Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beata Paczosa-Bator | -- | 2313 | 2023-11-22 07:17:20 | | | |
| 2 | Catherine Yang | Meta information modification | 2313 | 2023-11-22 07:43:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lenar, N.; Piech, R.; Wardak, C.; Paczosa-Bator, B. Metal Oxide Nanoparticles in Potentiometric Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/51886 (accessed on 12 January 2026).
Lenar N, Piech R, Wardak C, Paczosa-Bator B. Metal Oxide Nanoparticles in Potentiometric Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/51886. Accessed January 12, 2026.
Lenar, Nikola, Robert Piech, Cecylia Wardak, Beata Paczosa-Bator. "Metal Oxide Nanoparticles in Potentiometric Sensors" Encyclopedia, https://encyclopedia.pub/entry/51886 (accessed January 12, 2026).
Lenar, N., Piech, R., Wardak, C., & Paczosa-Bator, B. (2023, November 22). Metal Oxide Nanoparticles in Potentiometric Sensors. In Encyclopedia. https://encyclopedia.pub/entry/51886
Lenar, Nikola, et al. "Metal Oxide Nanoparticles in Potentiometric Sensors." Encyclopedia. Web. 22 November, 2023.
Copy Citation
Metal oxides, because of their remarkable properties including high electrical capacity and mixed ion-electron conductivity, have found applications as both sensing layers (e.g., of screen-printing pH sensors) or solid-contact layers and paste components in solid-contact and paste-ion-selective electrodes.
metal oxides
constructions of sensors
solid-contact electrodes
1. Metal Oxides
1.1. Characterization of Metal Oxides as Electrode Materials
One of the most known constructions of a potentiometric sensor is the solid-contact electrode. When choosing the material for solid-contact layers in potentiometric sensors, some key aspects must be taken into careful consideration.
Firstly, the material must enable an efficient ion-electron exchange process at the electrode | ion-selective membrane interface. Those processes are associated with either high redox capacitance or high double-layer capacitance. Redox capacitance is associated with the electron and ionic conductivity of materials in which exchange processes occur through reversible redox reactions, while double-layer capacitance surface development results from the nanometric size of particles or pores in the material structure.
Due to the nanometric size of its particles, nanostructured metal oxides are characterized by a high surface area. Besides its favorable morphology, some metal oxide nanomaterials such as ruthenium or iridium dioxide exhibit excellent redox properties. One of the mechanisms proposed by Fog et al. in [1] is that in contact with hydrogen ions, the oxide undergoes a redox reaction in which ions and protons are exchanged. This mechanism decides the ability of the oxide to transduce both ions and electrons through the layer, which simplifies the processes on the interface between the ion-selective membrane and the electronic conductor. The combination of those two properties—high surface area and high redox capacitance [2]—in one material makes it a great choice for the solid-contact layer.
In addition to the basic criterion of the presence of mixed ion-electron conductivity and high electrical capacitance, materials for solid-contact layers must meet a number of other requirements, including high hydrophobicity, chemical stability of the material, and the absence of side reactions occurring during the electron-ion exchange process [3][4][5][6].
The wettability of the solid-contact material is of particular importance in the context of the formation of an undesirable aqueous layer between the ion-selective membrane and the electron conductor. In the literature, hydrophilic materials (characterized by low values of the wetting angle below 90°) introduced into the electrode design did not prevent the formation of a water layer, unlike hydrophobic materials (with wetting angles above 90°), which have been repeatedly proven to protect sensors from the unwanted water layer and susceptibility to drop off of the ion-selective membrane [7][8][9]. Metal oxides, especially hydrous forms of oxides, are characterized by low wetting angle values and are defined as a hydrophilic material. Besides this property, even after prolonged use and conditioning of the electrodes in aqueous solutions, an aqueous layer did not form. This may be due to a specific reaction occurring on the surface of the ruthenium oxide layer [1] and/or large surface development that conditions the solid-contact layer to adhere strongly to the membrane, preventing it from peeling off the electrode surface.
The opposite requirements concerning wettability relate to electrodes without an ion-selective membrane. In this construction of an electrode, the metal oxide nanoparticle layer contacts the analyzed fluid and must be easily wettable by aqueous solutions. Taking this into account, hydrophilic metal oxides with contact angles below 30 degrees are perfect for the sensing layer in potentiometric sensors for pH measurement.
