
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stavros P. Papadakos | -- | 4028 | 2023-11-20 15:46:02 | | | |
| 2 | Jessie Wu | + 13 word(s) | 4041 | 2023-11-21 03:48:53 | | |
Video Upload Options
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and is a leading cause of cancer-related death worldwide. Immunotherapy has emerged as the mainstay treatment option for unresectable HCC. Toll-like receptor 4 (TLR4) plays a crucial role in the innate immune response by recognizing and responding primarily to bacterial lipopolysaccharides. In addition to its role in the innate immune system, TLR4 has also been implicated in adaptive immunity, including specific anti-tumor immune responses. In particular, the TLR4 signaling pathway seems to be involved in the regulation of several cancer hallmarks, such as the continuous activation of cellular pathways that promote cell division and growth, the inhibition of programmed cell death, the promotion of several invasion and metastatic mechanisms, epithelial-to-mesenchymal transition, angiogenesis, drug resistance, and epigenetic modifications. Emerging evidence further suggests that TLR4 signaling holds promise as a potential immunotherapeutic target in HCC
1. Toll-like Receptor 4 Signaling as a Platform for Hepatocellular Carcinoma Vaccines
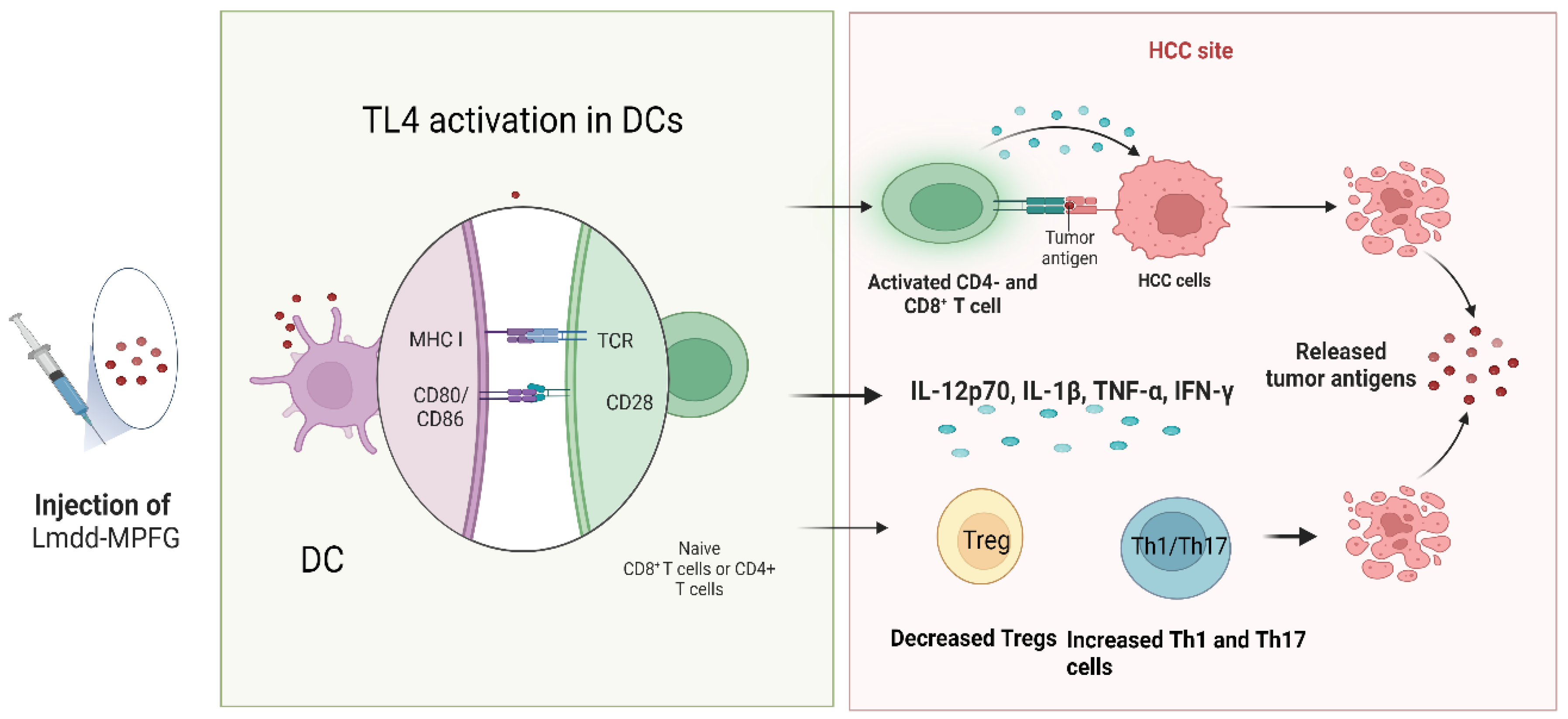
2. Toll-like Receptor 4 Signaling as Regulator of Hepatocellular Carcinoma Tumor Immune Landscape
2.1. Regulation of T and B lymphocytes by Toll-like Receptor 4 Signaling
2.2. Regulation of Dendritic Cells by Toll-like Receptor 4 Signaling
2.3. Regulation of Neutrophils by Toll-like Receptor 4 Signaling
2.4. Regulation of Myeloid-Derived Suppressor Cells by Toll-like Receptor 4 Signaling
2.5. Regulation of Macrophages by Toll-like Receptor 4 Signaling
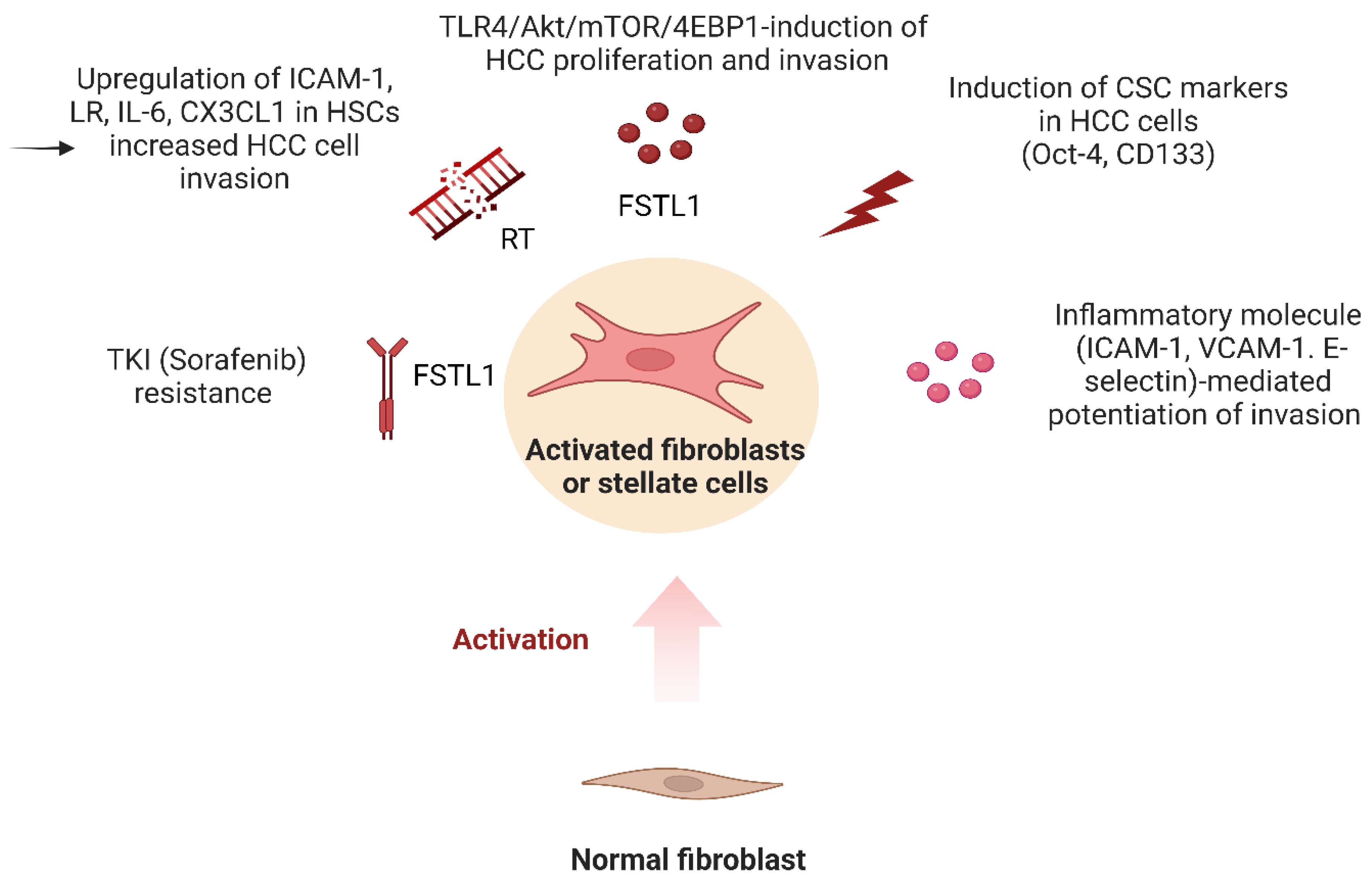
2.6. Regulation of Fibroblasts by Toll-like Receptor 4 Signaling
3. Targeting Toll-like Receptor 4 Signaling to Enhance PD-1 Blockade
References
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926.
