Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
Developing low-cost and stable materials for converting solar energy into electricity is vital in meeting the world’s energy demand. Metal-organic frameworks (MOFs) have gained attention for solar cells due to their natural porous architectures and tunable chemical structures. They are built by high-symmetry metal clusters as secondary building units and organic carboxylate/azolate ligands as linkers.
- MOFs
- hydrothermal method
- sol-gel
- porous materials
- solar cells
- photoanodes
1. Introduction
In recent years, melting ice, drought, and flooding have increased significantly. This is a direct result of global warming, attributed to the increased greenhouse gases released from using conventional fossil fuels [1,2,3,4,5,6,7,8,9,10]. Therefore, there needs to be a shift from fossil fuel to renewable energy to meet the increasing demands of power without causing much environmental contamination [11,12,13,14,15,16,17,18,19,20]. Among renewable energy sources, solar energy is considered an ideal solution due to zero CO2 emission and abundant resources [21,22,23,24,25,26]. Nonetheless, problems of solar cells are expensive, and restricted scale. Solar cells based on silicon are the most widely used for converting solar energy to electricity to satisfy human energy demands. Until now, Si-based materials displayed the highest performance for converting solar energy into electricity [27,28,29,30,31]. However, solar cell-based Si materials are ineffective with inside sunlight due to their unsuitable band gap. As a result, various types of solar cells involving dye-sensitized solar cells (DSSC), perovskite solar cells (PSC), and organic solar cells (OSC) have been explored with a large number of publications in recent years (Figure 1) [32,33,34].
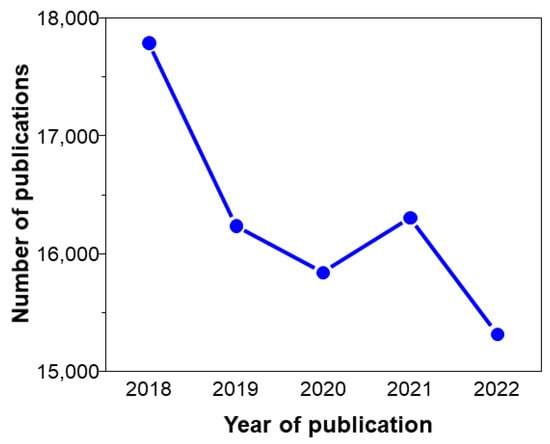
Figure 1. Number of publications resulting from the search of “solar cells” on Web of Science, accessed on 12 September 2023.
Regarding DSSC, TiO2 was identified as a typical photoanode material, whereas Pt-based materials are the most effective counter electrodes (CE). However, the fast excitons recombination of TiO2 and the high cost of Pt have driven scientists to explore the novelty alternatives in solar cell applications. Furthermore, stability is an issue of concern for PSC and OSC researchers due to the instability of organic components. As a result, ultra-stable materials and integrated methodologies for increasing the long-term stability of PSC and OSC must be investigated [35,36,37].
2. Synthetic Methods of MOFs
2.1. Hydrothermal Technique
The hydrothermal process is a typical method for producing MOFs and materials developed from them for solar cells. Various sizes and structures of MOFs were created by varying the temperature, reaction duration, and pressure [52]. Despite the effectiveness of obtaining MOFs, it was plagued by low yield and expansiveness. Furthermore, the reaction pathway of MOFs and their derived materials remains to be discovered.
2.2. Solvothermal Method
The solvothermal technique is generally acknowledged as an effective method for synthesizing MOFs and their composites by virtue of its high yield and facial control. The solvent is vital in the solvothermal reaction process. Until now, the most often employed organic solvents for MOF fabrication are N, N-dimethylformamide, ethanol, and methanol. These organic solvents might shift MOFs’ structure and size for specific applications. However, organic solvents’ toxicity and environmental unfriendliness limit their widespread use. Furthermore, organic solvents are difficult to decontaminate and recycle. As a result, developing safe and ecologically acceptable solvents for the solvothermal process is critical [53].
