploring these avenues. The development of a compatible nutraceutical enriched with flaxseed proteins and the analysis of its anti-metastatic effects contribute significantly to promoting healthy aging. These initiatives not only address existing gaps but also pave the way for novel strategies that can enhance both cancer treatment and overall well-being.
References
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical Composition and Health Benefits of Flaxseed. Austin J. Nutr. Food Sci. 2014, 2, 1045.
- Lowcock, E.C.; Cotterchio, M.; Boucher, B.A. Consumption of Flaxseed, a Rich Source of Lignans, Is Associated with Reduced Breast Cancer Risk. Cancer Causes Control 2013, 24, 813–816.
- McCann, S.E.; Hootman, K.C.; Weaver, A.M.; Thompson, L.U.; Morrison, C.; Hwang, H.; Edge, S.B.; Ambrosone, C.B.; Horvath, P.J.; Kulkarni, S.A. Dietary Intakes of Total and Specific Lignans Are Associated with Clinical Breast Tumor Characteristics. J. Nutr. 2012, 142, 91.
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945.
- Pal, P.; Hales, K.; Petrik, J.; Hales, D.B. Pro-Apoptotic and Anti-Angiogenic Actions of 2-Methoxyestradiol and Docosahexaenoic Acid, the Biologically Derived Active Compounds from Flaxseed Diet, in Preventing Ovarian Cancer. J. Ovarian Res. 2019, 12, 49.
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15.
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118.
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Kajla, P.; Goyal, N.; Bangar, S.P.; Chaudhary, V.; Lorenzo, J.M. Flaxseed Proteins (Linum Usitassimum): Thermal, Functional and Spectroscopic Characterization. Food Anal. Methods 2023, 16, 459–467.
- Mueed, A.; Madjirebaye, P.; Shibli, S.; Deng, Z. Flaxseed Peptides and Cyclolinopeptides: A Critical Review on Proteomic Approaches, Biological Activity, and Future Perspectives. J. Agric. Food Chem. 2022, 70, 14600–14612.
- Sammour, R.H. Proteins of Linseed (Linum usitatissimum L.), Extraction and Characterization by Electrophoresis. Bot. Bull. Acad. Sin. 1999, 40, 121–126.
- Ayad, A.A. Characterization and Properties of Flaxseed Protein Fractions. Ph.D. Thesis, Mcgill University, Montreal, QC, Canada, 2010.
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A Potential Source of Food, Feed and Fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222.
- Sharma, M.; Saini, C.S. Amino Acid Composition, Nutritional Profiling, Mineral Content and Physicochemical Properties of Protein Isolate from Flaxseeds (Linum usitatissimum). J. Food Meas. Charact. 2022, 16, 829–839.
- Peng, D.; Ye, J.; Jin, W.; Yang, J.; Geng, F.; Deng, Q. A Review on the Utilization of Flaxseed Protein as Interfacial Stabilizers for Food Applications. JAOCS J. Am. Oil Chem. Soc. 2022, 99, 723–737.
- Oomah, B.D.; Mazza, G. Flaxseed Proteins—A Review. Food Chem. 1993, 48, 109–114.
- Arntfield, S.D. Proteins from Oil-Producing Plants. In Proteins in Food Processing, 2nd ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 187–221.
- Xie, M.; Liu, D.; Yang, Y. Anti-Cancer Peptides: Classification, Mechanism of Action, Reconstruction and Modification. Open Biol. 2020, 10, 200004.
- Fillería, S.G.; Nardo, A.E.; Paulino, M.; Tironi, V. Peptides Derived from the Gastrointestinal Digestion of Amaranth 11S Globulin: Structure and Antioxidant Functionality. Food Chem. Mol. Sci. 2021, 3, 100053.
- Taghizadeh, S.F.; Rezaee, R.; Mehmandoust, M.; Badibostan, H.; Karimi, G. Assessment of in Vitro Bioactivities of Pis v 1 (2S Albumin) and Pis v 2.0101 (11S Globulin) Proteins Derived from Pistachio (Pistacia vera L.). J. Food Meas. Charact. 2020, 14, 1054–1063.
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-Inflammatory and Antioxidant Effects of Peptides Released from Germinated Amaranth during in Vitro Simulated Gastrointestinal Digestion. Food Chem. 2021, 343, 128394.