In the context of the design of paste electrodes, the material used as a paste should meet certain requirements. These include providing a quick and reversible transition from ionic to electronic conductivity and having a nonpolarizable surface between the paste and the high-capacity red-ox or double-layer ISM. In addition, the paste should be hydrophobic and characterized by low water absorption, which prevents the formation of a water layer. Moreover, the materials for the paste should be readily available and non-toxic. Similar to the case of solid-contact electrodes, metal oxides were proven to be great materials for paste electrode modification and production [10].
In addition, metal oxides exhibit a combination of unique properties, such as high thermal and chemical stability and low resistivity [11].
1.2. The Action Mechanism of Metal Oxides
The action mechanism is described based on the behavior of ruthenium oxide after contacting the electrolyte. According to the reaction mechanism proposed by Fog and Buck [1], ruthenium oxide in contact with hydrogen ions undergoes redox reactions, in which both ions and charged elementary parts are exchanged. The action mechanism of metal oxides is based on the redox reactions. These processes are initiated by electrons (e−) and protons (H+) introduced to the surface of the dioxide from the electrolyte, as presented in the following reaction [12]:
RuO2 + xH + xe = RuO2 − x(OH)x, where 0 ≤ x ≤ 2
This mechanism is used indirectly when designing sensors for pH determination with metal oxides acting both as a sensing layer and as a solid-contact layer.
In the literature [13], it was noted that the insertion of other cations, for example, potassium ions, into the porous dioxide layer also participates in the mentioned mechanism. As presented by Wen et al. [14], monovalent cations may act as hydrogen ions in the presented reaction and, as protons, lead to faradaic reactions. This feature was used when designing potentiometric sensors with an ion-selective membrane based on metal oxides for potassium determination [15].
The schematic representation of the charge storage mechanism that occurs via the proton-electron transfer for the metal oxide (MOx) layer is presented in Figure 1.
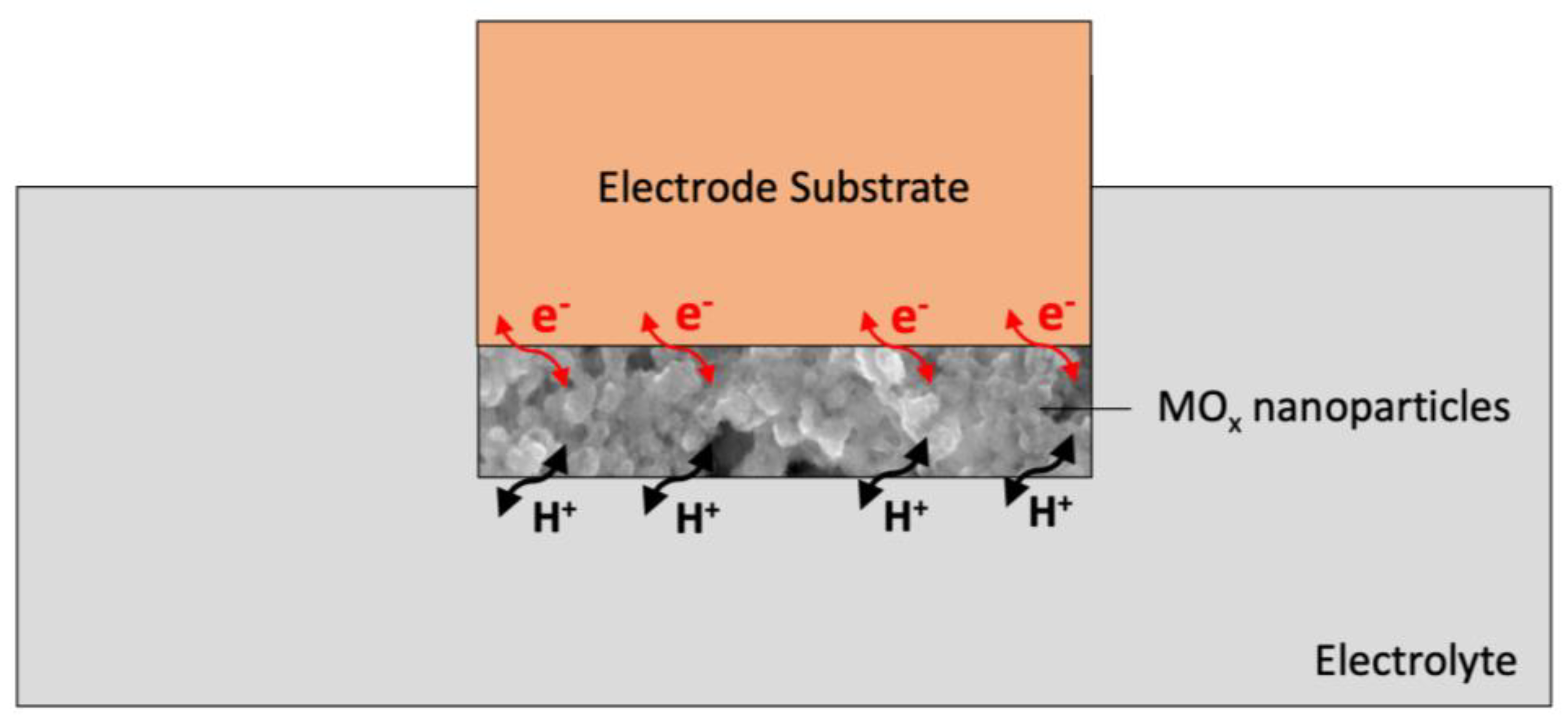
Figure 1. Mechanism of ion-electron exchange at phase boundaries using a metal oxide (MOx) layer.