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; De Vries, I.J.M.; Figdor, C.G. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906.
- Wan, X.; Cheng, C.; Lin, Z.; Jiang, R.; Zhao, W.; Yan, X.; Tang, J.; Yao, K.; Sun, B.; Chen, Y. The attenuated hepatocellular carcinoma-specific Listeria vaccine Lmdd-MPFG prevents tumor occurrence through immune regulation of dendritic cells. Oncotarget 2015, 6, 8822–8838.
- Silva, L.; Egea, J.; Villanueva, L.; Ruiz, M.; Llopiz, D.; Repáraz, D.; Aparicio, B.; Lasarte-cia, A.; Lasarte, J.J.; de Galarreta, M.R.; et al. Cold-inducible rna binding protein as a vaccination platform to enhance immunotherapeutic responses against hepatocellular carcinoma. Cancers 2020, 12, 3397.
- Sakurai, T.; Yada, N.; Watanabe, T.; Arizumi, T.; Hagiwara, S.; Ueshima, K.; Nishida, N.; Fujita, J.; Kudo, M. Cold-inducible RNA-binding protein promotes the development of liver cancer. Cancer Sci. 2015, 106, 352–358.
- Zheng, Q.C.; Yang, J.; Li, M. Emerging role of Toll-like receptor 4 in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 11.
- Sepehri, Z.; Kiani, Z.; Kohan, F.; Alavian, S.M.; Ghavami, S. Toll like receptor 4 and hepatocellular carcinoma; A systematic review. Life Sci. 2017, 179, 80–87.
- Giannou, A.D.; Lücke, J.; Kleinschmidt, D.; Shiri, A.M.; Steglich, B.; Nawrocki, M.; Zhang, T.; Zazara, D.E.; Kempski, J.; Zhao, L.; et al. A Critical Role of the IL-22-IL-22 Binding Protein Axis in Hepatocellular Carcinoma. Cancers 2022, 14, 6019.
- Kuang, D.M.; Xiao, X.; Zhao, Q.; Chen, M.M.; Li, X.F.; Liu, R.X.; Wei, Y.; Ouyang, F.Z.; Chen, D.P.; Wu, Y.; et al. B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets. J. Clin. Investig. 2014, 124, 4657–4667.
- Yang, J.; Zhang, J.X.; Wang, H.; Wang, G.L.; Hu, Q.G.; Zheng, Q.C. Hepatocellular carcinoma and macrophage interaction induced tumor immunosuppression via treg requires TLR4 signaling. World J. Gastroenterol. 2012, 18, 2938–2947.
- Li, C.X.; Ling, C.C.; Shao, Y.; Xu, A.; Li, X.C.; Ng, K.T.P.; Liu, X.B.; Ma, Y.Y.; Qi, X.; Liu, H.; et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J. Hepatol. 2016, 65, 944–952.
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283.
- Xiao, X.; Lao, X.-M.; Chen, M.-M.; Liu, R.-X.; Wei, Y.; Ouyang, F.-Z.; Chen, D.-P.; Zhao, X.-Y.; Zhao, Q.; Li, X.-F.; et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016, 6, 546–559.
- Hargadon, K.M. Tumor-altered dendritic cell function: Implications for anti-tumor immunity. Front. Immunol. 2013, 4, 192.
- Yamamoto, M.; Tatsumi, T.; Miyagi, T.; Tsunematsu, H.; Aketa, H.; Hosui, A.; Kanto, T.; Hiramatsu, N.; Hayashi, N.; Takehara, T. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clin. Exp. Immunol. 2011, 165, 211–219.
