Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Puangrat Kajitvichyanukul | -- | 4296 | 2023-11-23 06:52:26 | | | |
| 2 | Lindsay Dong | Meta information modification | 4296 | 2023-11-24 07:14:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Udomkun, P.; Boonupara, T.; Sumitsawan, S.; Khan, E.; Pongpichan, S.; Kajitvichyanukul, P. Sampling and Analysis of Airborne Pesticides. Encyclopedia. Available online: https://encyclopedia.pub/entry/51969 (accessed on 04 March 2026).
Udomkun P, Boonupara T, Sumitsawan S, Khan E, Pongpichan S, Kajitvichyanukul P. Sampling and Analysis of Airborne Pesticides. Encyclopedia. Available at: https://encyclopedia.pub/entry/51969. Accessed March 04, 2026.
Udomkun, Patchimaporn, Thirasant Boonupara, Sulak Sumitsawan, Eakalak Khan, Siwatt Pongpichan, Puangrat Kajitvichyanukul. "Sampling and Analysis of Airborne Pesticides" Encyclopedia, https://encyclopedia.pub/entry/51969 (accessed March 04, 2026).
Udomkun, P., Boonupara, T., Sumitsawan, S., Khan, E., Pongpichan, S., & Kajitvichyanukul, P. (2023, November 23). Sampling and Analysis of Airborne Pesticides. In Encyclopedia. https://encyclopedia.pub/entry/51969
Udomkun, Patchimaporn, et al. "Sampling and Analysis of Airborne Pesticides." Encyclopedia. Web. 23 November, 2023.
Copy Citation
The escalating utilization of pesticides has led to pronounced environmental contamination, posing a significant threat to agroecosystems. The extensive and persistent global application of these chemicals has been linked to a spectrum of acute and chronic human health concerns.
pesticide analysis
atmospheric contaminants
airborne sampling
extraction approaches
1. Introduction
Acute or chronic exposure to airborne pesticides has the potential to negatively affect human health. Some pesticides are known to be toxic, and long-term exposure to low levels of them has been associated with adverse health effects, including respiratory issues, neurodevelopmental disorders, hormonal disruption, and certain types of cancers [1][2][3][4]. Furthermore, the environmental impact of airborne pesticides extends to non-target organisms, including wildlife, beneficial insects, and aquatic ecosystems [5][6].
Accurately measuring pesticides in the air is of paramount importance. However, routine monitoring and reporting of these pesticides in the air are lacking. Furthermore, analyzing these pesticides in both indoor and outdoor air presents complexities that require specific procedures. Various techniques and methods are used for this purpose. For example, active sampling methods involve actively pulling air through samplers using pumps or fans [7], while passive sampling methods rely on the diffusion of pesticides into a sorbent material [8].
After sample collection, extraction techniques are employed to isolate the pesticide compounds from the collected air samples. Common extraction methods include solid-phase extraction (SPE), liquid-liquid extraction (LLE), and solid-phase microextraction (SPME). With the increasing focus on miniaturization and the adoption of green chemistry principles, environmentally friendly sample preparation methods have gained popularity for pesticide extraction. One such method is pressurized liquid extraction (PLE), which offers high efficiency and low consumption of organic solvents for extracting pesticides from particulate matter (PM) [9]. Microwave-assisted extraction (MAE) is another technique that has been utilized for pesticide extraction, offering similar advantages [10]. In contrast, miniaturized systems based on ultrasonic-assisted extraction (UAE) have emerged as a simple and cost-effective alternative for extracting pollutants from PM [11]. Moreover, different solvents and sorbents can be utilized depending on the properties of the target pesticides. Analytical methods such as gas chromatography (GC), liquid chromatography (LC), or their combination with mass spectrometry (MS) are commonly employed for the analysis and quantification of airborne pesticides [12]. In addition to the traditional methods, alternative techniques like ion mobility spectrometry (IMS) and attenuated total reflectance–Fourier-transform infrared spectroscopy (ATR-FTIR) have been applied to measure airborne pesticides [13][14]. These methods allow for the identification and quantification of specific pesticide compounds present in the samples. Additionally, high-resolution mass spectrometry (HRMS) techniques offer improved sensitivity and selectivity in pesticide analysis [15].
2. Pesticide Concentrations in Air
2.1. Indoor Air
The potential adverse health effects stemming from pesticide exposure have garnered substantial public attention. While numerous studies have extensively examined and reported on their presence in water and soil, less focus has been directed towards their occurrence in the air. This is chiefly due to the fact that pesticides generally exist in the air as trace-level pollutants, ranging from picograms per cubic meter (pg m−3) to nanograms per cubic meter (ng m−3). Pesticides can manifest in the air in diverse states, encompassing solids, gases, and liquids [16]. Agricultural spraying activities, for instance, contribute to around 30–50% dispersion of most pesticides into the air [17], primarily facilitated through drift (wind-carried) and evaporation. Subsequently, pesticides undergo volatilization from soil and plants, degradation, and photolysis, eventually integrating into the atmospheric environment. Airborne pesticides present a dual concern, as they not only pose immediate health risks through inhalation but can also settle on surfaces, leading to prolonged indoor contamination. Some studies have consistently highlighted the prevalence of organochlorine and organophosphate pesticides as the prominent groups detected in indoor air.
Studies have examined the temporal analysis of pesticide residues to pinpoint factors influencing the transportation and redistribution of these compounds within indoor environments. For example, Obendorf et al. [18] discovered that the occurrence and quantity of pesticide residues, particularly chlorpyrifos, were elevated during the summer months—a correlation attributed to agricultural and horticultural practices. Conversely, greater quantities of insecticides like mecoprop, resmethrin, and tetramethrin were detected on flat surfaces during the winter, suggesting household application and potential redistribution within the indoor environment. In addition, a study conducted by Berger-Preiss and Elflein [19] observed the presence of pesticides (such as pyrethroids, pyrethrum, and the synergist piperonyl butoxide) in an experimental house over a two-year period. The concentrations of these pesticides were the highest at the top of the room and gradually decreased towards the middle and lower areas [20]. While some pesticides may degrade quickly through photodecomposition, others like DDT, chlordane, heptachlor, methoxychlor, dieldrin, and pentachlorophenol tend to persist indoors [21].
Household pesticides are also found in dust and PM. The arena of dust and suspended particles becomes another tableau for household pesticides, as Rudel et al. [21] revealed the prevalence of permethrins and the synergist piperonyl butoxide, woven into concentrations ranging from 1.7 to 17 µg g−1. Wang et al. [22] gathered dust samples from floors within residential homes and office spaces. Among the array of pesticides, it was observed that hexachlorobenzene’s presence in indoor settings could be attributed to its transfer from outdoor sources [23]. On the other hand, chlordanes were linked to historical household usage [24], while pyrethroids showed an association with ongoing household applications [25].