2. Metal Oxide Nanoparticles as a Sensing Material
Metal oxides as sensing materials are mostly used in pH sensors. Historically, the use of metal oxides in potentiometric sensors began in the 1980s when Fog and Buck introduced them for the first time as a sensing material for pH detection [1]. Some of the metal oxides were successfully applied as pH sensor materials and showed a near-Nernstian response in the pH range of 2–12. This research was followed by many publications by scientists searching for a replacement for the glass-membrane electrode.
Among the oxides used for the fabrication of pH sensors, the most commonly known and used are ruthenium oxide RuO2 and iridium oxide IrO2, characterized by remarkable sensitivity to hydrogen ions and high accuracy. Furthermore, the researchers can distinguish tantalum(V) oxide Ta2O5, titanium dioxide TiO2, tin(IV) oxide SnO2, cerium dioxide CeO2, tungsten trioxide WO3, lead dioxide PbO2, and mixed oxides: Bi2O3-Nb2O5, IrO2-TiO2, and RuO2-TiO2 for pH sensor applications. In addition to this, some work has been conducted in the area of metal/MOx-based pH sensors such as those based on antimony and bismuth. In these couples, the redox equilibrium between the metal/MOx phases (e.g., Sb-Sb2O3) is attributed to the origin of their pH sensitivity [16][17][18]. The sensitivity of the MOx pH sensor significantly depends on the type of material composition and the deposition method since both factors can influence the microstructure, porosity, surface homogeneity, and crystalline structure of the material. This leads to variation in the sensitivity of the electrodes. The sensitivity of the pH sensors is expressed in terms of the Nernstian response (the sensor is expected to show 59.14 mV/pH at 25 °C derived from the Nernst equation). Table 1 presents the comparison of analytical parameters of MOx-based sensors such as pH measurement range, slope of calibration curve, and time of response.
Table 1. pH sensors based on metal oxides—metal oxides as sensing materials.
| Material | pH Range | Slope [mV/pH] | Response Time | Reference |
|---|---|---|---|---|
| RuO2 | 1–13 | 55.64 | - | [19] |
| RuO2-TiO2 | 2–12 | 56.03 | - | [20] |
| RuO2-Ni | 1.5–12.5 | 52 | - | [21] |
| RuO2-NT | 2–12 | 55 | <40 s | [22] |
| RuO2-Ta2O5 | 2–12 | 56 | <8 s | [23] |
| RuO2-SnO2 | 2–12 | 56.5 | <5 s | [24] |
| IrO2 | 2–10 | 63.5 | ~1 min | [25] |
| IrO2-TiO2 | 1–13 | 59.1 | 120 s | [26] |
| Ta2O5 | 2–12 | 58–59 | <0.3 s | [27] |
| SnO2 | 2–12 | 58.1 | - | [28] |
| TiO2 | 1–11 | 58.73 | - | [29] |
| WO3 | 1–7 | 44.85 | - | [30] |
| WO3-NT | 2–12 | 41 | <90 s | [31] |
| CeO2 | 7.2–10.8 | 38 | - | [32] |
| PbO2 | 1.5–12.5 | 64.82 | <1 s | [33] |
| MnO2 | 2–12 | 78.3 | few seconds | [34] |
| CoO2 | 1–12 | 54.9 | <1 min | [35] |
List of abbreviations: NT—nanotubes, IrO2—iridium dioxide, RuO2—ruthenium dioxide, MnO2—manganium dioxide, CeO2—cerium dioxide, TiO2—titanium dioxide, Ta2O5—tantalum(V) oxide, SnO2—tin(IV) oxide, WO3—tungsten trioxide, PbO2—lead dioxide, CoO2—cobalt(II) oxide.
