Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodriguez-Amaya Delia | -- | 6553 | 2023-11-15 02:57:00 | | | |
| 2 | Jessie Wu | Meta information modification | 6553 | 2023-11-15 03:55:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Carotenoid Colorants from Plants and Microalgae. Encyclopedia. Available online: https://encyclopedia.pub/entry/51571 (accessed on 07 February 2026).
Rodriguez-Amaya DB, Esquivel P, Meléndez-Martínez AJ. Carotenoid Colorants from Plants and Microalgae. Encyclopedia. Available at: https://encyclopedia.pub/entry/51571. Accessed February 07, 2026.
Rodriguez-Amaya, Delia B., Patricia Esquivel, Antonio J. Meléndez-Martínez. "Carotenoid Colorants from Plants and Microalgae" Encyclopedia, https://encyclopedia.pub/entry/51571 (accessed February 07, 2026).
Rodriguez-Amaya, D.B., Esquivel, P., & Meléndez-Martínez, A.J. (2023, November 15). Carotenoid Colorants from Plants and Microalgae. In Encyclopedia. https://encyclopedia.pub/entry/51571
Rodriguez-Amaya, Delia B., et al. "Carotenoid Colorants from Plants and Microalgae." Encyclopedia. Web. 15 November, 2023.
Copy Citation
The substitution of synthetic food dyes with natural colorants continues to be assiduously pursued. The current list of natural carotenoid colorants consists of plant-derived annatto (bixin and norbixin), paprika (capsanthin and capsorubin), saffron (crocin), tomato and gac fruit lycopene, marigold lutein, and red palm oil (α- and β-carotene), along with microalgal Dunaliella β-carotene and Haematococcus astaxanthin and fungal Blakeslea trispora β-carotene and lycopene. Potential microalgal sources are being sought, especially in relation to lutein, for which commercial plant sources are lacking.
plant-derived colorants
microalgal carotenoids
microencapsulation
nanoencapsulation
green extraction
by-product valorization
1. Introduction
The world’s food color market continues to be dominated by artificial color additives but driven by concerns about possible adverse health effects, their substitution with those obtained from natural sources has been widely advocated. Research and commercial interest in natural colorants have intensified with mounting scientific evidence for their health benefits, along with consumers’ demand for food to be as natural as possible [1][2]. The transition from synthetic to natural colorants, however, is not easily accomplished, considering that the latter are usually unstable, more costly, and not as easily utilized. Moreover, they have weaker tinctorial strength, a limited range of hues, and may interact with other food components [3][4][5]. Research on natural colorants should therefore focus on obtaining a wider variety of colors, using pigments with health benefits, increasing shelf life, and lowering production costs [6].
Cheaper and faster to produce, carotenoids in the market (e.g., β-carotene, astaxanthin, canthaxanthin, zeaxanthin, and β-apo-8′carotenol) are mostly products of chemical synthesis [7]. However, some natural carotenoid colorants produced from plant sources and by microbial fermentation are also available, and demand for these products is increasing.
Aside from the provitamin A activity, carotenoids have been associated with a reduced risk of developing certain types of cancer, cardiovascular diseases, cataracts, and macular degeneration [8][9][10]. More recently, other health-promoting effects have been attributed to these bioactive compounds, such as maintenance of cognitive functions [11][12][13], reduced level of depressive symptoms [14][15], and reduced risk of osteoporosis and fracture [16][17][18].
Carotenoids, however, pose daunting challenges to researchers and food processors due to their instability, lack of solubility in water, and low bioavailability [19]. Research has therefore been directed to addressing these problems, whether the carotenoids are inherent constituents of foods or are added as colorants.
2. The Carotenoid Chromophore
The vast majority of carotenoids absorb maximally in the wavelength range of 400–500 nm. The centrally located conjugated double-bond system constitutes the light-absorbing chromophore that confers the carotenoid’s attractive color [19]. At least seven conjugated double bonds are needed for a carotenoid to have perceptible color (faint yellow). As the number of conjugated double bonds increases, the color changes from yellow to orange, and then to red. The acyclic crocin with nine double bonds is yellow and the acyclic bixin, having a total of eleven conjugated double bonds, is orange–red (Figure 1). One of the double bonds of bixin is in the Z(cis)-configuration; the absorption maxima of Z-double bonds are slightly shifted to shorter wavelengths. The acyclic lycopene, with 11 conjugated E(trans)-double bonds, is red. Cyclization takes the π electrons of the ring double bond out of plane with those of the polyene chain. Thus, although also possessing 11 conjugated double bonds, β-carotene is yellow–orange because two of the double bonds are in β-rings. Lutein and α-carotene with 10 conjugated double bonds, one of which is in a ring, are yellow.