2.2. Outdoor Air
Various pesticide groups, including organochlorine insecticides, and organophosphate insecticides, herbicides, and fungicides, have been detected in outdoor air samples from several countries such as France, Spain, China, Pakistan, Malaysia, South Africa, and the United States. Mirroring the trend observed with indoor pesticides, the organochlorine insecticides that have consistently surfaced as the most frequently detected compounds over the past 15 years include 4,4′-DDE, 4,4′-DDD, and endosulfan. Their concentrations ranged from 0.002 to 25.6 ng m−3, 0.011 to 154 ng m−3, and 0.0001 to 81.3 ng m−3, respectively. Much like indoor environments, outdoor air contains traces of organophosphate insecticides such as chlorpyrifos, malathion, and diazinon, although typically at lower concentrations. However, their concentrations are commonly higher in indoor air compared to outdoor air due to their prevalent use for indoor pest control. Factors such as their proximity to indoor application sources, limited dispersion indoors, volatility, persistence, surface deposition, and potential re-suspension contribute to their higher concentrations. Outdoor air, in contrast, experiences lower exposure to these compounds due to differences in usage patterns and dispersion dynamics.
3. Advancements in Pesticide Sampling Techniques
3.1. Active Sampling Applications and Limitations
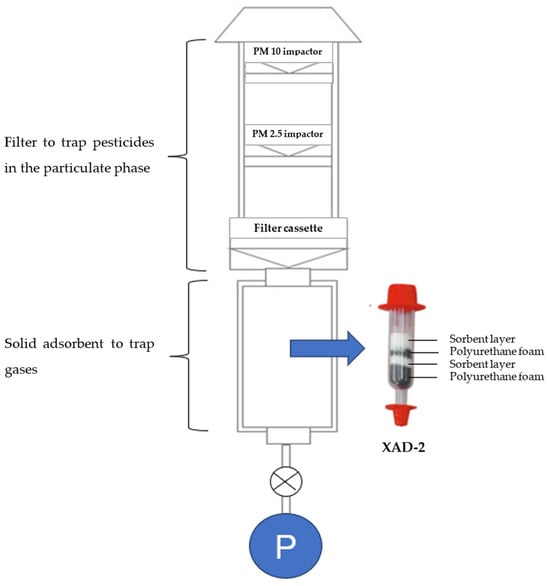
Active air sampling (AAS) is a technique that involves using a pump to collect gases, vapors, and particulates in a tube equipped with a sorbent bed or a size-selective sampler with a filter [26][27] (Figure 1). The volume of air sampled can be accurately measured using a flow meter. AAS is commonly employed for short-duration sampling, ranging from hours to days, making it suitable for monitoring daily or weekly variations [28]. However, AAS can also be utilized for long-term sampling, requiring hundreds or thousands of samples to obtain annual data. For instance, Hung et al. [29] utilized high-volume air samplers with a glass fiber filter and polyurethane foam (PUF) to monitor persistent organic pollutants (POPs) such as organochlorine pesticides and dichlorodiphenyltrichloroethanes in the Arctic region from 1993 to 2012. High-volume samplers effectively capture a higher amount of airborne pesticides compared to low-volume samplers [30]. However, the use of high-volume sampling techniques may introduce some minor errors, such as gaseous compounds being adsorbed on deposited particles or filters (blow-on), while volatile compounds may desorb from the filter (blow-off) [31]. To obtain a sufficient volume of pesticides in the air, Yusà et al. [30] suggested considering the sampling objective, sampler flow rate, and the analytical method’s limit of detection. In addition to high-volume samplers, diffusion denuder systems have been proposed for measuring semi-volatile organic compounds (SVOCs) such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), or carbonyl compounds in the atmosphere [32][33].

Figure 1. Diagram illustrating the active air sampling technique.
In terms of the filters employed in active air sampling, various materials have been used. Glass fiber filters [34] and quartz fiber filters [35] have been commonly utilized. According to Yusà et al. [30], the diameter of these filters typically ranges from 9 to 30 cm, depending on the specific sampling technique.
When it comes to adsorbents for capturing gases, several materials have been employed. Polymeric phases such as polytetrafluoroethylene membranes (PTFE) [13], Tenax TA [36], XAD®-2 resins [37], XAD-4 [38], and PUF [38] have been used. Among these, XAD®-2 resins, which are hydrophobic copolymers of styrene-divinylbenzene resin, are widely preferred due to their high efficiency in trapping various types of herbicides, fungicides, and insecticides. PUF has also been extensively applied for monitoring OCPs and other pollutants such as PCBs, polybrominated diphenyl ethers (PBDEs), PAHs, and polychlorinated dibenzo-para-dioxins (PCDDs) [30][39][40][41].
3.2. Passive Sampling Applications and Limitations
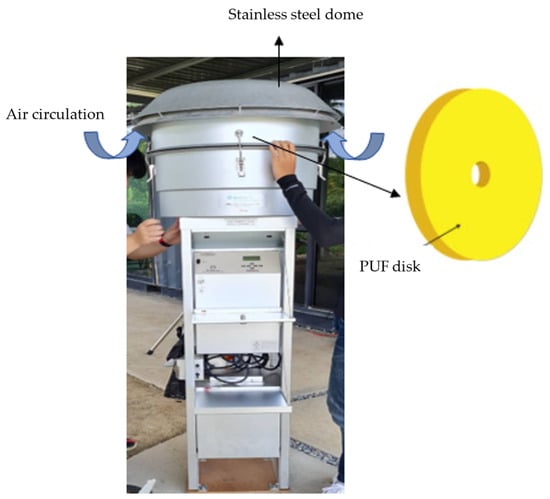
Passive air sampling (PAS) is a technique that relies on the natural diffusion of gaseous pesticides through adsorbents, eliminating the need for a pump [42]. PAS typically operates at rates below 5 m3 day−1 [28]. A passive air sampler consists of a commercial accumulating medium with a high retention capacity for the target analytes (Figure 2). The adoption of PAS has gained global popularity over active air sampling (AAS) due to several reasons. Firstly, PAS offers convenience for integrative sampling in remote locations, as it does not require electricity [43][44]. Secondly, it allows for longer sampling durations, ranging from weeks to months, enabling the representation of monthly to yearly average exposure while minimizing spikes from episodic incidents [28][30][43]. Thirdly, PAS exhibits a broader range for sampling different types of pesticides, including both persistent and less persistent ones [45].

Figure 2. Diagram illustrating the passive air sampling technique.
PAS generally employs various sorbent polymeric phases to capture pesticides. These include Tenax TA®, PUF disks, sorbent-impregnated PUF (SIP), semi-permeable membrane devices, and carbon-based foams [27][45][46][47][48][49][50]. PUF has been widely used since 2002, particularly for monitoring persistent compounds, but it has also been effective for sampling less persistent pesticides [45]. In response to the limitation of PUF in detecting glyphosate, TIEM Integrated Environmental Engineering from Germany has developed a polyester filter that effectively captures both glyphosate and aminomethylphosphonic acid in ambient air [51][52].