3. Metal Oxide Nanoparticles as Solid-Contact Layers
One of the observed trends in the development of potentiometric sensors is the search for new functional materials for the construction of solid-contact electrodes. These electrodes are characterized by sandwich-like construction with a solid-contact layer between the substrate electrode and the ion-selective membrane. The properties of the solid-contact layer directly translate into the properties of the sensors, allowing them to achieve lower and lower limits of determination, higher potential stability, and lower response times.
As already mentioned, one of the trends in modern potentiometry is the search for new materials for solid-contact layers, which, because of their properties, will improve the analytical and performance of solid-contact ion-selective electrodes. Since the 1990s, when conductive polymers were first introduced as a solid-contact layer, up to the present day, various types of materials have been introduced into electrode design with varying results [2][36].
A group of metal oxides can be classified as high redox capacity and high double-layer capacity materials, as they exhibit both high redox sensitivity and high surface area [1]. The implementation of metal oxide macro/nanomaterials as solid-contact layers, to support the ion-to-electron transduction processes, relies on two strategies: High specific surface area and high redox activity. Some of the tested metal oxides also appeared to meet all the other requirements for solid-contact layers; hence, they were considered promising materials for solid-contact electrode design.
The mechanism of the selective response to hydrogen ions, described and applied by Fog and Buck [1], was later implemented by many research groups in the design of ion-selective electrodes with solid-contact layers based on metal oxide.
The first implementation of metal oxide in membrane-based potentiometric sensors dates back to 2013 when the Khun group introduced ZnO nanorods as solid-contact material for Cr2+-ISEs and ZnO nanotubes in I-ISEs [37]. The hollow structures of ZnO nanotubes can provide a larger surface area than ZnO nanorods. Later, the same group presented the use of CuO nanoflowers in Cd2+-ISEs [38]. These electrodes exhibit a very advantageous LOD (level of detection) at the nanomolar level. In other studies, the Qin group proposed MoO2 microspheres as an ion-to-electron transducer (2017) [39]. Compared to the coated-disc electrodes, the introduction of MoO2 microspheres improves the sensor performance to some extent as a result of the enhanced double-layer capacitance. In 2019, Yang et al. manufactured solid-contact K+-ISEs based on MnO2 nanosheets for the determination of K+ in the blood [40]. The MnO2 nanosheets turned out to have a wrinkled morphology, thereby preventing their dense accumulation and maintaining the advantage of a large surface area, which is advantageous for achieving a large double-layer capacitance. Also in 2019, the Paczosa-Bator group introduced hydrous RuO2 nanoparticles into solid-contact H+—and K+-ISEs as ion-to-electron transducers. Remarkably, these RuO2 nanoparticle-based sensors exhibit a large redox capacitance at the millifarad level. Other oxides introduced by the Paczosa-Bator group in the construction of ion-selective electrodes were hydrous IrO2 (2021) [41], and a year later, hydrous CeO2 (2022) [42]. In 2022, Wardak’s group presented a paper on the use of ZnO, CuO, and Fe2O3 in potassium-sensitive electrodes [43].
Metal Oxide Nanoparticles-Based Hybrid Materials as Solid-Contact Layers
Metal oxides were also used as components of hybrid materials with remarkable success. In 2019, Zeng et al. introduced TiO2-PANI material into the Pb2+- selective electrodes. This material possessed a large specific capacitance, thereby effectively promoting ion-to-electron transduction and enhancing the potential response stability of electrodes [44].
Later, the Paczosa-Bator group presented composite materials containing RuO2 nanoparticles and conducting polymers POT (RuO2–POT) [45] and PEDOT:PSS [46] and composite material containing ruthenium dioxide and carbon nanomaterials [47]. All the mentioned hybrids were applied as solid-contact layers in K+- selective electrodes.