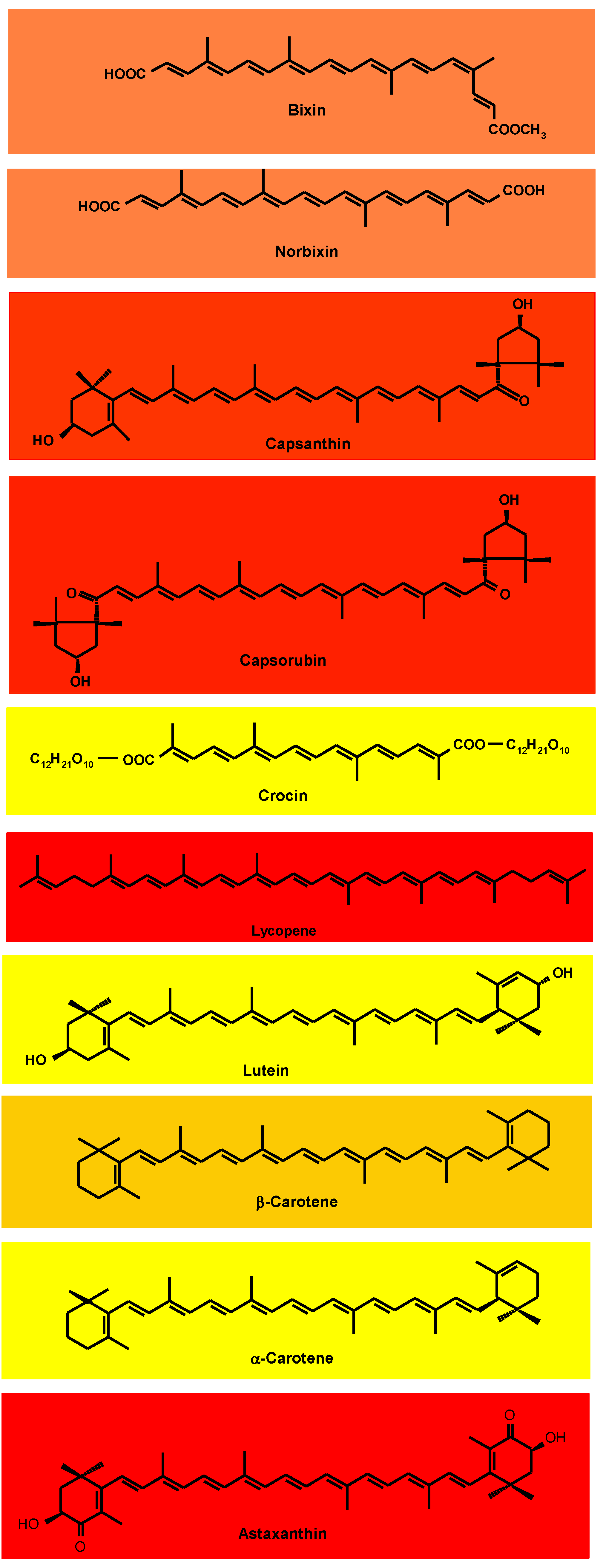
Figure 1. Structures of the principal carotenoids of commercial natural carotenoid colorants.
The red capsanthin has a conjugated double bond system consisting of the carbonyl group double bond, nine double bonds in the polyene chain, and one in the β-ring (totaling eleven conjugated double bonds) (Figure 1) [19]. Also red, capsorubin’s chromophore consists of nine conjugated double bonds in the polyene chain extended by the double bonds of two carbonyl groups. Red astaxanthin has nine conjugated double bonds in the polyene chain, extended by two double bonds in β-rings and 2 C=O.
On exposure to light, heat, and acids, carotenoids undergo E-Z isomerization, which results in a small spectral shift to shorter wavelengths, thus a slight lightening of the color [19].
Apart from the chemical structure, the color of carotenoids is also dependent on other factors such as concentration and aggregation or interaction with proteins. Indeed, carotenoproteins extend the colors of carotenoids to blue, purple, green, and brown [20].