The choice of sorbent and housing design significantly affects the mass and type of collected particles. PUF and SIP can collect a representative portion of the particle phase, while XAD resins are suited for the gas phase [53]. PUF-based PAS has been widely utilized due to its high capacity for detecting atmospheric pollutants, simplicity in structure, cost-effectiveness, and ease of operation [48][54].
PUF disks have been employed to collect various airborne pesticides, including POPs and SVOCs such as OCPs and OPs [55][56][57][58][59][60]. Nonetheless, Hayward et al. [61] noted that while the PUF-PAS might have reached equilibrium with the atmosphere during deployment, the average air concentrations over extended periods did not significantly differ from those determined by AAS. They also suggested that for evaluating long-term air concentration trends in a cost-effective manner, utilizing fewer samples, the preferred approach would involve year-long XAD-PAS deployments. Moreover, PUF’s low effective surface area and limited reusability pose challenges, especially when using PLE at high temperatures [27]. Despite the advantages of PAS, accurately measuring the specific volume of air passing through the adsorbent during exposure remains a drawback. To address this issue, Lévy et al. [27] recommended calculating the sampling rate based on field data.
3.3. Evaluation of Sampling Techniques for Measuring Airborne Pesticides
The assessment of each factor’s impact was conducted on a scale from 1 to 4, with 1 denoting a low impact, 2 indicating a moderate low impact, 3 suggesting a moderate high impact, and 4 representing a high impact. As known, active sampling methods involve deliberately directing airflow through a sampler, leading to swift sample collection. In contrast, passive sampling relies on natural diffusion, which extends the sampling duration. Nonetheless, it is important to consider that this extended exposure can cause degradation, affecting both the chemical compounds being measured and the sorbent material itself. As a result, compound-specific degradation may introduce variability in sampler calibrations [62]. Active sampling often achieves higher precision due to controlled airflow and consistent sample collection, while the precision of passive sampling is influenced by environmental factors like wind speed, temperature, and air concentrations. Various refinements in PAS have been introduced to address and control these factors, including the calibration of sampling rates, design modifications in sampler housing, and the use of performance reference compounds. However, despite these efforts, environmental variables can still introduce bias and errors in estimated air concentrations [62][63][64].
4. Emerging Trends in Pesticide Extraction Techniques: Enhancing Efficiency and Analytical Performance
4.1. Pesticide Extraction Techniques
The extraction step plays a crucial role in separating pesticide residues after sampling. The most commonly used technique for extracting target analytes from the samplers is liquid-solid extraction, with Soxhlet extraction (SE) being the prevailing method [65]. SE involves the use of a single solvent such as acetone or dichloromethane, or binary solvents like hexane-dichloromethane, dichloromethane-light petroleum, cyclohexane-acetone, or hexane-acetone [30]. However, traditional SE has certain drawbacks, including its time-consuming nature and the potential for environmental harm due to solvent release [66].
To address the concerns associated with SE and promote more environmentally friendly extraction techniques, alternative methods have been developed. These include liquid-solid extraction (LSE) [13], PLE [30][67], UAE [68], and MAE [69]. These techniques offer advantages over traditional SE, such as reduced extraction time, decreased solvent consumption, and enhanced extraction efficiency. LSE, PLE, UAE, and MAE have emerged as more environmentally friendly alternatives, contributing to the advancement of pesticide residue extraction methods. These approaches provide researchers with options that not only are efficient but also mitigate the potential negative impacts associated with traditional SE.
LSE is a commonly used technique for extracting pesticide residues but is often time-consuming, solvent-intensive, and laborious [70]. In contrast, alternative extraction methods offer advantages such as rapidity, automation, selectivity, and reduced solvent consumption, making them more environmentally friendly options [70]. Among these alternatives, PLE, also known as accelerated solvent extraction (ASE), has gained significant attention for the extraction of airborne pesticides due to its short extraction time, low solvent consumption, high contaminant yield, improved selectivity, and user-friendly system [71][72]. PLE involves using an extracting solvent to flush a solid or semi-solid sample under intense heat (50–200 °C) and high pressures (500–3000 psi) for a short duration (around 10 min) [30][73]. The efficiency of PLE depends on critical variables such as solvent selection, pressure, temperature, flush volume, extraction time, sorbent type, and the number of extraction cycles, necessitating optimization procedures [71].
UAE, also known as sonication, is an environmentally friendly technique used as an alternative method for extracting particle pollution. This method utilizes acoustic waves to generate cavitation bubbles, which enhance the solubility of analytes and the diffusion of solvents within the matrix [68]. UAE offers several advantages, including a significant reduction in extraction time, lower solvent usage, fewer opportunities for contamination and analyte losses, and the development of eco-friendly and cost-effective methods with increased productivity [73][74][75][76].
It should be noted that UAE may be less precise than automated methods like PLE or MAE, especially when applied to matrices with strong interferences [77]. Furthermore, the sonication involved in UAE has the potential to damage sampling filters, which could result in the release of particles during the extraction process [73]. These factors should be taken into consideration when selecting the appropriate extraction method for airborne pesticide analysis. In summary, UAE offers a greener alternative for extracting pesticides from PM, providing advantages such as reduced extraction time, decreased solvent usage, and enhanced productivity. Despite its limitations in certain matrix types and the potential for filter damage, UAE has demonstrated successful applications in extracting airborne pesticides from solids and PM.
4.2. Analytical Performance of Pesticide Extraction Methods
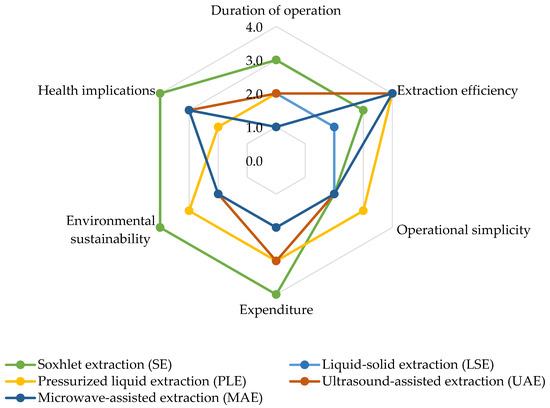
When conducting a comparative assessment of various extraction methods for analyzing airborne pesticides through the utilization of a spider chart (Figure 3), several crucial factors emerge. For instance, the operational time varies, with SE entailing time-consuming processes due to prolonged extraction cycles. In contrast, methods like PLE and MAE expedite procedures by capitalizing on elevated temperature and pressure or microwave heating. Extraction efficiency also displays variability: SE and PLE excel in efficiency, particularly for heat-sensitive compounds in the case of PLE, while UAE and MAE ensure effectiveness through improved mass transfer or swift and uniform heating. The spectrum of operational simplicity ranges from complexity with SE to relative simplicity with UAE and MAE.

