Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ha Huu Do | -- | 1780 | 2023-11-15 12:08:54 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 1782 | 2023-11-17 02:17:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Do, H.H.; Kim, S.Y. Metal-Organic Frameworks Based Multifunctional Materials for Solar Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/51606 (accessed on 08 February 2026).
Do HH, Kim SY. Metal-Organic Frameworks Based Multifunctional Materials for Solar Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/51606. Accessed February 08, 2026.
Do, Ha Huu, Soo Young Kim. "Metal-Organic Frameworks Based Multifunctional Materials for Solar Cells" Encyclopedia, https://encyclopedia.pub/entry/51606 (accessed February 08, 2026).
Do, H.H., & Kim, S.Y. (2023, November 15). Metal-Organic Frameworks Based Multifunctional Materials for Solar Cells. In Encyclopedia. https://encyclopedia.pub/entry/51606
Do, Ha Huu and Soo Young Kim. "Metal-Organic Frameworks Based Multifunctional Materials for Solar Cells." Encyclopedia. Web. 15 November, 2023.
Copy Citation
Developing low-cost and stable materials for converting solar energy into electricity is vital in meeting the world’s energy demand. Metal-organic frameworks (MOFs) have gained attention for solar cells due to their natural porous architectures and tunable chemical structures. They are built by high-symmetry metal clusters as secondary building units and organic carboxylate/azolate ligands as linkers.
MOFs
hydrothermal method
sol-gel
porous materials
solar cells
photoanodes
1. Introduction
In recent years, melting ice, drought, and flooding have increased significantly. This is a direct result of global warming, attributed to the increased greenhouse gases released from using conventional fossil fuels [1][2][3][4][5][6][7][8][9][10]. Therefore, there needs to be a shift from fossil fuel to renewable energy to meet the increasing demands of power without causing much environmental contamination [11][12][13][14][15][16][17][18][19][20]. Among renewable energy sources, solar energy is considered an ideal solution due to zero CO2 emission and abundant resources [21][22][23][24][25][26]. Nonetheless, problems of solar cells are expensive, and restricted scale. Solar cells based on silicon are the most widely used for converting solar energy to electricity to satisfy human energy demands. Until now, Si-based materials displayed the highest performance for converting solar energy into electricity [27][28][29][30][31]. However, solar cell-based Si materials are ineffective with inside sunlight due to their unsuitable band gap. As a result, various types of solar cells involving dye-sensitized solar cells (DSSC), perovskite solar cells (PSC), and organic solar cells (OSC) have been explored with a large number of publications in recent years (Figure 1) [32][33][34].
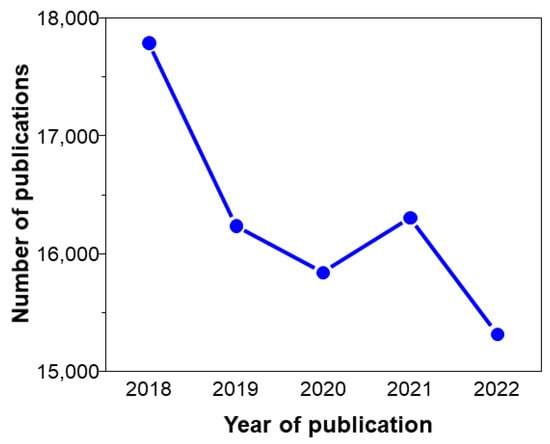
Figure 1. Number of publications resulting from the search of “solar cells” on Web of Science, accessed on 12 September 2023.
Regarding DSSC, TiO2 was identified as a typical photoanode material, whereas Pt-based materials are the most effective counter electrodes (CE). However, the fast excitons recombination of TiO2 and the high cost of Pt have driven scientists to explore the novelty alternatives in solar cell applications. Furthermore, stability is an issue of concern for PSC and OSC researchers due to the instability of organic components. As a result, ultra-stable materials and integrated methodologies for increasing the long-term stability of PSC and OSC must be investigated [35][36][37].
2. Synthetic Methods of Metal-Organic Frameworks (MOFs)
2.1. Hydrothermal Technique
The hydrothermal process is a typical method for producing MOFs and materials developed from them for solar cells. Various sizes and structures of MOFs were created by varying the temperature, reaction duration, and pressure [38]. Despite the effectiveness of obtaining MOFs, it was plagued by low yield and expansiveness. Furthermore, the reaction pathway of MOFs and their derived materials remains to be discovered.