The conjugated double-bond system is also responsible for the susceptibility of carotenoids to oxidation [19]. Color fading is a well-known problem during processing and storage of carotenogenic foods. As oxidative degradation advances, the conjugated double bond system is progressively shortened until the number of conjugated double bonds falls below seven and the carotenoid molecule becomes colorless [19][21][22].
3. Carotenoid Colorants Derived from Higher Plants
Recent investigations on plant-derived colorants focus on: (a) optimizing extraction to obtain the maximum pigment yield and to turn to green extraction, (b) stabilizing the colorant by microencapsulation or nanoencapsulation, and (c) additional benefits (aside from color) of the main coloring carotenoids, especially health promoting effects. A summary of commercially available natural colorants is presented in Figure 2.

Figure 2. Summary of commercially available natural carotenoid colorants for food use.
A major problem with plant-derived carotenoid colorants is batch-to-batch variation in the carotenoid concentrations of the raw materials due to cultivar/varietal differences, seasonal and geographic variability, maturity at harvest, climatic conditions, etc. [19]. According to Bogacz-Radomska and Harasym [23], the main disadvantages of the production of carotenoid colorants from plant materials are the high cost, geographic determinants, and seasonality of the raw material.
It is now widely accepted that the reduction of the risk of chronic diseases by plant foods is not due to a single food component or a single class of compounds but to the coexistence of different bioactive compounds [19]. They have different modes of action, act at different stages of disease development, and demonstrate additive or even synergistic effects. Thus, plant extracts used as colorants, which usually contain bioactive compounds other than the carotenoids, can be more beneficial for preventing the risk of some chronic diseases than individual carotenoids.
3.1. Annatto Bixin and Norbixin
The orange-to-red annatto is derived from the resinous, thin seed coats of the capsular fruits of Bixa orellana, a tropical tree believed to be native to Central and South America [19]. Annual world production of the seeds is approximately 14,500 tons dry weight (DW) [24]. Two-thirds of the production is commercialized as dried seeds and the rest as colorants. Latin America produces 60% of the total world production, followed by Africa (27%) and Asia (12%). The main producers in Latin America are Peru, Brazil, and Mexico.
Annatto is available as an oil-soluble extract, water-soluble extract, suspension, emulsion, encapsulated product, and dried powder. It is used worldwide to color a wide range of products, such as butter, cheese, margarine, mayonnaise, sauces, salad dressings, mustard, soups, juices, ice cream, bakery products, snacks, soft drinks, desserts, meat products, and macaroni [19].
The coloring agent in oil-soluble annatto preparations is bixin, a monomethyl ester of a dicarboxylic apocarotenoid (carotenoids in which the carbon skeleton has been shortened by removal of fragments from one or both ends of the usual C-40 structure) (Figure 1) [19]. Hydrolysis (saponification) liberates the dicarboxylic, water-soluble norbixin.
Unlike most carotenoids, which occur in nature in the all-E-configuration, bixin is normally in the 9Z-form (Figure 1). In general, carotenoids undergo E-Z isomerization and oxidative degradation during processing and storage of foods [19][21][22]. The latter consists of epoxidation, cleavage to apocarotenoids, and finally, cleavage to low-mass (volatile) compounds. On exposure of bixin to light, heat, or acids during extraction, processing, and storage, Z-E isomerization occurs, slightly accentuating the red color. Bixin’s conjugated double bond system is also prone to oxidative degradation, eventually leading to its cleavage to low-mass (volatile) compounds [25], manifested by the loss of color and sometimes by off-flavor.
It is well known that bixin is unstable in the presence of oxygen, light, high pH (alkali), and heat. Light, reduced pH, and metal ions, with and without H2O2, increase the bleaching of norbixin, whereas chelators and the natural antioxidants, ascorbic acid and tocopherol, reduce the bleaching [26].
Norbixin in buffered aqueous solutions was stored under light and in the dark and analyzed by mass spectrometry [27]. Compounds with both higher and lower masses than norbixin were detected, suggesting that oxidation products and oxidative cleavage products of norbixin were produced. The compounds formed were not identified, however. The norbixin concentration decreased during storage, the loss occurring faster under light.