Figure 3. Comparative evaluation of extraction methods for airborne pesticide analysis. Level of impact: 1 = Low impact; 2 = Middle low impact; 3 = Middle high impact; 4 = High impact.
5. Advancements in Pesticide Analytical Methods
5.1. Advances in GC for Pesticide Detection in Air
The measurement of atmospheric pesticides poses a significant challenge, particularly when dealing with concentrations below 2 ng/m3 [43]. In both AAS and PAS, an extraction process is necessary to release the trapped pesticides from the media. Two common techniques for extraction are organic solvent desorption and thermal desorption (TD). The organic solvent method involves multiple extraction and concentration steps, resulting in higher uncertainty and a more time-consuming process [43]. In contrast, TD eliminates the need for a concentration step, and it can be coupled with GC-MS to achieve lower quantification limits compared to organic solvent extraction. GC is widely utilized for pesticide measurements in combination with various element-specific detectors, such as atomic emission (AED), electron capture (ECD), sulfur chemiluminescence (SCD), nitrogen-phosphorus detection (NPD), and MS.
GC-ECD is commonly used for the analysis of OCPs in food and environmental samples due to its high separation efficiency, sensitivity, and cost-effectiveness [78][79][80]. Non-polar or semi-polar columns with dimensions of 30 m × 0.32 mm ID × 0.25 mm film thickness are typically employed, and helium is commonly used as the carrier gas [30]. However, it is important to note that GC-ECD is particularly well suited for the analysis of thermally stable compounds due to its thermal desorption capabilities. Given this consideration, Liu et al. [80] recommended using GC-negative chemical ionization-mass spectrometry (GC-NCI-MS) instead of GC-ECD for analyzing OCPs and hexabromobiphenyls (HBBs) in atmospheric PM and soil samples. They found that GC-NCI-MS provided better sensitivity and robustness compared to GC-ECD.
Nowadays, GC-MS is the predominant technique used for determining pesticides in the air, primarily due to the volatility of most compounds [81]. Capillary columns with various trade names, such as MDN-5, DB-5, TR-5MS, SGE-BPX5, or V5-MS, are commonly employed [9][15][82][83][84]. Helium gas with a purity of 99.99% or argon C50 gas with a purity of 99.99% is typically used as the carrier gas [82]. In GC-MS analysis, the major application for the MS analyzer is the use of quadrupole in selected ion monitoring (SIM) mode, which offers higher sensitivity compared to the full scan mode [30]. The MS analyzer is primarily operated in the electron ionization (EI) mode, especially in multi-residue analysis.
Pesticide quantification is based on the GC-MS peak area, and external calibration curves are generated by directly injecting analytical standards of the target pesticides. However, the use of tandem mass spectrometry (MS/MS) has recently been proposed as a valuable tool due to its increased selectivity and reduced mass spectral noise [85]. Lee and Jo [86] and Wu [87] emphasized the excellent selectivity and sensitivity provided by GC-MS/MS with a triple quadrupole (QqQ) analyzer. It can be operated in multiple reaction monitoring (SRM) mode, enabling more reliable identification and quantification of target analytes.
To ensure satisfactory sensitivity for each pesticide, the precise optimization of MS/MS variables is required. The initial step involves selecting the parent ions from each pesticide using a full scanning spectra mode. Subsequently, the precursor ions are accumulated and isolated in the ion trap, followed by fragmentation through collision-induced dissociation [30]. Among the resulting product ions for each congener, the two most prominent ones are selected. The optimization process can be carried out using either the approach of changing one factor at a time or the design of experiment procedure [88]. This optimization ensures that the MS/MS settings are fine-tuned for optimal sensitivity and the accurate identification of target pesticides.
5.2. Advances in LC for Pesticide Detection in Air
GC-MS in SIM mode and GC-MS/MS-QqQ are commonly used for GC-amenable pesticides. However, for non-GC amenable pesticides, such as polar, non-volatile, and thermolabile compounds like herbicides, carbamates, triazines, phenoxy acids, or neonicotinoids, HPLC has been proposed as an alternative method. HPLC is suitable for the separation and quantification of these pesticides, especially when they require derivatization to enhance volatility, thermal stability, and sensitivity, or to address the limitations of GC-MS methods. Despite the prevalence of GC-MS techniques, there are limited studies that have applied HPLC methods for pesticide analysis in ambient air.
5.3. Other Advances in Pesticide Detection Methods
IMS has recently gained significant prominence as a robust separation technique owing to its unique design, high sensitivity, rapid response time, operation at ambient pressure, and capability to effectively separate isomeric compounds. This versatile technique finds applications in diverse fields such as chemical weapons detection, explosives analysis, pharmaceutical screening, and environmental monitoring [89]. In IMS, gas-phase ions are generated by ionizing neutral molecules, and subsequently, they are separated based on their distinct velocities within an electric field before quantification. The separation efficiency is influenced by factors such as temperature, pressure, and the molecular properties of the drift gas.
Attenuated total reflectance–Fourier-transform infrared spectroscopy (ATR–FTIR) spectroscopy is a widely utilized technique for the chemical characterization of environmental samples [90][91]. ATR, as a sampling mode, enhances the FTIR signal obtained from the surfaces of samples, making it a promising tool for detecting organic pesticides and hazardous mineral compounds such as asbestos, which is a re-emerging contaminant in environmental matrices [14]. While ATR-FTIR offers rapid and non-invasive analysis, with improved reproducibility compared to traditional analytical methods, it has limitations in the molecular-level identification of contaminants of emerging concern. To address this challenge, a complementary spectroscopic analysis data of non-target analytes is recommended.
Synchrotron radiation–attenuated total reflectance–Fourier-transform infrared spectroscopy (SR-ATR-FTIR) represents a sophisticated analytical approach leveraging synchrotron light’s unique qualities [92][93]. Unlike traditional FTIR, it offers brightness, spatial precision, and energy versatility, with distinct advantages for airborne pesticide analysis in environmental samples. This technique requires the preliminary processing or extraction of environmental components like soil, water, or plant tissue. With SR-FTIR, the interaction between emitted infrared radiation and the sample generates molecular fingerprints. Exposure to potent synchrotron radiation enables the selective absorption of IR light by pesticide functional groups, yielding specific spectral peaks. The exceptional resolution capability of SR-ATR-FTIR facilitates microscale exploration, uncovering pesticide traces undetected by conventional methods like GC-MS and LC-MS. Spectral examination aids in identifying pesticide-specific absorption peaks, enabling the qualitative and quantitative assessment of residues using reference spectra. Overall, SR-ATR-FTIR stands as a powerful tool for intricate airborne pesticide analysis within complex environmental settings.