2.3. Sol-Gel
Recently, the sol-gel approach has been frequently used to produce inorganic nanomaterials. The synthesis procedure causes ion co-precipitation, which results in the generation of the desirable structures. This approach is more effective than the hydrothermal process for synthesizing MOFs. MOFs with variable sizes and shapes might be created by optimizing experimental parameters such as reaction temperature, duration, etc. Furthermore, MOFs might be employed as a precursor to create derivatives with outstanding properties [54].
2.4. Other Methods
Besides the hydrothermal technique, solvothermal process, and sol-gel methods, MOFs could be prepared by other strategies involving the electrochemical technique, microwave, and ultrasound methods [55,56,57].
3. Solar Cells
Solar cells are electronic devices for converting the sun’s energy directly into electricity. Generally, a solar cell comprises a semiconductor with a photovoltaic effect once illuminated. In terms of DSSC, the mechanism of the photovoltaic device includes three steps: light adsorption, charge production, and charge transport. Notably, the first process is the light adsorption of the dye molecule to change the excited state (dye*), after which this dye provides electrons for the conduction band of the semiconductor, accompanied by the oxidation process of dye*. Then, the injected electrons will move to the photoanode before transferring to CE through an external circuit [58]. In terms of PSC, this device generally includes five parts: FTO (Fluorine-doped Tin Oxide) glass as a conductive substrate, an electron transfer layer (ETL), a hole transfer layer (HTL), a perovskite layer, along with a metal electrode. PSC’s operation may be broken down into three steps: light adsorption and production of excitons, electron-hole separation, and charge transfer process. In particular, the perovskite layer will absorb solar energy to create electrons in the conduction band and holes in the valance band. The electrons then move to ETL before being transported to the anode, whereas the holes flow to HTL before being transferred to the cathode [59]. The process includes four steps for OSC’s working principle: light adsorption/electron-hole pairs production, charge diffusion, charge dissociation, and charge transport to the electrodes [60]. In particular, when a p-type material absorbed solar energy, electrons will be excited from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). Then these electrons will move to the LUMO of the acceptor before transferring into the cathode.
4. MOFs for Dye-Sensitized Solar Cells
4.1. MOFs as Photoanodes
Over the past decades, DSSC have gained considerable interests of scientists around the world because of their low-price, facial preparation, and high η. A typical DSSC is constructed from a photoanode, a CE, and a redox solution. TiO2 was evaluated as a conventional photoanode material. But, the fast excitons recombination of TiO2 has driven researchers to find the novelty photoanode in solar cell applications. On the one hand, MOFs could be utilized as photosensitizers for the conversion of sunlight into electricity. For example, Lee et al. conducted hole doping on various structures, including Co-BDC (BDC = benzenedicarboxylate) and Co-NDC (NDC = 2,6-naphthalendicarboxylic acid) for DSSCs [61]. The results revealed that I2-doped Co-based MOFs as photoanodes displayed a potential performance for energy conversion. Notably, I2-doped Co-NDC/TiO2 gave an efficiency of 1.12%, whereas this quantity is 0.96% for I2-doped Co-NDC/TiO2. The improved performance was attributed to the well-interaction between I2 and π-electrons of linkers.
4.2. MOFs as Counter Electrodes
The counter electrodes (CE) play vital roles in the construction of photovoltaic devices and in determining efficiency. The CE is created from depositing an electrocatalyst on the conductive template. Herein, Pt is generally utilized as electrocatalyst. Although Pt-based thin layers are the best CE for DSSC, high cost leads to an increase in the cost of solar cells. MOFs with a well-distribution of metal active sites were appreciated as promising CE in DSSC. For instance, Chen et al. blended MOF-525 with a conductive polymer, followed by coating on the carbon cloth to form an efficient CE in DSSC [68]. This device displayed a high cell performance of 8.91%, comparable with Pt CE-based solar cells. The effectiveness of using MOFs for CEs has been exemplified in a study by Zhao et al. [69]. The authors found that using ZIF-8 for fabrication CE brings higher power conversion efficiency than without MOF in DSSC.
