
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masakuni Noguchi | -- | 1874 | 2023-11-16 23:52:18 | | | |
| 2 | Mona Zou | Meta information modification | 1874 | 2023-11-17 09:54:20 | | |
Video Upload Options
Axillary lymph node dissection (ALND) has traditionally been performed to assess nodal status, prevent axillary recurrence, and possibly improve survival. However, the procedure has been associated with postoperative morbidities, including arm lymphedema, shoulder dysfunction, and paresthesia. Sentinel lymph node (SLN) biopsy was introduced as an alternative approach to assess axillary nodal status and potentially eliminate the need for ALND in patients with clinically node-negative (cN0) breast cancer. Despite this progress, eliminating ALND for all breast cancer patients still seems premature at this time. Various forms of conservative axillary surgery have been developed to replace or supplement conventional ALND. Conservative axillary surgery may be promising in reducing the incidence of arm lymphedema without increasing the risk of axillary recurrence.
1. Conservative Axillary Surgery
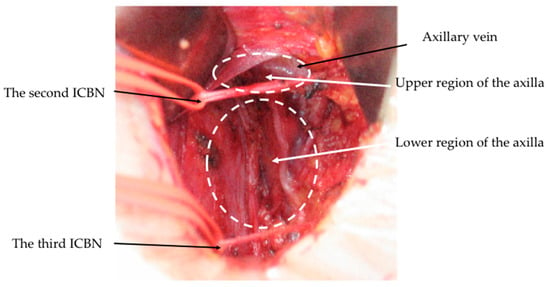
2. Partial Lower ALND for cN0 Patients
| Patients/Methods | No. of Patients |
Systemic Chemotherapy |
Radiotherapy | Follow-Up Period |
Axillary Recurrence |
Arm Lymphedema |
|---|---|---|---|---|---|---|
| (a) cN0 patients [6] | ||||||
| Partial lower ALND | 1043 | 100% | Not reported | 72 months | 0.6% | 0.0% |
| Conventional ALND | 1084 | 100% | Not reported | 120 months | 0.1% * | 11.8% # |
| (b) cN0 and cN+ patients [7] | ||||||
| CARE procedure | 587 | 49.3% | 25.9% | 5.1 years | 0.5% | 3.4% |
3. Conservative Axillary Regional Excision (CARE)
4. Conservative ALND with Axillary Reverse Mapping (ARM)
| Authors/ Surgical Procedures with ARM |
Identification Rate of SLNs |
Identification Rate of ARM Nodes or Lymphatics |
Crossover Rate between SLN and ARM Nodes |
Involved Rate of ARM Node |
Axillary Recurrence |
Objective Lymphedema |
|---|---|---|---|---|---|---|
| (a) Boneti et al. [19] | ||||||
| SLN biopsy (n = 220) | 97.2% (214/220) | 40.6% (87/214) | 2.8% (6/214) | 0% (0/15) | / | |
| and/or ALND (n = 47) | / | 5.4% (2/51) | ||||
| (b) Noguchi M, et al. [20] | ||||||
| SLN biopsy alone (n = 437) | 98% (499/507) | 63% (321/507) | 28% (140/499) | 0.9% (4/429) | 0.7% (3/429) | |
| ALND after SLN biopsy (n = 70) | 93% (65/70) | 34% (24/70) | 5.7% (4/70) # | 2.9% (2/70) | 21% (15/70) * |
| Authors/Methods | No. of Patients |
Adjuvant Chemotherapy |
Radiotherapy | Follow-Up Periods |
Axillary Recurrence |
Arm Swelling |
|---|---|---|---|---|---|---|
| (a) Yue et al. [17] | ||||||
| ARM-guided ALND | 138 | None | Not reported | 20 months | 0% | 5.9% |
| Conventional ALND | 127 | None | Not reported | 20 months | 0% * | 33.1% # |
| (b) Yuan et al. [12] | ||||||
| ARM-guided ALND | 543 & | None | 56.5% | 37 months | 1.4% | 3.3% |
| Conventional ALND | 648 & | None | 60.6% | 37 months | 1.2% * | 15.3% # |
5. Tailored Axillary Surgery (TAS) for cN+ Patients
References
- Noguchi, M.; Inokuchi, M.; Yokoi-Noguchi, M.; Morioka, E.; Haba, Y. Conservative axillary surgery is emerging in the surgical management of breast cancer. Breast Cancer 2023, 30, 14–22.