- Martínez, J.H.; Velázquez, F.; Burrieza, H.P.; Martínez, K.D.; Paula Domínguez Rubio, A.; dos Santos Ferreira, C.; del Pilar Buera, M.; Pérez, O.E. Betanin Loaded Nanocarriers Based on Quinoa Seed 11S Globulin. Impact on the Protein Structure and Antioxidant Activity. Food Hydrocoll. 2019, 87, 880–890.
- Luo, X.; Wu, W.; Feng, L.; Treves, H.; Ren, M. Short Peptides Make a Big Difference: The Role of Botany-Derived Amps in Disease Control and Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 11363.
- Paterson, S.; Fernández-Tomé, S.; Hernández-Ledesma, B. Modulatory Effects of a Lunasin-Enriched Soybean Extract on Immune Response and Oxidative Stress-Associated Biomarkers. Biol. Life Sci. Forum 2022, 12, 10.
- Caponio, G.R.; Wang, D.Q.H.; Di Ciaula, A.; De Angelis, M.; Portincasa, P. Regulation of Cholesterol Metabolism by Bioactive Components of Soy Proteins: Novel Translational Evidence. Int. J. Mol. Sci. 2021, 22, 227.
- Capraro, J.; De Benedetti, S.; Heinzl, G.C.; Scarafoni, A.; Magni, C. Bioactivities of Pseudocereal Fractionated Seed Proteins and Derived Peptides Relevant for Maintaining Human Well-Being. Int. J. Mol. Sci. 2021, 22, 3543.
- Ateeq, M.; Adeel, M.M.; Kanwal, A.; Qamar, M.T.U.; Saeed, A.; Khaliq, B.; Saeed, Q.; Atiq, M.N.; Bilal, M.; Alharbi, M.; et al. In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna aconitifolia (Jacq.) Marechal) Seeds. Molecules 2022, 27, 3251.
- Chen, Y.J.; Chen, Y.Y.; Wu, C.T.; Yu, C.C.; Liao, H.F. Prolamin, a Rice Protein, Augments Anti-Leukaemia Immune Response. J. Cereal Sci. 2010, 51, 189–197.
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and Anti-Inflammatory Activity of Peptides from Foxtail Millet (Setaria italica) Prolamins in HaCaT Cells and RAW264.7 Murine Macrophages. Food Biosci. 2020, 36, 100636.
- Jimenez-Pulido, I.J.; Daniel, R.; Perez, J.; Martínez-Villaluenga, C.; De Luis, D.; Martín Diana, A.B. Impact of Protein Content on the Antioxidants, Anti-Inflammatory Properties and Glycemic Index of Wheat and Wheat Bran. Foods 2022, 11, 2049.
- Montserrat-de la Paz, S.; Rodriguez-Martin, N.M.; Villanueva, A.; Pedroche, J.; Cruz-Chamorro, I.; Millan, F.; Millan-Linares, M.C. Evaluation of Anti-Inflammatory and Atheroprotective Properties of Wheat Gluten Protein Hydrolysates in Primary Human Monocytes. Foods 2020, 9, 854.
- Aqeel, T.; Gurumallu, S.C.; Bhaskar, A.; Hashimi, S.M.; Lohith, N.C.; Javaraiah, R. Protective Role of Flaxseed Lignan Secoisolariciresinol Diglucoside against Lead-Acetate-Induced Oxidative-Stress-Mediated Nephrotoxicity in Rats. Phytomedicine Plus 2021, 1, 100038.
- Rajesha, J.; Ranga Rao, A.; Madhusudhan, B.; Karunakumar, M. Antibacterial Properties of Secoisolariciresinol Diglucoside Isolated from Indian Flaxseed Cultivars. Curr. Trends Biotechnol. Pharm. 2010, 4, 551–560.
- Nguyen, N.P.T.; Cong, T.L.; Tran, T.T.H.; Do, B.N.; Nguyen, S.T.; Vu, B.T.; Nguyen, L.H.T.; Van Ngo, M.; Dinh, H.T.; Huy, H.D.; et al. Lower Plasma Albumin, Higher White Blood Cell Count and High-Sensitivity C-Reactive Protein Are Associated with Femoral Artery Intima-Media Thickness Among Newly Diagnosed Patients with Type 2 Diabetes Mellitus. Int. J. Gen. Med. 2022, 15, 2715–2725.