5. MOFs for Perovskite Solar Cells
5.1. MOFs as Interfacial Layers (IL)
PSCs have been proven as potential candidates for replacing inorganic solar cell by virtue of excellent optical adsorption, inexpensiveness, and fast charge transfer. Recently, works related to the enhancement of PSC’s long-term durability have been implemented through a strategy of compositional engineering. Notably, by using inorganic ions to substitute unstable organic components, stability of PSC could be substantially improved. Also, interfacial engineering is an effective strategy to enhance stability of PSC. Recently, MOFs with high stability were employed as IL in PSC to improve the particle size and perovskite crystallinity and hinder electron-hole recombination, leading to increased solar cell efficiency. For instance, Shen et al. inserted ZIF-8 IL between the PSC application’s mesoporous TiO2 and perovskite component [72]. This strategy improved the solar cell efficiency from 14.75% (without ZIF-8) to 16.99% (with ZIF-8), attributed to the improved crystal quality and efficient suppression of exciton recombination. Ahmadian-Yazdi et al. used ZIF-8 as an interfacial modifier in PSC [73]. To confirm the ability of charge extraction, Photoluminescence (PL) was analyzed with various samples. The outcome revealed that c-TiO2/ZIF-8-10/perovskite displayed the smallest intensity of PL, implying the most effective charge extraction. As a result, this material exhibited the highest solar cell performance of 16.8%. Recently, Jin et al. utilized ZIF-8 as a host material to confine methylammonium chloride (MACl) to form an effective IL between SnO2 and the perovskite laye.
5.2. MOFs as Charge Transfer Layers
MOFs have been identified as promising materials for charge transporting in PSC. On the one hand, MOFs have been applied to improve electron transfer in PSC. For instance, Ryu et al. mixed a Ti-based MOF with commercial carbon material (PCBM) to create an electron transfer layer (ETL) [75]. This approach not only improves charge transport but also prevents the recombination of excitons. As a result, the rigid nTi-MOF/PCBM device exhibits a solar cell efficiency of 18.94%, whereas its flexible device has a performance of 17.43%. Nguyen et al. used a metal doping strategy on TiO2 derived from Ti-based MOF to produce an efficient ETL in PSC [76].
6. MOFs for Organic Solar Cells
OSC are a potential new energy conversion device that has significant prospects to deliver excellent η by utilizing inexpensive, adjustable conductive polymers or organic molecules. Moreover, these materials could be applied for industrial applications, implying that large-scale preparation is achievable. However, the stability of OSC is a headache problem for scientists. Therefore various strategies were conducted to improve η as well as stability of OSC devices. Interfacial engineering was identified as a primary strategy for increasing power conversion efficiency. The interfacial layers in OSCs could take various roles, involving shifting the energetic barrier at the metal/photoactive material interface, providing contact selectivity, hindering chemical or photophysical interaction between the photoactive component and the electrode, and acting as an optical spacer to maximize photocurrent. Compared to the rising uses in DSSC and PSC, MOFs have yet to be fully investigated in OSCs due to their low semiconducting characteristics. Up to now, only a few works related to MOFs for OSC application.
7. Conclusions
In summary, MOFs have emerged as a new topic in solar applications, which introduced tremendous theoretical and experimental works to improve the photovoltaic capabilities of MOFs. In particular, MOFs could be utilized as counter electrodes or photoanodes for DSSC with potential efficiency. Moreover, interface modifiers and charge transport components could be made from MOFs for PSC. Additionally, several works related to the usage of MOFs for OSC could bring a novelty direction in photovoltaic applications
This entry is adapted from the peer-reviewed paper 10.3390/sym15101830
This entry is offline, you can click here to edit this entry!