- Arelaki, S.; Koletsa, T.; Sinakos, E.; Papadopoulos, V.; Arvanitakis, K.; Skendros, P.; Akriviadis, E.; Ritis, K.; Germanidis, G.; Hytiroglou, P. Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 2022, 481, 455–465.
- Arvanitakis, K.; Mitroulis, I.; Germanidis, G. Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers 2021, 13, 2899.
- Yang, L.Y.; Luo, Q.; Lu, L.; Zhu, W.W.; Sun, H.T.; Wei, R.; Lin, Z.F.; Wang, X.Y.; Wang, C.Q.; Lu, M.; et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 2020, 13, 3.
- Zhan, X.; Wu, R.; Kong, X.; You, Y.; He, K.; Sun, X.; Huang, Y.; Chen, W.; Duan, L. Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma. Cancer Commun. 2022, 43, 225–245.
- Chen, Y.; Ramjiawan, R.R.; Reiberger, T.; Ng, M.R.; Hato, T.; Huang, Y.; Ochiai, H.; Kitahara, S.; Unan, E.C.; Reddy, T.P.; et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015, 61, 1591–1602.
- Marvel, D.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015, 125, 3356–3364.
- Hoechst, B.; Ormandy, L.A.; Ballmaier, M.; Lehner, F.; Krüger, C.; Manns, M.P.; Greten, T.F.; Korangy, F. A New Population of Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Induces CD4+CD25+Foxp3+ T Cells. Gastroenterology 2008, 135, 234–243.
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014, 6, 237ra67.
- Liu, H.; Ling, C.C.; Yeung, W.H.O.; Pang, L.; Liu, J.; Zhou, J.; Zhang, W.Y.; Liu, X.B.; Ng, T.P.K.; Yang, X.X.; et al. Monocytic MDSC mobilization promotes tumor recurrence after liver transplantation via CXCL10/TLR4/MMP14 signaling. Cell Death Dis. 2021, 12, 489.
- Hu, C.E.; Gan, J.; Zhang, R.D.; Cheng, Y.R.; Huang, G.J. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand. J. Gastroenterol. 2011, 46, 156–164.
- Arvanitakis, K.; Koletsa, T.; Mitroulis, I.; Germanidis, G. Tumor-Associated Macrophages in Hepatocellular Carcinoma Pathogenesis, Prognosis and Therapy. Cancers 2022, 14, 226.
- Wan, S.; Zhao, E.; Kryczek, I.; Vatan, L.; Sadovskaya, A.; Ludema, G.; Simeone, D.M.; Zou, W.; Welling, T.H. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014, 147, 1393–1404.
- Miura, K.; Ishioka, M.; Minami, S.; Horie, Y.; Ohshima, S.; Goto, T.; Ohnishi, H. Toll-like receptor 4 on macrophage promotes the development of steatohepatitis-related hepatocellular carcinoma in mice. J. Biol. Chem. 2016, 291, 11504–11517.
- Li, W.; Xiao, J.; Zhou, X.; Xu, M.; Hu, C.; Xu, X.; Lu, Y.; Liu, C.; Xue, S.; Nie, L.; et al. STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma. J. Clin. Investig. 2015, 125, 4239–4254.
- Min, L.; Wang, H.; Qi, H. Astragaloside IV inhibits the progression of liver cancer by modulating macrophage polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am. J. Transl. Res. 2022, 14, 1551–1566.
- Pan, C.; Wu, Q.; Wang, S.; Mei, Z.; Zhang, L.; Gao, X.; Qian, J.; Xu, Z.; Zhang, K.; Su, R.; et al. Combination with Toll-like receptor 4 (TLR4) agonist reverses GITR agonism mediated M2 polarization of macrophage in Hepatocellular carcinoma. Oncoimmunology 2022, 11, 2073010.
- Yao, R.R.; Li, J.H.; Zhang, R.; Chen, R.X.; Wang, Y.H. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J. Surg. Oncol. 2018, 16, 9.
- Zhou, J.; Che, J.; Xu, L.; Yang, W.; Zhou, W.; Zhou, C. Tumor-derived extracellular vesicles containing long noncoding RNA PART1 exert oncogenic effect in hepatocellular carcinoma by polarizing macrophages into M2. Dig. Liver Dis. 2022, 54, 543–553.