- Li, J.; Zhang, Y.; Zhang, W.; Jia, S.; Gu, X.; Ma, Y.; Li, D. Intercostobrachial nerves as a novel anatomic landmark for dividing the axillary space in lymph node dissection. ISRN Oncol. 2013, 2013, 279013.
- Cirocchi, R.; Amabile, M.I.; De Luca, A.; Frusone, F.; Tripodi, D.; Gentile, P.; Tabola, R.; Pironi, D.; Forte, F.; Monti, M.; et al. New classifications of axillary lymph nodes and their anatomical-clinical correlations in breast surgery. World J. Surg. Oncol. 2021, 19, 93.
- Ponzone, R.; Cont, N.T.; Maggiorotto, F.; Cassina, E.; Minina, P.; Sismondi, P. Extensive nodal disease may impair axillary reverse mapping in patients with breast cancer. J. Clin. Oncol. 2009, 27, 5547–5551.
- Clough, K.B.; Nasr, R.; Nos, C.; Vieira, M.; Inguenault, C.; Poulet, B. New anatomical classification of the axilla with implications for sentinel node biopsy. Br. J. Surg. 2010, 97, 1659–1665.
- Kodama, H.; Mise, K.; Kan, N. Partial lower axillary dissection for patients with clinically node-negative breast cancer. J. Int. Med. Res. 2012, 40, 2336–2345.
- Cowher, M.S.; Grobmyer, S.R.; Lyons, J.; O’Rourke, C.; Baynes, D.; Crowe, J.P. Conservative axillary surgery in breast cancer patients undergoing mastectomy: Long-term results. J. Am. Coll. Surg. 2014, 218, 819–824.
- Lyman, G.H.; Giuliano, A.E.; Somerfield, M.R.; Benson, A.B., III; Bodurka, D.C.; Burstein, H.J.; Cochran, A.J.; Cody, H.S., III; Edge, S.B.; Galper, S.; et al. American society of clinical oncology guideline Recommendations for sentinel lymph node biopsy in early-stage breast cancer. J. Clin. Oncol. 2005, 23, 7703–7720.
- Noguchi, M.; Tsugawa, K.; Kawahara, F.; Bando, E.; Miwa, K.; Minato, H.; Nonomura, A. Dye-guided sentinel lymphadenectomy in clinically node-negative and node-positive breast cancer patients. Breast Cancer 1998, 5, 381–387.
- Thompson, M.; Korourian, S.; Henry-Tillman, R.; Adkins, L.; Mumford, S.; Westbrook, K.C.; Klimberg, V.S. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann. Surg. Oncol. 2007, 14, 1890–1895.
- Nos, C.; Lesieur, B.; Clough, K.B.; Lecuru, F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann. Surg. Oncol. 2007, 14, 2490–2496.
- Yuan, Q.; Wu, G.; Xiao, S.Y.; Hou, J.; Ren, Y.; Wang, H.; Wang, K.; Zhang, D. Identification and preservation of arm lymphatic system in axillary dissection for breast cancer to reduce arm lymphedema events: A randomized clinical trial. Ann. Surg. Oncol. 2019, 26, 3446–3454.
- Faisal, M.; Sayed, M.G.; Antonious, K.; Bakr, A.A.; Farag, S.H. Prevention of lymphedema via axillary reverse mapping for arm lymph-node preservation following breast cancer surgery: A randomized controlled trial. Patient Saf. Surg. 2019, 13, 35.