- Lessomo, F.Y.N.; Fan, Q.; Wang, Z.Q.; Mukuka, C. The Relationship between Leukocyte to Albumin Ratio and Atrial Fibrillation Severity. BMC Cardiovasc. Disord. 2023, 23, 67.
- Gupta, D.; Lis, C.G. Pretreatment Serum Albumin as a Predictor of Cancer Survival: A Systematic Review of the Epidemiological Literature. Nutr. J. 2010, 9, 69.
- Nazha, B.; Moussaly, E.; Zaarour, M.; Weerasinghe, C.; Azab, B. Hypoalbuminemia in Colorectal Cancer Prognosis: Nutritional Marker or Inflammatory Surrogate? World J. Gastrointest. Surg. 2015, 7, 370.
- Van De Wouw, J.; Joles, J.A. Albumin Is an Interface between Blood Plasma and Cell Membrane, and Not Just a Sponge. Clin. Kidney J. 2022, 15, 624–634.
- Cho, H.; Jeon, S.I.; Ahn, C.H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728.
- Li, H.; Wang, Z.; Yu, S.; Chen, S.; Zhou, Y.; Qu, Y.; Xu, P.; Jiang, L.; Yuan, C.; Huang, M. Albumin-Based Drug Carrier Targeting Urokinase Receptor for Cancer Therapy. Int. J. Pharm. 2023, 634, 122636.
- Ranaivo, H.R.; Hodge, J.N.; Choi, N.; Wainwright, M.S. Albumin Induces Upregulation of Matrix Metalloproteinase-9 in Astrocytes via MAPK and Reactive Oxygen Species-Dependent Pathways. J. Neuroinflamm. 2012, 9, 68.
- von Au, A.; Vasel, M.; Kraft, S.; Sens, C.; Hackl, N.; Marx, A.; Stroebel, P.; Hennenlotter, J.; Todenhöfer, T.; Stenzl, A.; et al. Circulating Fibronectin Controls Tumor Growth. Neoplasia 2013, 15, 925–938.
- Zhao, P.; Wang, Y.; Wu, A.; Rao, Y.; Huang, Y. Roles of Albumin-Binding Proteins in Cancer Progression and Biomimetic Targeted Drug Delivery. ChemBioChem 2018, 19, 1796–1805.
- Givant-Horwitz, V.; Davidson, B.; Reich, R. Laminin-Induced Signaling in Tumor Cells. Cancer Lett. 2005, 223, 1–10.
- Radovic, R.S.; Maksimovic, R.V.; Brkljacic, M.J.; Varkonji Gasic, I.E.; Savic, P.A. 2S Albumin from Buckwheat (Fagopyrum esculentum Moench) Seeds. J. Agric. Food Chem. 1999, 47, 1467–1470.
- Souza, P.F.N. The Forgotten 2S Albumin Proteins: Importance, Structure, and Biotechnological Application in Agriculture and Human Health. Int. J. Biol. Macromol. 2020, 164, 4638–4649.
- Bueno-Díaz, C.; Martín-Pedraza, L.; Parrón, J.; Cuesta-Herranz, J.; Cabanillas, B.; Pastor-Vargas, C.; Batanero, E.; Villalba, M. Characterization of Relevant Biomarkers for the Diagnosis of Food Allergies: An Overview of the 2s Albumin Family. Foods 2021, 10, 1235.
- Khan, S.; Ali, S.A.; Yasmin, T.; Ahmed, M.; Khan, H. Purification and Characterization of 2S Albumin from Nelumbo Nucifera. Biosci. Biotechnol. Biochem. 2016, 80, 2109–2114.
- Shidal, C.; Inaba, J.I.; Yaddanapudi, K.; Davis, K.R. The Soy-Derived Peptide Lunasin Inhibits Invasive Potential of Melanoma Initiating Cells. Oncotarget 2017, 8, 25525–25541.
- Vuyyuri, S.B.; Shidal, C.; Davis, K.R. Development of the Plant-Derived Peptide Lunasin as an Anticancer Agent. Curr. Opin. Pharmacol. 2018, 41, 27–33.
- Seber, L.E.; Barnett, B.W.; McConnell, E.J.; Hume, S.D.; Cai, J.; Boles, K.; Davis, K.R. Scalable Purification and Characterization of the Anticancer Lunasin Peptide from Soybean. PLoS ONE 2012, 7, e35409.