- Tsai, Y.T.; Li, C.Y.; Huang, Y.H.; Chang, T.S.; Lin, C.Y.; Chuang, C.H.; Wang, C.Y.; Anuraga, G.; Chang, T.H.; Shih, T.C.; et al. Galectin-1 orchestrates an inflammatory tumor-stroma crosstalk in hepatoma by enhancing TNFR1 protein stability and signaling in carcinoma-associated fibroblasts. Oncogene 2022, 41, 3011–3023.
- Kobayashi, H.; Enomoto, A.; Woods, S.L.; Burt, A.D.; Takahashi, M.; Worthley, D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 282–295.
- Zhu, G.-Q.; Tang, Z.; Huang, R.; Qu, W.-F.; Fang, Y.; Yang, R.; Tao, C.-Y.; Gao, J.; Wu, X.-L.; Sun, H.-X.; et al. CD36+ cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. 2023, 9, 25.
- Song, I.J.; Yang, Y.M.; Inokuchi-Shimizu, S.; Roh, Y.S.; Yang, L.; Seki, E. The contribution of toll-like receptor signaling to the development of liver fibrosis and cancer in hepatocyte-specific TAK1-deleted mice. Int. J. Cancer 2018, 142, 81–91.
- Liu, W.T.; Jing, Y.Y.; Gao, L.; Li, R.; Yang, X.; Pan, X.R.; Yang, Y.; Meng, Y.; Hou, X.J.; Zhao, Q.D.; et al. Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts constitutes the hepatocarcinogenesis-associated microenvironment. Cell Death Differ. 2020, 27, 85–101.
- Lu, Y.; Xu, J.; Chen, S.; Zhou, Z.; Lin, N. Lipopolysaccharide promotes angiogenesis in mice model of HCC by stimulating hepatic stellate cell activation via TLR4 pathway. Acta Biochim. Biophys. Sin. 2017, 49, 1029–1034.
- Loh, J.J.; Li, T.W.; Zhou, L.; Wong, T.L.; Liu, X.; Ma, V.W.S.; Lo, C.M.; Man, K.; Lee, T.K.; Ning, W.; et al. FSTL1 secreted by activated fibroblasts promotes hepatocellular carcinoma metastasis and stemness. Cancer Res. 2021, 81, 5692–5705.
- Ding, S.M.; Lu, J.F.; Edoo, M.I.A.; Zhou, L.; Xie, H.Y.; Zheng, S.S.; Li, Q.Y. MRC-5 cancer-associated fibroblasts influence production of cancer stem cell markers and inflammation-associated cell surface molecules, in liver cancer cell lines. Int. J. Med. Sci. 2019, 16, 1157–1170.
- Shen, X.; Zhao, J.; Wang, Q.; Chen, P.; Hong, Y.; He, X.; Chen, D.; Liu, H.; Wang, Y.; Cai, X. The Invasive Potential of Hepatoma Cells Induced by Radiotherapy is Related to the Activation of Hepatic Stellate Cells and Could be Inhibited by EGCG Through the TLR4 Signaling Pathway. Radiat. Res. 2022, 197, 365–375.
- Fulgenzi, C.A.M.; Scheiner, B.; Korolewicz, J.; Stikas, C.-V.; Gennari, A.; Vincenzi, B.; Openshaw, M.R.; Silletta, M.; Pinter, M.; Cortellini, A.; et al. Efficacy and safety of frontline systemic therapy for advanced HCC: A network meta-analysis of landmark phase III trials. JHEP Rep. 2023, 5, 100702.
- Minaei, N.; Ramezankhani, R.; Tamimi, A.; Piryaei, A.; Zarrabi, A.; Aref, A.R.; Mostafavi, E.; Vosough, M. Immunotherapeutic approaches in Hepatocellular carcinoma: Building blocks of hope in near future. Eur. J. Cell Biol. 2023, 102, 151284.
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, 7250–7257.
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420.
- Lv, T.; Xiong, X.; Yan, W.; Liu, M.; Xu, H.; He, Q. Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J. Immunother. Cancer 2022, 10, e004763.
- Xu, G.; Feng, D.; Yao, Y.; Li, P.; Sun, H.; Yang, H.; Li, C.; Jiang, R.; Sun, B.; Chen, Y. Listeria-based hepatocellular carcinoma vaccine facilitates anti-PD-1 therapy by regulating macrophage polarization. Oncogene 2019, 39, 1429–1444.