5.4. Analytical Performance of Pesticide Detection Techniques
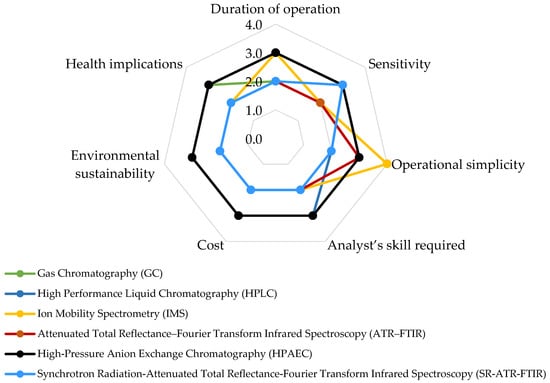
When employing a spider chart to assess various techniques for measuring airborne pesticides across different factors (Figure 4), several pivotal considerations become apparent. In terms of operational time, GC techniques exhibit variations depending on the specific method utilized (e.g., GC-MS, GC-FID). This encompasses stages such as sample preparation, injection, column separation, and detection, collectively contributing to a distinct timeframe. Similarly, the HPLC method requires several sequential processes, making it relatively time-consuming. In contrast, IMS expedites analyses due to its rapid ion mobility separation process. ATR–FTIR and SR-ATR-FTIR offer quicker analysis due to minimal sample preparation and the efficiency of FTIR techniques. Likewise, leveraging synchrotron sources for rapid scanning enables time-resolved studies, facilitating insights into pesticide degradation and transformation in the environment. HPAEC involves multiple steps similar to GC and HPLC, contributing to a moderately paced operational timeframe.

Figure 4. Comparative assessment of analytical techniques for airborne pesticide analysis. Level of impact: 1 = Low impact; 2 = Middle low impact; 3 = Middle high impact; 4 = High impact.
Regarding sensitivity, both GC and HPLC methodologies are renowned for their exceptional sensitivity, enabling the detection of trace compounds, including airborne pesticides, within complex matrices. Similarly, IMS also demonstrates sensitivity to specific compound classes, although it might not reach the same level as chromatography-based methods. ATR–FTIR recognizes functional groups and chemical bonds, albeit sensitivity toward specific pesticides may exhibit variance. HPAEC’s sensitivity extends to anions, encompassing selective pesticides and aligning with specific applications. SR-ATR-FTIR offers high sensitivity, making it suitable for detecting trace amounts of pesticide residues.
In assessing operational simplicity, GC and HPLC demand adept analysts and intricate hardware, diminishing their operational ease. IMS stands out as comparatively user-friendly, necessitating minimal analyst expertise when compared to chromatographic methods. ATR–FTIR demonstrates relative straightforwardness with minimal sample preparation and swift analysis. HPAEC introduces a degree of complexity, manageable with proper training. Meanwhile, SR-ATR-FTIR demands a certain level of skill but offers a harmonious blend of advanced capabilities and user-friendliness.
When evaluating the requisite analyst skill, GC and HPLC demand proficient analysts for meticulous method development, sample preparation, and data interpretation. IMS calls for less analyst expertise in comparison to intricate chromatographic approaches. ATR–FTIR and SR-ATR-FTIR mandate basic training for operational competence and spectral interpretation. HPAEC, on the other hand, necessitates adept analysts, proficient in method development, sample preparation, and data interpretation.
In terms of cost analysis, GC and HPLC instruments, along with their consumables and maintenance, incur moderate expenses. IMS instruments are often affordable, with generally lower operational costs in comparison to chromatography. ATR–FTIR instruments fall within a moderate cost range, with routine analyses proving cost-effective. For HPAEC, instruments and consumables hold moderate costs, justified by the technique’s specificity. On the other hand, SR-ATR-FTIR entails higher costs due to synchrotron access and equipment requirements, but its capabilities substantiate the investment.
From an environmental sustainability standpoint, GC and HPLC utilize solvents that can potentially impact the environment. However, ongoing efforts are directed towards adopting greener practices to mitigate these effects. In contrast, IMS stands out for its reduced solvent usage and minimized waste generation, which contribute to a more environmentally sustainable approach. Both ATR–FTIR and SR-ATR-FTIR require minimal reagents and generate limited waste, further reinforcing their positive environmental profile.
Turning to health implications, GC and HPLC demand the vigilant handling of solvents and analytes to mitigate potential health risks associated with hazardous substances. On the other hand, IMS involves fewer harmful chemicals, alleviating health concerns for analysts. The ATR–FTIR and SR-ATR-FTIR methods entail minimal exposure to hazardous chemicals, which significantly reduces health risks for practitioners. HPAEC necessitates the proper management of solvents, reagents, and analytes to effectively minimize potential health hazards. This comprehensive evaluation highlights the significance of considering environmental sustainability and health aspects when choosing among these analytical techniques.
References
- Kamel, F. Paths from pesticides to Parkinson’s. Science 2013, 341, 722–723.
- Mascarelli, A. Growing up with pesticides. Science 2013, 341, 740–741.
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Environmental exposure to pesticides and cancer risk in multiple human organ systems. Toxicol. Lett. 2013, 230, 157–165.
- Yan, D.; Zhang, Y.; Liu, L.; Yan, H. Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32222.
- Brühl, C.A.; Bakanov, N.; Köthe, S.; Eichler, L.; Sorg, M.; Hörren, T. Direct pesticide exposure of insects in nature conservation areas in Germany. Sci. Rep. 2021, 11, 24144.
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210.
- Amstrong, J.L.; Yost, M.G.; Fenske, R.A. Development of passive air sampler to measure airborne organophosphorus pesticides and oxygen analogs in an agricultural community. Chemosphere 2014, 111, 135–143.
- Martin, S.; Dévier, M.-H.; Cruz, J.; Duporté, G.; Barron, E.; Gaillard, J.; Le Menach, K.; Pardon, P.; Augagneur, S.; Flaud, P.-M. Passive sampling as a tool to assess atmospheric pesticide contamination related to vineyard land use. Atmosphere 2022, 13, 504.
- Raeppel, C.; Fabritius, M.; Nief, M.; Appenzeller, B.M.R.; Millet, M. Coupling ASE, sylilation and SPME–GC/MS for the analysis of current-used pesticides in atmosphere. Talanta 2014, 121, 24–29.
- Masiá, A.; Vásquez, K.; Campo, J.; Picó, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges: Application to the Túria River Basin. J. Chromatogr. 2015, 1378, 19–31.
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC-Trends Anal. Chem. 2015, 71, 100–109.
- Samsidar, A.; Siddiquee, S.; Shaarani, S. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201.
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 161, 632–639.
- El-Zahhar, A.A.; Idris, A.M.; Fawy, K.F.; Arshad, M. SEM, SEM-EDX, μ-ATR-FTIR and XRD for urban street dust characterization. Int. J. Environ. Anal. Chem. 2021, 101, 988–1006.
- López, A.; Coscollà, C.; Yusà, V.; Armenta, S.; Guardia, M.; Esteve-Turrillas, F.A. Comprehensive analysis of airborne pesticides using hard cap espresso extraction-liquid chromatography-high-resolution mass spectrometry. J. Chromatogr. A 2017, 1506, 27–36.
- Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E. Occurrence of pesticides in the atmosphere in France. Agronomie 2002, 22, 35–49.
- Van den Berg, F.; Kubiak, R.; Benjey, W.G.; Majewsjki, M.S.; Yates, S.R.; Reeves, G.L.; Smelt, J.H.; Van der Linden, A.M.A. Emission of pesticides in the air. In Fate of Pesticides in the Atmosphere, Implications for Environmental Risk Assessment; Water, Air, Soil Pollution; van Dijk, H.F.G., van Pul, W.A.J., de Voogt, P., Eds.; Kluwer Academic Publishers: Dordrecht/Boston/London, UK, 1999; Volume 115, pp. 195–218.
- Obendorf, S.K.; Lemley, A.T.; Hedge, A.; Kline, A.A.; Tan, K.; Dokuchayeva, T. Distribution of pesticide residues within homes in central New York State. Arch. Environ. Contam. Toxicol. 2006, 50, 31–44.
- Berger-Preiss, E.; Elflein, L. Determination of household insecticides in indoor air by Gas Chromatography-Mass Spectrometry. In Pesticide Protocols; Humana Press: Totowa, NJ, USA, 2006; pp. 179–190.
- Ramesh, A.; Vijayalakshmi, A. Monitoring of allethrin, deltamethrin, esbiothrin, prallethrin and transfluthrin in air during the use of household mosquito repellents. J. Environ. Monit. 2001, 3, 191–193.
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553.
- Wang, X.; Banks, A.P.W.; He, C.; Drage, D.S.; Gallen, C.L.; Li, Y.; Li, Q.; Thai, P.K.; Mueller, J.F. Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and legacy and current pesticides in indoor environment in Australia–occurrence, sources and exposure risks. Sci. Total Environ. 2019, 693, 133588.
- Reed, L.; Buchner, V.; Tchounwou, P.B. Environmental toxicology and health effects associated with hexachlorobenzene exposure. Rev. Environ. Health 2007, 22, 213–243.
- Mendes, V.; Ribeiro, C.; Delgado, I.; Peleteiro, B.; Aggerbeck, M.; Distel, E.; Annesi-Maesano, I.; Sarigiannis, D.; Ramos, E. The association between environmental exposures to chlordanes, adiposity and diabetes-related features: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 14546.
- Yoshida, T.; Mimura, M.; Sakon, N. Estimating household exposure to pyrethroids and the relative contribution of inhalation pathway in a sample of Japanese children. Environ. Sci. Pollut. Res. Int. 2021, 28, 19310–19324.
- Schummer, C.; Mothiron, E.; Appenzeller, B.M.R.; Rizet, A.L.; Wennig, R.; Millet, M. Temporal variations of concentrations of currently used pesticides in the atmosphere of Strasbourg, France. Environ. Pollut. 2010, 158, 576–584.
- Lévy, M.; Ba, H.; Pallares, C.; Pham-Huu, C.; Millet, M. Comparison and calibration of diverse passive samplers used for the air sampling of pesticides during a regional sampling monitoring campaign. Atmos. Pollut. Res. 2020, 11, 1217–1225.
- Zhang, X.; Saini, A.; Hao, C.; Harner, T. Passive air sampling and nontargeted analysis for screening POP-like chemicals in the atmosphere: Opportunities and challenges. TrAC Trends Anal. Chem. 2020, 132, 116052.
- Hung, H.; Katsoyiannis, A.A.; Brorstrom-Lunden, E.; Olafsdottir, K.; Aas, W.; Breivik, K.; Bohlin-Nizzetto, P.; Sigurdsson, A.; Hakola, H.; Bossi, R.; et al. Temporal trends of Persistent Organic Pollutants (POPs) in arctic air: 20 years of monitoring under the Arctic Monitoring and Assessment Programme (AMAP). Environ. Pollut. 2016, 217, 52–61.
- Yusà, V.; Coscollà, C.; Mellouki, W.; Pastor, A.; de la Guardia, M. Sampling and analysis of pesticides in ambient air. J. Chromatogr. A 2009, 1216, 2972–2983.
- Sauret, N.; Wortham, H.; Putaud, J.P.; Mirabel, P. Study of the effects of environmental parameters on the gas/particle partitioning of current-use pesticides in urban air. Atmos. Environ. 2008, 42, 544–553.
- Peters, A.J.; Lane, D.A.; Gundel, L.A.; Northcott, G.L.; Jones, K.C. A comparison of high volume and diffusion denuder samplers for measuring semivolatile organic compounds in the atmosphere. Environ. Sci. Technol. 2000, 34, 5001–5006.
- Feng, Y.L.; Mu, C.C.; Fu, Z.R.; Chen, Y.J. Determination of airborne dicarbonyls by HPLC analysis using annular denuder/filter system coated with 2,4-dinitrophenylhydrazine. Chin. J. Anal. Chem. 2011, 39, 1653–1658.
- Yao, Y.; Tuduri, L.; Blanchard, P.; Harner, P.; Waite, D.; Poissantd, L.; Murphy, C.; Belzerf, W.; Aulagnierd, F.; Li, F.; et al. Spatial and temporal distribution of pesticide in air concentrations in Canadian agricultural regions. Atmos. Environ. 2006, 40, 4339–4351.
- Alegria, H.; Bidleman, T.F.; Figueroa, M.S. Organochlorine pesticides in the ambient air of Chiapas, Mexico. Environ. Pollut. 2006, 140, 483–491.
- Clément, M.; Arzel, S.; Le Bot, B.; Seux, R.; Millet, M. Adsorption/thermal desorption-GC/MS for the analysis of pesticides in the atmosphere. Chemosphere 2000, 40, 49–56.
- Al-Alam, J.; Lévy, M.; Ba, H.; Pham-Huu, C.; Millet, M. Measuring current-use pesticides in air: A comparison of silicon carbide foam to XAD as passive air samplers. Environ. Technol. Innov. 2021, 24, 101876.
- Pellicer-Castell, E.; Belenguer-Sapiña, C.; Amorós, P.; El Haskouri, J.; Herrero-Martínez, J.M.; Mauri-Aucejo, A.R. Comparison of silica-based materials for organophosphorus pesticides sampling and occupational risk assessment. Anal. Chim. Acta 2020, 1110, 26–34.
- Mari, M.; Harrison, R.M.; Schuhmacher, M.; Domingo, J.L.; Pongpiachan, S. Inferences over the sources and processes affecting polycyclic aromatic hydrocarbons in the atmosphere derived from measured data. Sci. Total Environ. 2010, 408, 2387–2393.
- Pongpiachan, S.; Ho, K.F.; Lee, S.C. A study of gas-particle partitioning of PAH according to adsorptive models and season. In Air Pollution Xviii; Wit Press: Southampton, UK, 2010; pp. 37–48.