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin Potentiates the Effect of Oxaliplatin Preventing Outgrowth of Colon Cancer Metastasis, Binds to α 5β 1 Integrin and Suppresses FAK/ERK/NF-ΚB Signaling. Cancer Lett. 2011, 313, 167–180.
- Fernández-tomé, S.; Xu, F.; Han, Y.; Hernández-ledesma, B.; Xiao, H. Inhibitory Effects of Peptide Lunasin in Colorectal Cancer Hct-116 Cells and Their Tumorsphere-Derived Subpopulation. Int. J. Mol. Sci. 2020, 21, 537.
- Dia, V.P.; De Mejia, E.G. Lunasin Induces Apoptosis and Modifies the Expression of Genes Associated with Extracellular Matrix and Cell Adhesion in Human Metastatic Colon Cancer Cells. Mol. Nutr. Food Res. 2011, 55, 623–634.
- Jiang, Q.; Pan, Y.; Cheng, Y.; Li, H.; Liu, D.; Li, H. Lunasin Suppresses the Migration and Invasion of Breast Cancer Cells by Inhibiting Matrix Metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-ΚB Signaling Pathways. Oncol. Rep. 2016, 36, 253–262.
- Aggarwal, B.B.; Gehlot, P. Inflammation and Cancer: How Friendly Is the Relationship for Cancer Patients? Curr. Opin. Pharmacol. 2009, 9, 351–369.
- Wan, X.; Liu, H.; Sun, Y.; Zhang, J.; Chen, X.; Chen, N. Lunasin: A Promising Polypeptide for the Prevention and Treatment of Cancer. Oncol. Lett. 2017, 13, 3997–4001.
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Antioxidant and Anti-Inflammatory Properties of Cancer Preventive Peptide Lunasin in RAW 264.7 Macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808.
- McConnell, E.J.; Devapatla, B.; Yaddanapudi, K.; Davis, K.R. The Soybean-Derived Peptide Lunasin Inhibits Non-Small Cell Lung Cancer Cell Proliferation by Suppressing Phosphorylation of the Retinoblastoma Protein. Oncotarget 2015, 6, 4649–4662.
- Hsieh, C.C.; Hernández-Ledesma, B.; de Lumen, B.O. Lunasin, a Novel Seed Peptide, Sensitizes Human Breast Cancer MDA-MB-231 Cells to Aspirin-Arrested Cell Cycle and Induced Apoptosis. Chem. Biol. Interact. 2010, 186, 127–134.
- Youle, R.J.; Huang, A.H.C. Occurrence of low molecular weight and high cysteine containing albumin storage proteins in oilseeds of diverse species. Am. J. Bot. 1981, 68, 44–48.
- Madhusudhan, K.T.; Singh, N. Isolation and Characterization of a Small Molecular Weight Protein of Linseed Meal. Phytochemistry 1985, 24, 2507–2509.
- Dev, D.K.; Sienkiewicz, T. Isolation and Subunit Composition of 11 S Globulin of Linseed (Linum usitatissimum L.). Food/Nahr. 1987, 31, 767–769.
- El-Saadany, A.S.; Hanafy, M.M.; Elkomy, A.E. Flaxseed and Agnus-Castuson Vitex as a Source of Phytoestrogens and Their Impact on Productive Performance, Some Blood Constituents, and Blood Oestradiol Profile of Aged Laying Hens. Ital. J. Anim. Sci. 2022, 21, 821–830.
- Singh, A.; Meena, M.; Kumar, D.; Dubey, A.K.; Hassan, M.I. Structural and Functional Analysis of Various Globulin Proteins from Soy Seed. Crit. Rev. Food Sci. Nutr. 2015, 55, 1491–1502.
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine Kinase—Role and Significance in Cancer. Int. J. Med. Sci. 2012, 1, 101–115.
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Kapusta, I.; Świeca, M. Antioxidant Content and Antioxidant Capacity of the Protein-Rich Powdered Beverages Enriched with Flax Seeds Gum. Antioxidants 2022, 11, 582.
- Yousif, A.N. Effect of Flaxseed on Some Hormonal Profile and Genomic DNA Concentration in Karadi Lambs. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 012035.