The RuO2–POT composite exhibits superhydrophobic properties (a contact angle of 149°). The results of electrochemical techniques demonstrate that these solid-contact electrodes possess a large capacitance of approximately 1.2 mF, which is much larger than that of the coated-disc electrode. Compared to single-component counterparts, K+-ISE based on the RuO2-POT composite exhibits a shorter equilibration time and better potential stability and reproducibility.
The RuO2-PEDOT:PSS composite allowed the acquisition of electrodes with an electrical capacitance of as much as 7 mF. It is worth mentioning that the value of 7.2 mF is one of the highest values presented in the literature for this type of electrode.
The addition of graphene, the representative of the carbon materials group, to ruthenium dioxide allowed the capacitance to increase to 2.6 mF, which is 2.5 times higher compared to the oxide alone.
In 2021, Su et al. designed electrodes with MoS2-Fe3O4 as an ion-electron transduction layer for the determination of serum potassium [48]. These sensors present high performance in potential reproducibility and long-term stability.
In 2022, Lenar et al. presented a composite material based on iridium dioxide, creating a hybrid material of not two but three different components. The layer of IrO2-POT-MWCNT turned out to be superhydrophobic with a contact angle of 178°, which resulted in great stability in potentiometric response and long lifetime. This layer was introduced in K+ [49] and H+ [50] selective electrodes.
A year later, the group of Wardak presented the CuO-MWCNT material, applied as a solid-contact layer in Cu2+-selective electrodes [51]. These electrodes were characterized by a low detection limit (1.5 × 10–8 mol L–1) and very good potential stability.
4. Metal Oxides Nanoparticles in Paste Electrodes
Both metal oxides and their composites were used as a component in the paste electrodes. Niemiec et al. designed carbon black paste electrodes modified with poly(3-octylthiophene-2,5-diyl) and transition metal oxides: Ruthenium and iridium dioxide [12].
In this research, electrodes with carbon paste based on carbon black and modified with hydrated transition metal oxides, namely hydrous ruthenium dioxide and hydrous iridium dioxide, were presented. The paste with hydrous ruthenium dioxide, characterized by the best capacitance (470 μF), was extra modified by poly(3-octylthiophene-2,5-diyl). This modification results in a PVC-based electrode with the highest capacitance (130 μF) and, consequently, the lowest drift of the potentiometric response. The developed electrodes can be used to determine nitrate ions in liquid samples and soil samples [12].
References
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146.
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26.
- Nikolskii, B.P.; Materova, E. Solid contact in membrane ion-selective electrodes. Ion. Select. Electr. Rev. 1985, 7, 3–39.
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937.
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. TrAC-Trends Anal. Chem. 2013, 51, 79–86.
- De Marco, R.; Veder, J.-P.; Clarke, G.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Evidence of a water layer in solid-contact polymeric ion sensors. Phys. Chem. Chem. Phys. 2008, 10, 73–76.
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; De Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion- selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292.
- Papp, S.; Bojtár, M.; Gyurcsányi, R.E.; Lindfors, T. Potential Reproducibility of Potassium-Selective Electrodes Having Perfluorinated Alkanoate Side Chain Functionalized Poly(3,4-ethylenedioxytiophene) as a Hydrophobic Solid Contact. Anal. Chem. 2019, 91, 9111–9118.
- Guzinski, M.; Jarvis, J.M.; D’Orazio, P.; Izadyar, A.; Pendley, B.D.; Lindner, E. Solid-Contact pH Sensor without CO2 Interference with a Superhydrophobic PEDOT-C14 as Solid Contact: The Ultimate “Water Layer” Test. Anal. Chem. 2017, 89, 8468–8475.
- Niemiec, B.; Piech, R.; Paczosa-Bator, B. All-Solid-State Carbon Black Paste Electrodes Modified by Poly(3-octylthiophene-2,5-diyl) and Transition Metal Oxides for Determination of Nitrate Ions. Molecules 2023, 28, 4313.
- Prataap, R.K.V.; Arunachalam, R.; Pavul Raj, R.; Mohan, S.; Peter, L. Effect of electrodeposition modes on ruthenium oxide electrodes for supercapacitors. Curr. Appl. Phys. 2018, 18, 1143–1148.
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614.
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications. ChemElectroChem 2019, 6, 4343–4372.