- Pongpiachan, S.; Wiriwutikorn, T.; Rungruang, C.; Yodden, K.; Duangdee, N.; Sbrilli, A.; Gobbi, M.; Centeno, C. Impacts of micro-emulsion system on polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) reduction from industrial boilers. Fuel 2016, 172, 58–64.
- Lévy, M.; Al-Alam, J.; Ridacker, C.; Massemin, S.; Millet, M. Use of XAD®-2 passive air samplers for monitoring environmental trends of PAHs, PCBs and pesticides in three different sites in Strasbourg and its vicinity (east of France). Atmos. Environ. 2018, 195, 12–23.
- Decuq, C.; Bourdat-Deschamps, M.; Benoit, P.; Bertrand, C.; Benabdallah, R.; Esnault, B.; Durand, B.; Loubet, B.; Fritsch, C.; Pelosi, C.; et al. A multiresidue analytical method on air and rainwater for assessing pesticide atmospheric contamination in untreated areas. Sci. Total Environ. 2022, 823, 153582.
- Namiesnik, J.; Zabiegala, B.; Kot-Wasik, A.; Partyka, M.; Wasik, A. Passive sampling and/or/extraction techniques in environmental analysis, a review. Anal. Bioanal. Chem. 2005, 381, 279–301.
- Gamboa, C.L.; Diaz, S.K.; Ruepert, C.; van Wendel de Joode, B. Passive monitoring techniques to evaluate environmental pesticide exposure: Results from the Infant’s Environmental Health study (ISA). Environmetal Res. 2020, 184, 109243.
- Raeppel, C.; Appenzeller, B.M.; Millet, M. Determination of seven pyrethroids biocides and their synergist in indoor air by thermal-desorption gas chromatography/mass spectrometry after sampling on Tenax TA® passive tubes. Talanta 2015, 131, 309–314.
- Al-Alam, J.; Lévy, M.; Ba, H.; Pham-Huu, C.; Millet, M. Passive air samplers based on ceramic adsorbent for monitoring of organochlorine pesticides, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in outdoor air. Environ. Technol. Innov. 2020, 20, 11094.
- Chaemfa, C.; Barber, J.L.; Gocht, T.; Harner, T.; Holoubek, I.; Klanova, J.; Jones, K.C. Field calibration of polyurethane foam (PUF) disk passive air samplers for PCBs and OC pesticides. Environ. Pollut. 2008, 156, 1290–1297.
- Koblizkova, M.; Lee, S.C.; Harner, T. Sorbent impregnated polyurethane foam disk passive air samplers for investigating current-use pesticides at the global scale. Atmos. Pollut. Res. 2012, 3, 456–462.
- Galon, L.; Bragagnolo, L.; Korf, E.P. Mobility and environmental monitoring of pesticides in the atmosphere—A review. Environ. Sci. Pollut. Res. 2021, 28, 32236–32255.
- Morshed, M.; Omar, R.D.; Mohamad, S.; Abd, S.W. Determination of glyphosate through passive and active sampling methods in a treated field atmosphere. Afr. J. Agric. Res. 2011, 6, 4010–4018.
- Kruse-Plaß, M.; Hofmann, F.; Wosniok, W.; Schlechtriemen, U.; Kohlschütter, N. Pesticides and pesticide-related products in ambient air in Germany. Environ. Sci. Eur. 2021, 33, 1–21.
- Lai, F.Y.; Rauert, C.; Gobelius, L.; Ahrens, L. A critical review on passive sampling in air and water for per- and polyfluoroalkyl substances (PFASs). TrAC Trends Anal. Chem. 2019, 121, 115311.
- Lee, S.C.; Harner, T.; Pozo, K.; Shoeib, M.; Wania, F.; Muir, D.C.G.; Barrie, L.A.; Jones, K.C. Polychlorinated naphthalenes in the global atmospheric passive sampling (GAPS) study. Environ. Sci. Technol. 2007, 41, 2680–2687.
- Strandberg, B.; Österman, C.; Koca Akdeva, H.; Moldanová, J.; Langer, S. The use of Polyurethane Foam (PUF) passive air samplers in exposure studies to PAHs in Swedish seafarers polycyclic aromatic compounds. TrAC-Trends Anal. Chem. 2022, 42, 448–459.
- Alani, R.; Zhao, S.; Liu, X.; Akinrinade, O.; Agunbiade, F.; Ayejuyo, O.; Zhang, G. Concentrations, profiles and exposure risks of polycyclic aromatic hydrocarbons (PAHs) in passive air samples from Lagos, Nigeria. Atmos. Pollut. Res. 2021, 12, 101162.
- Vuong, Q.T.; Kim, S.J.; Nguyen, T.N.T.; Thang, P.Q.; Lee, S.J.; Ohura, T.; Choi, S.D. Passive air sampling of halogenated polycyclic aromatic hydrocarbons in the largest industrial city in Korea: Spatial distributions and source identification. J. Hazard. Mater. 2020, 382, 121238.
- Pozo, K.; Harner, T.; Lee, S.C.; Sinha, R.K.; Sengupta, B.; Loewen, M.; Geethalakshmi, V.; Kannan, K.; Volpi, V. Assessing seasonal and spatial trends of persistent organic pollutants (POPs) in Indian agricultural regions using PUF disk passive air samplers. Environ. Pollut. 2011, 159, 646–653.
- Chakraborty, P.; Zhang, G.; Cheng, H.; Balasubramanian, P.; Li, J.; Jones, K.C. Passive air sampling of polybrominated diphenyl ethers in New Delhi, Kolkata, Mumbai and Chennai: Levels, homologous profiling and source apportionment. Environ. Pollut. 2017, 231, 1181–1187.
- Odabasi, M.; Bayram, A.; Elbir, T.; Dumanoglu, Y.; Kara, M.; Altiok, H.; Cetin, B. Investigation of seasonal variations and sources of atmospheric polychlorinated naphthalenes (PCNs) in an urban area. Atmos. Pollut. Res. 2012, 3, 477–484.
- Hayward, S.J.; Gouin, T.; Wania, F. Comparison of four active and passive sampling techniques for pesticides in air. Environ. Sci. Technol. 2010, 44, 3410–3416.
- Melymuk, L.; Bohlin, P.; Sáňka, O.; Pozo, K.; Klánová, J. Current challenges in air sampling of semivolatile organic contaminants: Sampling artifacts and their influence on data comparability. Environ. Sci. Technol. 2014, 48, 14077–14091.
- Tuduri, L.; Harner, T.; Hung, H. Polyurethane foam (PUF) disks passive air samplers: Wind effect on sampling rates. Environ. Pollut. 2006, 144, 377–383.
- Zhang, X.; Brown, T.N.; Ansari, A.; Yeun, B.; Kitaoka, K.; Kondo, A.; Lei, Y.D.; Wania, F. Effect of wind on the chemical uptake kinetics of a passive air sampler. Environ. Sci. Technol. 2013, 47, 7868–7875.