2.2. Solvothermal Method
The solvothermal technique is generally acknowledged as an effective method for synthesizing MOFs and their composites by virtue of its high yield and facial control. The solvent is vital in the solvothermal reaction process. Until now, the most often employed organic solvents for MOF fabrication are N, N-dimethylformamide, ethanol, and methanol. These organic solvents might shift MOFs’ structure and size for specific applications. However, organic solvents’ toxicity and environmental unfriendliness limit their widespread use. Furthermore, organic solvents are difficult to decontaminate and recycle. As a result, developing safe and ecologically acceptable solvents for the solvothermal process is critical [39].
2.3. Sol-Gel
Recently, the sol-gel approach has been frequently used to produce inorganic nanomaterials. The synthesis procedure causes ion co-precipitation, which results in the generation of the desirable structures. This approach is more effective than the hydrothermal process for synthesizing MOFs. MOFs with variable sizes and shapes might be created by optimizing experimental parameters such as reaction temperature, duration, etc. Furthermore, MOFs might be employed as a precursor to create derivatives with outstanding properties [40].
2.4. Other Methods
Besides the hydrothermal technique, solvothermal process, and sol-gel methods, MOFs could be prepared by other strategies involving the electrochemical technique, microwave, and ultrasound methods [41][42][43].
3. Solar Cells
Solar cells are electronic devices for converting the sun’s energy directly into electricity. Generally, a solar cell comprises a semiconductor with a photovoltaic effect once illuminated. In terms of DSSC, the mechanism of the photovoltaic device includes three steps: light adsorption, charge production, and charge transport. Notably, the first process is the light adsorption of the dye molecule to change the excited state (dye*), after which this dye provides electrons for the conduction band of the semiconductor, accompanied by the oxidation process of dye*. Then, the injected electrons will move to the photoanode before transferring to CE through an external circuit [44]. In terms of PSC, this device generally includes five parts: FTO (Fluorine-doped Tin Oxide) glass as a conductive substrate, an electron transfer layer (ETL), a hole transfer layer (HTL), a perovskite layer, along with a metal electrode. PSC’s operation may be broken down into three steps: light adsorption and production of excitons, electron-hole separation, and charge transfer process. In particular, the perovskite layer will absorb solar energy to create electrons in the conduction band and holes in the valance band. The electrons then move to ETL before being transported to the anode, whereas the holes flow to HTL before being transferred to the cathode [45]. The process includes four steps for OSC’s working principle: light adsorption/electron-hole pairs production, charge diffusion, charge dissociation, and charge transport to the electrodes [46]. In particular, when a p-type material absorbed solar energy, electrons will be excited from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). Then these electrons will move to the LUMO of the acceptor before transferring into the cathode.
4. MOFs for Dye-Sensitized Solar Cells
4.1. MOFs as Photoanodes
Over the past decades, DSSC have gained considerable interests of scientists around the world because of their low-price, facial preparation, and high η. A typical DSSC is constructed from a photoanode, a CE, and a redox solution. TiO2 was evaluated as a conventional photoanode material. But, the fast excitons recombination of TiO2 has driven researchers to find the novelty photoanode in solar cell applications. On the one hand, MOFs could be utilized as photosensitizers for the conversion of sunlight into electricity. For example, Lee et al. conducted hole doping on various structures, including Co-BDC (BDC = benzenedicarboxylate) and Co-NDC (NDC = 2,6-naphthalendicarboxylic acid) for DSSCs [47]. The results revealed that I2-doped Co-based MOFs as photoanodes displayed a potential performance for energy conversion. Notably, I2-doped Co-NDC/TiO2 gave an efficiency of 1.12%, whereas this quantity is 0.96% for I2-doped Co-NDC/TiO2. The improved performance was attributed to the well-interaction between I2 and π-electrons of linkers.