- Abdelhamid, M.I.; Bari, A.A.; Farid, M.; Nour, H. Evaluation of axillary reverse mapping (ARM) in clinically axillary node negative breast cancer patients—Randomized controlled trial. Int. J. Surg. 2020, 75, 174–178.
- Gennaro, M.; Maccauro, M.; Mariani, L.; Listorti, C.; Sigari, C.; De Vivo, A.; Chisari, M.; Maugeri, I.; Lorenzoni, A.; Alberti, G.; et al. Occurrence of breast-cancer-related lymphedema after reverse lymphatic mapping and selective axillary dissection versus standard surgical treatment of axilla: A two-arm randomized clinical trial. Cancer 2022, 128, 4185–4193.
- Beek, M.A.; Gobardhan, P.; Klompenhouwer, E.G.; Menke-Pluijmers, M.B.; Steenvoorde, P.; Merkus, J.W.S.; Rutten, H.J.T.; Voogd, A.C.; Luiten, E.J.T. A patient- and assessor-blinded randomized controlled trial of axillary reverse mapping (ARM) in patients with early breast cancer. Eur. J. Surg. Oncol. 2020, 46, 59–64.
- Yue, T.; Zhuang, D.; Zhou, P.; Zheng, L.; Fan, Z.; Zhu, J.; Hou, L.; Yu, F.; Dong, X.; Xiao, L.; et al. A prospective study to assess the feasibility of axillary reverse mapping and evaluate its effect on preventing lymphedema in breast cancer patients. Clin. Breast Cancer 2015, 15, 301–306.
- Suami, H.; Taylor, G.I.; Pan, W.-R. The lymphatic territories of the upper limb: Anatomical study and clinical implications. Plast. Recontr. Surg. 2007, 119, 1813–1822.
- Boneti, C.; Korourian, S.; Diaz, Z.; Santiago, C.; Mumford, S.; Adkins, L.; Klimberg, V.S. Scientific impact award: Axillary reverse mapping (ARM) to identify and protect lymphatics draining the arm during axillary lymphadenectomy. Am. J. Surg. 2009, 198, 482–487.
- Noguchi, M.; Inokuchi, M.; Yokoi-Noguchi, M.; Morioka, E. The involvement of axillary reverse mapping nodes in patients with clinically node-negative breast cancer. Breast Cancer 2022, 29, 209–215.
- Noguchi, M.; Yokoi, M.; Nakano, Y. Axillary reverse mapping with indocyanine fluorescence imaging in patients with breast cancer. J. Surg. Oncol. 2010, 101, 217–221.
- Giuliano, A.E.; McCall, L.; Beitsch, P.; Whitworth, P.W.; Blumencranz, P.; Leitch, M.; Saha, S.; Hunt, K.K.; Morrow, M.; Ballman, K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann. Surg. 2010, 252, 426–432; discussion 432–433.
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; Van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310.
- Noguchi, M.; Inokuchi, M.; Yokoi-Noguchi, M.; Morioka, E. The involvement of axillary reverse mapping nodes in patient with node-positive breast cancer. Eur. J. Surg. Oncol. 2023, 49, 106937.
- Maggi, N.; Nussbaumer, R.; Holzer, L.; Weber, W.P. Axillary surgery in node-positive breast cancer. Breast 2022, 62 (Suppl. S1), S50–S53.
- Weber, W.P.; Matrai, Z.; Hayoz, S.; Tausch, C.; Henke, G.; Zwahlen, D.R.; Gruber, G.; Zimmermann, F.; Seiler, S.; Maddox, C.; et al. Tailored axillary surgery in patients with clinically node-positive breast cancer: Pre-planned feasibility substudy of TAXIS (OPBC-03, SAKK 23/16, IBCSG 57–18, ABCSG-53, GBG 101). Breast 2021, 60, 98–110.
- Heidinger, M.; Knauer, M.; Tausch, C.; Weber, W.P. Tailored axillary surgery—A novel concept for clinically node positive breast cancer. Breast 2023, 69, 281–289.