- Russo, M.V.; Avino, P.; Cinelli, G.; Notardonato, I. Sampling of organophosphorus pesticides at trace levels in the atmosphere using XAD-2 adsorbent and analysis by gas chromatography coupled with nitrogen-phosphorus and ion trap mass spectrometry detectors. Anal. Bioanal. Chem. 2012, 404, 1517–1527.
- Adou, K.; Bontoyan, W.R.; Sweeney, P.J. Multiresidue method for the analysis of pesticide residues in fruits and vegetables by accelerated solvent xxtraction and capillary gas chromatography. J. Agric. Food Chem. 2001, 49, 4153–4160.
- Rodrigues, A.; Delhomme, O.; Millet, M. Use of PLE-ATD-GC/MSMS for the quantification of airborne pesticides in active and passive samples and in dust. J. Chromatogr. Sci. 2023, bmad020.
- Beristain-Montiel, E.; Villalobos-Pietrini, R.; Arias-Loaiza, G.E.; Gómez-Arroyo, S.L.; Amador-Muñoz, O. An innovative ultrasound assisted extraction micro-scale cell combined with gas chromatography/mass spectrometry in negative chemical ionization to determine persistent organic pollutants in air particulate matter. J. Chromatogr. A 2016, 1477, 100–107.
- Yusà, V.; Coscollà, C.; Millet, M. New screening approach for risk assessment of pesticides in ambient air. Atmos. Environ. 2014, 96, 322–330.
- Lesueur, C.; Gartner, M.; Mentler, A.; Fuerhacker, M. Comparison of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography–mass spectrometry and liquid chromatography–ion trap–mass spectrometry. Talanta 2008, 75, 284–293.
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721.
- Kim, I.; Lee, S.; Kim, S.D. Determination of toxic organic pollutants in fine particulate matter using selective pressurized liquid extraction and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1590, 39–46.
- Galmiche, M.; Delhomme, O.; François, Y.N.; Millet, M. Environmental analysis of polar and non-polar Polycyclic Aromatic Compounds in airborne particulate matter, settled dust and soot: Part I: Sampling and sample preparation. TrAC Trends Anal. Chem. 2021, 134, 116099.
- Aydin, M.E.; Ozcan, S.; Tor, A. Ultrasonic solvent extraction of persistent organic pollutants from airborne particles. Clean 2007, 35, 660–668.
- Bendicho, C.; De La Calle, I.; Pena, F.; Costas, M.; Cabaleiro, N.; Lavilla, I. Ultrasound-assisted pretreatment of solid samples in the context of green analytical chemistry. TrAC Trends Anal. Chem. 2012, 31, 50–60.
- Nascimento, M.M.; da Rocha, G.O.; de Andrade, J.B. Pesticides in fine airborne particles: From a green analysis method to atmospheric characterization and risk assessment. Sci. Rep. 2017, 7, 2267.
- Jonker, M.T.O.; Koelmans, A.A. Extraction of polycyclic aromatic hydrocarbons from soot and sediment: Solvent evaluation and implications for sorption mechanism. Environ. Sci. Technol. 2002, 36, 4107–4113.
- Garrido Frenich, A.; Martínez Vidal, J.L.; Moreno Frías, M. Determination of organochlorine pesticides by GC-ECD and GC-MS-MS techniques including an evaluation of the uncertainty associated with the results. Chromatographia 2003, 57, 213–220.
- Wang, G.L.; Ma, L.M.; Sun, J.H.; Zhang, G. Occurrence and distribution of organochlorine pesticides (DDT and HCH) in sediments from the middle and lower reaches of the Yellow River, China. Environ. Monit. Assess. 2010, 168, 511–521.
- Liu, Y.; Fu, X.; Tao, S.; Liu, L.; Li, W.; Meng, B. Comparison and analysis of organochlorine pesticides and hexabromobiphenyls in environmental samples by gas chromatography-electron capture detector and gas chromatography-mass spectrometry. J. Chromatogr. Sci. 2015, 53, 197–203.
- Maceira, A.; Marcé, R.M.; Borrull, F. Analytical methods for determining organic compounds present in the particulate matter from outdoor air. TrAC Trends Anal. Chem. 2019, 122, 115707.
- Coscollà, C.; Castillo, M.; Pastor, A.; Yusà, V. Determination of 40 currently used pesticides in airborne particulate matter (PM 10) by microwave-assisted extraction and gas chromatography coupled to triple quadrupole mass spectrometry. Anal. Chim. Acta 2011, 693, 72–81.
- Katzman, D.; Bohbot-Raviv, Y.; Dubowski, Y. Does polyacrylamide-based adjuvant actually reduce primary drift of airborne pesticides? Sci. Total Environ. 2021, 775, 145816.
- Kazos, E.A.; Stalikas, C.D.; Nanos, C.G.; Konidari, C.N. Determination of dithiocarbamate fungicide propineb and its main metabolite propylenethiourea in airborne samples. Chemosphere 2007, 68, 2104–2110.
- Zhang, F.; Yu, C.; Wang, W.; Fan, R.; Zhang, Z.; Guo, Y. Rapid simultaneous screening and identification of multiple pesticide residues in vegetables. Anal. Chim. Acta 2012, 757, 39–47.
- Lee, K.G.; Jo, E.K. Multiresidue pesticide analysis in Korean ginseng by gas chromatography–triple quadrupole tandem mass spectrometry. Food Chem. 2012, 134, 2497–2503.
- Wu, C.C. Multiresidue method for the determination of pesticides in Oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 580–587.
- Yusà, V.; Pastor, A.; de la Guardia, M. Microwave-assisted extraction of polybrominated diphenyl ethers and polychlorinated naphthalenes concentrated on semipermeable membrane devices. Anal. Chim. Acta 2006, 565, 103–111.
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123.
- Mathew, M.L.; Gopalakrishnan, A.; Aravindakumar, C.T.; Aravind, U.K. Low—Cost multilayered green fiber for the treatment of textile industry waste water. J. Hazard. Mater 2019, 365, 297–305.
- Obinaju, B.E.; Martin, F.L. ATR-FTIR spectroscopy reveals polycyclic aromatic hydrocarbon contamination despite relatively pristine site characteristics: Results of a field study in the Niger Delta. Environ. Int. 2016, 89–90, 93–101.
- Pongpiachan, S.; Thumanu, K.; Chantharakhon, C.; Phoomalee, C.; Tharasawatpipat, C.; Apiratikul, R.; Poshyachinda, S. Applying synchrotron radiation-based attenuated total reflection-Fourier transform infrared to evaluate the effects of shipping emissions on fluctuations of PM10-bound organic functional groups and ionic species. Atmos. Pollut. Res. 2022, 13, 101517.
- Yu, P. Application of advanced synchrotron radiation-based Fourier transform infrared (SR-FTIR) microspectroscopy to animal nutrition and feed science: A novel approach. Br. J. Nutr. 2004, 92, 869–885.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
24 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