4.2. MOFs as Counter Electrodes
The counter electrodes (CE) play vital roles in the construction of photovoltaic devices and in determining efficiency. The CE is created from depositing an electrocatalyst on the conductive template. Herein, Pt is generally utilized as electrocatalyst. Although Pt-based thin layers are the best CE for DSSC, high cost leads to an increase in the cost of solar cells. MOFs with a well-distribution of metal active sites were appreciated as promising CE in DSSC. For instance, Chen et al. blended MOF-525 with a conductive polymer, followed by coating on the carbon cloth to form an efficient CE in DSSC [48]. This device displayed a high cell performance of 8.91%, comparable with Pt CE-based solar cells. The effectiveness of using MOFs for CEs has been exemplified in a study by Zhao et al. [49]. The authors found that using ZIF-8 for fabrication CE brings higher power conversion efficiency than without MOF in DSSC.
5. MOFs for Perovskite Solar Cells
5.1. MOFs as Interfacial Layers (IL)
PSCs have been proven as potential candidates for replacing inorganic solar cell by virtue of excellent optical adsorption, inexpensiveness, and fast charge transfer. Recently, works related to the enhancement of PSC’s long-term durability have been implemented through a strategy of compositional engineering. Notably, by using inorganic ions to substitute unstable organic components, stability of PSC could be substantially improved. Also, interfacial engineering is an effective strategy to enhance stability of PSC. Recently, MOFs with high stability were employed as IL in PSC to improve the particle size and perovskite crystallinity and hinder electron-hole recombination, leading to increased solar cell efficiency. For instance, Shen et al. inserted ZIF-8 IL between the PSC application’s mesoporous TiO2 and perovskite component [50]. This strategy improved the solar cell efficiency from 14.75% (without ZIF-8) to 16.99% (with ZIF-8), attributed to the improved crystal quality and efficient suppression of exciton recombination. Ahmadian-Yazdi et al. used ZIF-8 as an interfacial modifier in PSC [51]. To confirm the ability of charge extraction, Photoluminescence (PL) was analyzed with various samples. The outcome revealed that c-TiO2/ZIF-8-10/perovskite displayed the smallest intensity of PL, implying the most effective charge extraction. As a result, this material exhibited the highest solar cell performance of 16.8%. Recently, Jin et al. utilized ZIF-8 as a host material to confine methylammonium chloride (MACl) to form an effective IL between SnO2 and the perovskite laye.
5.2. MOFs as Charge Transfer Layers
MOFs have been identified as promising materials for charge transporting in PSC. On the one hand, MOFs have been applied to improve electron transfer in PSC. For instance, Ryu et al. mixed a Ti-based MOF with commercial carbon material (PCBM) to create an electron transfer layer (ETL) [52]. This approach not only improves charge transport but also prevents the recombination of excitons. As a result, the rigid nTi-MOF/PCBM device exhibits a solar cell efficiency of 18.94%, whereas its flexible device has a performance of 17.43%. Nguyen et al. used a metal doping strategy on TiO2 derived from Ti-based MOF to produce an efficient ETL in PSC [53].
6. MOFs for Organic Solar Cells
OSC are a potential new energy conversion device that has significant prospects to deliver excellent η by utilizing inexpensive, adjustable conductive polymers or organic molecules. Moreover, these materials could be applied for industrial applications, implying that large-scale preparation is achievable. However, the stability of OSC is a headache problem for scientists. Therefore various strategies were conducted to improve η as well as stability of OSC devices. Interfacial engineering was identified as a primary strategy for increasing power conversion efficiency. The interfacial layers in OSCs could take various roles, involving shifting the energetic barrier at the metal/photoactive material interface, providing contact selectivity, hindering chemical or photophysical interaction between the photoactive component and the electrode, and acting as an optical spacer to maximize photocurrent. Compared to the rising uses in DSSC and PSC, MOFs have yet to be fully investigated in OSCs due to their low semiconducting characteristics. Up to now, only a few works related to MOFs for OSC application.
7. Conclusions
In summary, MOFs have emerged as a new topic in solar applications, which introduced tremendous theoretical and experimental works to improve the photovoltaic capabilities of MOFs. In particular, MOFs could be utilized as counter electrodes or photoanodes for DSSC with potential efficiency. Moreover, interface modifiers and charge transport components could be made from MOFs for PSC. Additionally, several works related to the usage of MOFs for OSC could bring a novelty direction in photovoltaic applications
References
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the synergistic effect between metal–organic frameworks and gas hydrates for CH4 storage and CO2 separation applications. Renew. Sustain. Energy Rev. 2022, 167, 112807.