- Wen, S.; Lee, J.; Yeo, I.; Park, J.; Mho, S. The role of cations of the electrolyte for the pseudocapacitive behavior of metal oxide electrodes, MnO2 and RuO2. Electrochim. Acta 2004, 50, 849–855.
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Ruthenium Dioxide as High-Capacitance Solid-Contact Layer in K+-Selective Electrodes Based on Polymer Membrane. J. Electrochem. Soc. 2019, 166, B1470–B1476.
- Edwall, G. Improved antimony-antimony (III) oxide pH electrodes. Med. Biol. Eng. Comput. 1978, 16, 661–669.
- Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955–4985.
- Głąb, S.; Hulanicki, A.; Edwall, G.; Folke, F.; Ingman, I.; Koch, W.F. Metal-metal oxide and metal oxide electrodes as pH sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47.
- Liao, Y.H.; Chou, J.C. Preparation and characteristics of ruthenium dioxide for pH array sensors with real-time measurement system. Sens. Actuators B Chem. 2008, 128, 603–612.
- Pocrifka, L.A.; Gonçalves, C.; Grossi, P.; Colpa, P.C.; Pereira, E.C. Development of RuO2–TiO2 (70–30) mol% for pH measurements. Sens. Actuators B Chem. 2006, 113, 1012–1016.
- da Silva, G.M.; Lemos, S.G.; Pocrifka, L.A.; Marreto, P.D.; Rosario, A.V.; Pereira, E.C. Development of low-cost metal oxide pH electrodes based on the polymeric precursor method. Anal. Chim. Acta 2008, 616, 36–41.
- Xu, B.; Zhang, W. Modification of vertically aligned carbon nanotubes with RuO2 for a solid-state pH sensor. Electrochim. Acta 2010, 55, 2859–2864.
- Manjakkal, L.; Zaraska, K.; Cvejin, K.; Kulawik, J.; Szwagierczak, D. Potentiometric RuO2–Ta2O5 pH sensors fabricated using thick film and LTCC technologies. Talanta 2016, 147, 233–240.
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Szwagierczak, D.; Stojanovic, G. Sensing mechanism of RuO2–SnO2 thick film pH sensors studied by potentiometric method and electrochemical impedance spectroscopy. J. Electroanal. Chem. 2015, 759, 82–90.
- Marzouk, S.A.M.; Ufer, S.; Buck, R.P.; Johnson, T.A.; Dunlap, L.A.; Cascio, W.E. Electrodeposited iridium oxide pH electrode for measurement of extracellular myocardial acidosis during acute ischemia. Anal. Chem. 1998, 70, 5054–5061.
- Shervedani, R.K.; Mehrdjardi, H.R.Z.; Ghahfarokhi, S.H.K. Electrochemical characterization and application of Ni-RuO2 as a pH sensor for determination of petroleum oil acid number. J. Iran. Chem. Soc. 2007, 4, 221–228.
- Kwon, D.H.; Cho, B.W.; Kim, C.S.; Sohn, B.K. Effects of heat treatment on Ta2O5 sensing membrane for low drift and high sensitivity pH-ISFET. Sens. Actuators B Chem. 1996, 34, 441–445.
- Tsai, C.N.; Chou, J.C.; Sun, T.P.; Hsiung, S.K. Study on the time-dependent slow response of the tin oxide pH electrode. IEEE Sens. J. 2006, 6, 1243–1249.
- Liao, Y.H.; Chou, J.C. Preparation and characterization of the titanium dioxide thin films used for pH electrode and procaine drug sensor by sol–gel method. Mater. Chem. Phys. 2009, 114, 542–548.
- Chiang, J.L.; Jan, S.S.; Chou, J.C.; Chen, Y.C. Study on the temperature effect, hysteresis and drift of pH-ISFET devices based on amorphous tungsten oxide. Sens. Actuators B Chem. 2001, 76, 624–628.
- Zhang, W.D.; Xu, B. A solid-state pH sensor based on WO3-modified vertically aligned multiwalled carbon nanotubes. Electrochem. Commun. 2009, 11, 1038–1041.
- Betelu, S.; Polychronopoulou, K.; Rebholz, C.; Ignatiadis, I. Novel CeO2-based screen-printed potentiometric electrodes for pH monitoring. Talanta 2011, 87, 126–135.