- Nowak, D.A.; Snyder, D.C.; Brown, A.J.; Demark-Wahnefried, W. The Effect of Flaxseed Supplementation on Hormonal Levels Associated with Polycystic Ovarian Syndrome: A Case Study. Curr. Top. Nutraceutical Res. 2007, 5, 177–181.
- Chang, V.C.; Cotterchio, M.; Boucher, B.A.; Jenkins, D.J.A.; Mirea, L.; McCann, S.E.; Thompson, L.U. Effect of Dietary Flaxseed Intake on Circulating Sex Hormone Levels among Postmenopausal Women: A Randomized Controlled Intervention Trial. Nutr. Cancer 2019, 71, 385–398.
- Ajibola, C.F.; Aluko, R.E. Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions. Molecules 2022, 27, 1059.
- Adachi, M.; Kanamori, J.; Masuda, T.; Yagasaki, K.; Kitamura, K.; Mikami, B.; Utsumi, S. Crystal Structure of Soybean 11S Globulin: Glycinin A3B4 Homohexamer. Proc. Natl. Acad. Sci. USA 2003, 100, 7395–7400.
- Quiroga, A.V.; Barrio, D.A.; Añón, M.C. Amaranth Lectin Presents Potential Antitumor Properties. LWT 2015, 60, 478–485.
- Mora-Escobedo, R.; Robles-Ramírez, M.d.C.; Ramón-Gallegos, E.; Reza-Alemán, R. Effect of Protein Hydrolysates from Germinated Soybean on Cancerous Cells of the Human Cervix: An In Vitro Study. Plant Foods Hum. Nutr. 2009, 64, 271–278.
- Wang, L.; Sun, Z.; Xie, W.; Peng, C.; Ding, H.; Li, Y.; Feng, S.; Wang, X.; Zhao, C.; Wu, J. 11S Glycinin Up-Regulated NLRP-3-Induced Pyroptosis by Triggering Reactive Oxygen Species in Porcine Intestinal Epithelial Cells. Front. Vet. Sci. 2022, 9, 677.
- Zou, X.G.; Hu, J.N.; Li, J.; Yang, J.Y.; Du, Y.X.; Yu, Y.F.; Deng, Z.Y. ICellular Uptake of -Linusorb B2 and -Linusorb B3 Isolated from Flaxseed, and Their Antitumor Activities in Human Gastric SGC-7901 Cells. J. Funct. Foods 2018, 48, 692–703.
- Silva-Sánchez, C.; Barba De La Rosa, A.P.; León-Galván, M.F.; De Lumen, B.O.; De León-Rodríguez, A.; González De Mejía, E. Bioactive Peptides in Amaranth (Amaranthus Hypochondriacus) Seed. J. Agric. Food Chem. 2008, 56, 1233–1240.
- Zou, X.G.; Hu, J.N.; Wang, M.; Du, Y.X.; Li, J.; Mai, Q.Y.; Deng, Z.Y. -Linusorb B2 and -Linusorb B3 Isolated from Flaxseed Induce G1 Cell Cycle Arrest on SGC-7901 Cells by Modulating the AKT/JNK Signaling Pathway. J. Funct. Foods 2019, 52, 332–339.
- Okinyo-Owiti, D.P.; Dong, Q.; Ling, B.; Jadhav, P.D.; Bauer, R.; Maley, J.M.; Reaney, M.J.T.; Yang, J.; Sammynaiken, R. Evaluating the Cytotoxicity of Flaxseed Orbitides for Potential Cancer Treatment. Toxicol. Rep. 2015, 2, 1014–1018.
- Deshpande, R.; Raina, P.; Shinde, K.; Mansara, P.; Karandikar, M.; Kaul-Ghanekar, R. Flax Seed Oil Reduced Tumor Growth, Modulated Immune Responses and Decreased HPV E6 and E7 Oncoprotein Expression in a Murine Model of Ectopic Cervical Cancer. Prostaglandins Other Lipid Mediat. 2019, 143, 106332.
- Sung, N.Y.; Jeong, D.; Shim, Y.Y.; Ratan, Z.A.; Jang, Y.J.; Reaney, M.J.T.; Lee, S.; Lee, B.H.; Kim, J.H.; Yi, Y.S.; et al. The Anti-Cancer Effect of Linusorb B3 from Flaxseed Oil through the Promotion of Apoptosis, Inhibition of Actin Polymerization, and Suppression of Src Activity in Glioblastoma Cells. Molecules 2020, 25, 5881.