- Li, L.; Jung, H.S.; Lee, J.W.; Kang, Y.T. Review on applications of metal–organic frameworks for CO2 capture and the performance enhancement mechanisms. Renew. Sustain. Energy Rev. 2022, 162, 112441.
- Parsaei, M.; Akhbari, K.; White, J. Modulating Carbon Dioxide Storage by Facile Synthesis of Nanoporous Pillared-Layered Metal–Organic Framework with Different Synthetic Routes. Inorg. Chem. 2022, 61, 3893–3902.
- Hang, X.; Zhao, J.; Xue, Y.; Yang, R.; Pang, H. Synergistic effect of Co/Ni bimetallic metal–organic nanostructures for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 628, 389–396.
- Guo, K.; Hussain, I.; Fu, Y.; Zhang, F.; Zhu, W. Strategies for improving the photocatalytic performance of metal-organic frameworks for CO2 reduction: A review. J. Environ. Sci. 2023, 125, 290–308.
- Tahir, M.; Ajiwokewu, B.; Bankole, A.A.; Ismail, O.; Al-Amodi, H.; Kumar, N. MOF based composites with engineering aspects and morphological developments for photocatalytic CO2 reduction and hydrogen production: A comprehensive review. J. Environ. Chem. Eng. 2023, 11, 109408.
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814.
- Karmakar, S.; Barman, S.; Rahimi, F.A.; Biswas, S.; Nath, S.; Maji, T.K. Developing post-modified Ce-MOF as a photocatalyst: A detail mechanistic insight into CO2 reduction toward selective C2 product formation. Energy Environ. Sci. 2023, 16, 2187–2198.
- Liu, J.-J.; Jiang, Z.-W.; Hsu, S.-W. Investigation of the Performance of Heterogeneous MOF-Silver Nanocube Nanocomposites as CO2 Reduction Photocatalysts by In Situ Raman Spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 6716–6725.
- Abid, H.R.; Hanif, A.; Keshavarz, A.; Shang, J.; Iglauer, S. CO2, CH4, and H2 Adsorption Performance of the Metal–Organic Framework HKUST-1 by Modified Synthesis Strategies. Energy Fuels 2023, 37, 7260–7267.
- Isikgor, F.H.; Zhumagali, S.; Merino, L.V.T.; De Bastiani, M.; McCulloch, I.; De Wolf, S. Molecular engineering of contact interfaces for high-performance perovskite solar cells. Nat. Rev. Mater. 2023, 8, 89–108.
- Bati, A.S.; Zhong, Y.L.; Burn, P.L.; Nazeeruddin, M.K.; Shaw, P.E.; Batmunkh, M. Next-generation applications for integrated perovskite solar cells. Commun. Mater. 2023, 4, 2.
- Bhattarai, S.; Hossain, M.K.; Pandey, R.; Madan, J.; Samajdar, D.; Rahman, M.F.; Ansari, M.Z.; Amami, M. Perovskite solar cells with dual light absorber layers for performance efficiency exceeding 30%. Energy Fuels 2023, 37, 10631–10641.
- Xu, Y.; Zhang, X.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. A critical review of research progress for metal alloy materials in hydrogen evolution and oxygen evolution reaction. Environ. Sci. Pollut. Res. 2023, 30, 11302–11320.
- Dogra, N.; Agrawal, P.; Pathak, S.; Saini, R.; Sharma, S. Hydrothermally synthesized MoSe2/ZnO composite with enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 26210–26220.
- Huang, Y.; Hu, Z.; Huang, L.-A.; Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Pan, J. Phosphorus-modified cobalt single-atom catalysts loaded on crosslinked carbon nanosheets for efficient alkaline hydrogen evolution reaction. Nanoscale 2023, 15, 3550–3559.
- Zeb, Z.; Huang, Y.; Chen, L.; Zhou, W.; Liao, M.; Jiang, Y.; Li, H.; Wang, L.; Wang, L.; Wang, H. Comprehensive overview of polyoxometalates for electrocatalytic hydrogen evolution reaction. Coord. Chem. Rev. 2023, 482, 215058.
- Wu, C.; Sun, Y.; Wen, X.; Zhang, J.-Y.; Qiao, L.; Cheng, J.; Zhang, K.H. Adjusting oxygen vacancies in perovskite LaCoO3 by electrochemical activation to enhance the hydrogen evolution reaction activity in alkaline condition. J. Energy Chem. 2023, 76, 226–232.