- Razmi, H.; Heidari, H.; Habibi, E. pH-sensing properties of PbO2 thin film electrodeposited on carbon ceramic electrode. J. Solid State Electrochem. 2008, 12, 1579–1587.
- Telli, L.; Brahimi, B.; Hammouche, A. Study of a pH sensor with MnO2 and montmorillonite-based solid-state internal reference. Solid State Ionics 2000, 128, 255–259.
- Qingwen, L.; Guoan, L.; Youqin, S. Response of nanosized cobalt oxide electrodes as pH sensors. Anal. Chim. Acta 2000, 409, 137–142.
- Bakker, E.; Telting-Diaz, M. Electrochemical Sensors. Anal. Chem. 2002, 74, 2781–2800.
- Ibupoto, Z.H.; Khun, K.; Willander, M. A Selective Iodide Ion Sensor Electrode Based on Functionalized ZnO Nanotubes. Sensors 2013, 13, 1984–1997.
- Khun, K.; Ibupoto, Z.H.; Willander, M. Urea Assisted Synthesis of Flower Like CuO Nanostructures and Their Chemical Sensing Application for the Determination of Cadmium Ions. Electroanalysis 2013, 25, 1425–1432.
- Zeng, X.; Qin, W. A solid-contact potassium-selective electrode with MoO2 microspheres as ion-to-electron transducer. Anal. Chim. Acta 2017, 982, 72–77.
- Yang, C.; Song, C.Q.; Zhang, Y.Q.; Qu, Y. Determination of Concentration of Serum Potassium Ion Using All-Solid-State Potassium Ion Selective Electrode Based on Two-Dimensional Manganese Dioxide Nanosheet. Chin. J. Anal. Chem. 2019, 47, 765–771.
- Lenar, N.; Piech, R.; Wyrwa, J.; Paczosa-Bator, B. Potassium-Selective Solid-Contact Electrode with High-Capacitance Hydrous Iridium Dioxide in the Transduction Layer. Membranes 2021, 11, 259.
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Hydrous Cerium Dioxide-Based Materials as Solid-Contact Layers in Potassium-Selective Electrodes. Membranes 2022, 12, 349.
- Pietrzak, K.; Krstulović, N.; Blažeka, D.; Car, J.; Malinowski, S.; Wardak, C. Metal oxide nanoparticles as solid contact in ion-selective electrodes sensitive to potassium ions. Talanta 2022, 243, 123335.
- Zeng, X.; Jiang, W.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Stable Pb2+ ion-selective electrodes based on polyaniline-TiO2 solid contacts. Anal. Chim. Acta 2020, 1094, 26–33.
- Lenar, N.; Paczosa-Bator, B.; Piech, R.; Królicka, A. Poly(3-octylthiophene-2,5-diyl)-nanosized ruthenium dioxide composite material as solid-contact layer in polymer membrane-based K+-selective electrodes. Electrochim. Acta 2019, 322, 134718.
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate. Materials 2021, 14, 1891.
- Lenar, N.; Piech, R.; Paczosa-Bator, B. High Capacity Nanocomposite Layers Based on Nanoparticles of Carbon Materials and Ruthenium Dioxide for Potassium Sensitive Electrode. Materials 2021, 14, 1308.
- Su, Y.; Liu, T.; Song, C.; Fan, A.; Zhu, N.; Sun, B.; Yang, C. Using MoS2/Fe3O4 as Ion-Electron Transduction Layer to Manufacture All-Solid-State Ion-Selective Electrode for Determination of Serum Potassium. Chemosensors 2021, 9, 155.
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Carbon Nanomaterials-Poly(3-octylthiophene-2,5-diyl)–Hydrous Iridium Dioxide Triple Composite Materials as Superhydrophobic Layers for Ion-Selective Electrodes. J. Electrochem. Soc. 2022, 169, 127508.
- Lenar, N.; Piech, R.; Paczosa-Bator, B. The New Reliable pH Sensor Based on Hydrous Iridium Dioxide and Its Composites. Materials 2023, 16, 192.
- Wardak, C.; Pietrzak, K.; Morawska, K. Nanocomposite of copper oxide nanoparticles and multi-walled carbon nanotubes as a solid contact of a copper-sensitive ion-selective electrode: Intermediate layer or membrane component–comparative studies. Appl. Nanosci. 2023, 13, 7017–7028.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
758
Revisions:
2 times
(View History)
Update Date:
22 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