- Lee, J.; Cho, K. Flaxseed Sprouts Induce Apoptosis and Inhibit Growth in MCF-7 and MDA-MB-231 Human Breast Cancer Cells. Vitr. Cell. Dev. Biol.-Anim. 2012, 48, 244–250.
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68.
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A Pilot Study Comparing the Effect of Flaxseed, Aromatase Inhibitor, and the Combination on Breast Tumor Biomarkers. Nutr. Cancer 2014, 66, 566–575.
- Lim, T.L.; Pietrofesa, R.A.; Arguiri, E.; Koumenis, C.; Feigenberg, S.; Simone, C.B.; Rengan, R.; Cengel, K.; Levin, W.P.; Christofidou-Solomidou, M.; et al. Phase II Trial of Flaxseed to Prevent Acute Complications After Chemoradiation for Lung Cancer. J. Altern. Complement. Med. 2021, 27, 824–831.
- Ristic-Medic, D.; Perunicic-Pekovic, G.; Rasic-Milutinovic, Z.; Takic, M.; Popovic, T.; Arsic, A.; Glibetic, M. Effects of Dietary Milled Seed Mixture on Fatty Acid Status and Inflammatory Markers in Patients on Hemodialysis. Sci. World J. 2014, 2014, 563576.
- de Mey, S.; Dufait, I.; De Ridder, M. Radioresistance of Human Cancers: Clinical Implications of Genetic Expression Signatures. Front. Oncol. 2021, 11, 761901.
- Fukui, R.; Saga, R.; Matsuya, Y.; Tomita, K.; Kuwahara, Y.; Ohuchi, K.; Sato, T.; Okumura, K.; Date, H.; Fukumoto, M.; et al. Tumor Radioresistance Caused by Radiation-Induced Changes of Stem-like Cell Content and Sub-Lethal Damage Repair Capability. Sci. Rep. 2022, 12, 1056.
- Engel, A.L.; Lorenz, N.I.; Klann, K.; Münch, C.; Depner, C.; Steinbach, J.P.; Ronellenfitsch, M.W.; Luger, A.L. Serine-Dependent Redox Homeostasis Regulates Glioblastoma Cell Survival. Br. J. Cancer 2020, 122, 1391–1398.
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of Metabolism in Cancer Cell Radioresistance and Radiosensitization Methods. J. Exp. Clin. Cancer Res. 2018, 37, 87.
- Okunieff, P.; Swarts, S.; Keng, P.; Sun, W.; Wang, W.; Kim, J.; Yang, S.; Zhang, H.; Liu, C.; Williams, J.P.; et al. Antioxidants reduce consequences of radiation exposure. Adv. Exp. Med. Biol. 2008, 614, 165.
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravatà, V. Portrait of Inflammatory Response to Ionizing Radiation Treatment. J. Inflamm. 2015, 12, 14.
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211.
- Mortazavi, S.M.J.; Mosleh-Shirazi, M.A.; Tavassoli, A.R.; Taheri, M.; Mehdizadeh, A.R.; Namazi, S.A.S.; Jamali, A.; Ghalandari, R.; Bonyadi, S.; Haghani, M.; et al. Increased Radioresistance to Lethal Doses of Gamma Rays in Mice and Rats after Exposure to Microwave Radiation Emitted by a GSM Mobile Phone Simulator. Dose-Response 2012, 11, 281–292.
- Wu, D.; Taibi, A.; Thompson, L.; Comelli, E. Flaxseed Alters Gut Microbiota-Mammary Gland MicroRNA Relationships Differently Than Its Oil and Lignan Components. Curr. Dev. Nutr. 2022, 6, 342.
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary Flaxseed Modulates the MiRNA Profile in Irradiated and Non-Irradiated Murine Lungs: A Novel Mechanism of Tissue Radioprotection by Flaxseed. Cancer Biol. Ther. 2014, 15, 930–937.
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary Flaxseed Prevents Radiation-Induced Oxidative Lung Damage, Inflammation and Fibrosis in a Mouse Model of Thoracic Radiation Injury. Cancer Biol. Ther. 2009, 8, 47–53.
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary Flaxseed Administered Post Thoracic Radiation Treatment Improves Survival and Mitigates Radiation-Induced Pneumonopathy in Mice. BMC Cancer 2011, 11, 269.