- Wang, B.; Yang, F.; Feng, L. Recent Advances in Co-Based Electrocatalysts for Hydrogen Evolution Reaction. Small 2023, 2302866.
- Mohamed, M.; Gondal, M.; Hassan, M.; Khan, A.; Surrati, A.; Almessiere, M. Exceptional co-catalysts free SrTiO3 perovskite coupled CdSe nanohybrid catalyst by green pulsed laser ablation for electrochemical hydrogen evolution reaction. Chem. Eng. J. Adv. 2022, 11, 100344.
- Duan, L.; Uddin, A. Progress in stability of organic solar cells. Adv. Sci. 2020, 7, 1903259.
- Mahmood, A.; Wang, J.-L. Machine learning for high performance organic solar cells: Current scenario and future prospects. Energy Environ. Sci. 2021, 14, 90–105.
- Chatterjee, S.; Jinnai, S.; Ie, Y. Nonfullerene acceptors for P3HT-based organic solar cells. J. Mater. Chem. A 2021, 9, 18857–18886.
- Xu, X.; Zhang, G.; Yu, L.; Li, R.; Peng, Q. P3HT-based polymer solar cells with 8.25% efficiency enabled by a matched molecular acceptor and smart green-solvent processing technology. Adv. Sci. 2019, 31, 1906045.
- Ghosekar, I.C.; Patil, G.C. Review on performance analysis of P3HT: PCBM-based bulk heterojunction organic solar cells. Semicond. Sci. Technol. 2021, 36, 045005.
- Nguyen, V.-H.; Do, H.H.; Van Nguyen, T.; Singh, P.; Raizada, P.; Sharma, A.; Sana, S.S.; Grace, A.N.; Shokouhimehr, M.; Ahn, S.H. Perovskite oxide-based photocatalysts for solar-driven hydrogen production: Progress and perspectives. Sol. Energy 2020, 211, 584–599.
- Labed, M.; Sengouga, N.; Meftah, A.; Meftah, A.; Rim, Y.S. Study on the improvement of the open-circuit voltage of NiOx/Si heterojunction solar cell. Opt. Mater. 2021, 120, 111453.
- Park, H.; Park, I.J.; Lee, M.G.; Kwon, K.C.; Hong, S.-P.; Kim, D.H.; Lee, S.A.; Lee, T.H.; Kim, C.; Moon, C.W. Water splitting exceeding 17% solar-to-hydrogen conversion efficiency using solution-processed Ni-based electrocatalysts and perovskite/Si tandem solar cell. ACS Appl. Mater. Interfaces 2019, 11, 33835–33843.
- Shen, H.; Walter, D.; Wu, Y.; Fong, K.C.; Jacobs, D.A.; Duong, T.; Peng, J.; Weber, K.; White, T.P.; Catchpole, K.R. Monolithic perovskite/Si tandem solar cells: Pathways to over 30% efficiency. Adv. Energy Mater. 2020, 10, 1902840.
- Kim, C.U.; Jung, E.D.; Noh, Y.W.; Seo, S.K.; Choi, Y.; Park, H.; Song, M.H.; Choi, K.J. Strategy for large-scale monolithic Perovskite/Silicon tandem solar cell: A review of recent progress. EcoMat 2021, 3, e12084.
- Wu, Y.; Zheng, P.; Peng, J.; Xu, M.; Chen, Y.; Surve, S.; Lu, T.; Bui, A.D.; Li, N.; Liang, W. 27.6% Perovskite/c-Si Tandem Solar Cells Using Industrial Fabricated TOPCon Device. Adv. Energy Mater. 2022, 12, 2200821.
- Chen, B.; Yang, Z.; Jia, Q.; Ball, R.J.; Zhu, Y.; Xia, Y. Emerging applications of metal-organic frameworks and derivatives in solar cells: Recent advances and challenges. Mater. Sci. Eng. R Rep. 2023, 152, 100714.
- Chueh, C.-C.; Chen, C.-I.; Su, Y.-A.; Konnerth, H.; Gu, Y.-J.; Kung, C.-W.; Wu, K.C.-W. Harnessing MOF materials in photovoltaic devices: Recent advances, challenges, and perspectives. J. Mater. Chem. A 2019, 7, 17079–17095.
- Heo, D.Y.; Do, H.H.; Ahn, S.H.; Kim, S.Y. Metal-organic framework materials for perovskite solar cells. Polymers 2020, 12, 2061.
- Butova, V.V.E.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280.
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–organic frameworks for separations. Chem. Rev. 2012, 112, 869–932.
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X. Solar cell efficiency tables (version 62). Prog. Photovolt. Res. Appl. 2023, 31, 651–663.
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425.
- Al Amery, N.; Abid, H.R.; Al-Saadi, S.; Wang, S.; Liu, S. Facile directions for synthesis, modification and activation of MOFs. Mater. Today Chem. 2020, 17, 100343.
- Sumida, K.; Liang, K.; Reboul, J.; Ibarra, I.A.; Furukawa, S.; Falcaro, P. Sol–gel processing of metal–organic frameworks. Chem. Mater. 2017, 29, 2626–2645.
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480.
- Ni, Z.; Masel, R.I. Rapid production of metal−organic frameworks via microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395.
- Vakili, R.; Xu, S.; Al-Janabi, N.; Gorgojo, P.; Holmes, S.M.; Fan, X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): Optimization and gas adsorption. Microporous Mesoporous Mater. 2018, 260, 45–53.
- Cole, J.M.; Pepe, G.; Al Bahri, O.K.; Cooper, C.B. Cosensitization in dye-sensitized solar cells. Chem. Rev. 2019, 119, 7279–7327.
- Bhattarai, S.; Mhamdi, A.; Hossain, I.; Raoui, Y.; Pandey, R.; Madan, J.; Bouazizi, A.; Maiti, M.; Gogoi, D.; Sharma, A. A detailed review of perovskite solar cells: Introduction, working principle, modelling, fabrication techniques, future challenges. Micro Nanostruct. 2022, 172, 207450.
- Marinova, N.; Valero, S.; Delgado, J.L. Organic and perovskite solar cells: Working principles, materials and interfaces. J. Colloid Interface Sci. 2017, 488, 373–389.
- Lee, D.Y.; Lim, I.; Shin, C.Y.; Patil, S.A.; Lee, W.; Shrestha, N.K.; Lee, J.K.; Han, S.-H. Facile interfacial charge transfer across hole doped cobalt-based MOFs/TiO2 nano-hybrids making MOFs light harvesting active layers in solar cells. J. Mater. Chem. A 2015, 3, 22669–22676.
- Chen, T.-Y.; Huang, Y.-J.; Li, C.-T.; Kung, C.-W.; Vittal, R.; Ho, K.-C. Metal-organic framework/sulfonated polythiophene on carbon cloth as a flexible counter electrode for dye-sensitized solar cells. Nano Energy 2017, 32, 19–27.
- Ahmed, A.S.; Xiang, W.; Amiinu, I.S.; Zhao, X. Zeolitic-imidazolate-framework (ZIF-8)/PEDOT: PSS composite counter electrode for low cost and efficient dye-sensitized solar cells. New J. Chem. 2018, 42, 17303–17310.
- Shen, D.; Pang, A.; Li, Y.; Dou, J.; Wei, M. Metal–organic frameworks at interfaces of hybrid perovskite solar cells for enhanced photovoltaic properties. Chem. Commun. 2018, 54, 1253–1256.
- Ahmadian-Yazdi, M.-R.; Gholampour, N.; Eslamian, M. Interface engineering by employing zeolitic imidazolate framework-8 (ZIF-8) as the only scaffold in the architecture of perovskite solar cells. ACS Appl. Energy Mater. 2020, 3, 3134–3143.
- Ryu, U.; Jee, S.; Park, J.-S.; Han, I.K.; Lee, J.H.; Park, M.; Choi, K.M. Nanocrystalline titanium metal–organic frameworks for highly efficient and flexible perovskite solar cells. ACS Nano 2018, 12, 4968–4975.
- Nguyen, T.M.H.; Bark, C.W. Synthesis of cobalt-doped TiO2 based on metal–organic frameworks as an effective electron transport material in perovskite solar cells. ACS Omega 2020, 5, 2280–2286.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
536
Revisions:
2 times
(View History)
Update Date:
17 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