The effects of different extraction methods on the degradation of bixin and the formation of undesirable volatile compounds were investigated by Chuyen and Eun [28]. Extraction with sodium hydroxide solution at 50 °C was recommended for extracting sufficient pigment from annatto seeds with minimum risk of forming harmful volatile compounds. Extraction with acetone in a Soxhlet extractor resulted in the highest bixin yield but produced the highest amount of xylene. Extraction with soybean oil at 120 °C gave the lowest bixin yield and caused significant bixin degradation. All three methods produced negligible amounts of toluene.
The impact of solvents (ethyl acetate, methanol, and ethanol) and varying process parameters such as time (10, 20, and 30 min), temperature (50, 60, and 70 °C), and seed-to-solvent ratio (1:5, 1:10, and 1:15) on bixin yield was evaluated by Jayakumar et al. [29]. Methanol was observed to be the optimal solvent; the optimum conditions were 70 °C, a seed-to-solvent ratio of 1:15, and a treatment time of 30 min.
Microwave dielectric heating and ultrasonic cavitation bubbles demonstrated substantial improvement in the extraction of carotenoids over conventional heating methods, with microwave heating being the optimal technique [30]. Using the optimum extraction parameters for annatto seeds (at least 30 min extraction with ethyl acetate, solvent/material ratio of 0.05 L/g), the yield of bixin reached about 85% with sonication and 95% with microwave heating.
Extraction obtained by using ultrasound (UAE) is mainly attributed to the effect of acoustic cavitations produced in the solvent as a result of ultrasound wave passage [31]. Compared to other extraction techniques such as microwave-assisted extraction (MAE) and supercritical fluid extraction (SFE), the ultrasonic device is less expensive and much easier in practice. UAE was found to be a more efficient process compared to conventional extraction.
Physical separation methods were used to obtain the pigment of semi-defatted annatto seeds, the residue produced after the extraction of the tocotrienol-rich oil using SFE [32]. The physical methods included mechanical fractionation and an integrated process of mechanical fractionation and low-pressure solvent extraction. The latter method required a significantly higher cost. The mechanical fractionation method was considered an adequate and low-cost process to obtain a pigment-rich product from semi-defatted annatto seeds.
Norbixin is water-soluble at neutral and alkaline pH but starts to precipitate below neutral pH [33]. The addition of whey protein isolate prevented norbixin precipitation between pH 2 and pH 7, except at pH 5. At the latter pH, isoelectric precipitation of whey protein isolate was prevented by the inclusion of alginate. Encapsulation of norbixin within liposomes was also shown to increase its water dispersibility and chemical stability under acidic pH conditions [34].
Annato extract, apart from being a colorant, has other desirable properties such as antimicrobial and antioxidant activities. The survival curve of the food-born pathogen Escherichia coli in mayonnaise with annatto reached zero during 15 and 12 days after inoculation at 4 and 25 °C, respectively [35]. All annatto extracts obtained by maceration with distilled water at various pH and temperature had the potential to inhibit E. coli and Staphylococcus aureus [36]. Paprika, lutein, and especially annatto, investigated against ten microorganisms, had antimicrobial effects, particularly against gram-positive bacteria, including Staphyloccus aureus, Staphylococcus epidermidis, Bacillus cereus, Bacillus subtilis, Listeria monocytogenes, and Streptococcus pyogenes [37]. The addition of 1 to 10% annatto to bread formulation adequately inhibited the growth of Aspergillus niger, Neurospora sitophila, Rhizopus stolonifer, major pathogens and spoilage microorganisms of bread and many other food products [38]. Antioxidant activity was also observed. The bread with annatto had a longer shelf life and acceptable sensory qualities.
β-carotene, α-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin have been the most studied carotenoids in terms of human health. Epidemiological and supplementation studies have been carried out and submitted to meta-analyses [10]. The health benefits of bixin and norbixin have not been the focus of research.
In several pathologies, erythrocytes exhibit high susceptibility to hemolysis because of the oxidation of cellular components. Beni et al. [39] investigated whether food-grade annatto carotenoids could increase human erythrocyte resistance to hemolysis in vitro and ex vivo. For the in vitro experiment, erythrocytes from healthy volunteers were isolated and coincubated with bixin or norbixin and 2,2′-azobis(2-amidinopropane) dihydrochloride, glucose, or sodium nitrite as hemolysis inducers. In the ex vivo study, healthy volunteers consumed a capsule containing bixin or norbixin or placebo for 7 days before blood sample collection. The results supported the hypothesis that supplementation with annatto carotenoids exerted antihemolytic properties by preventing the oxidative damage of human erythrocytes.
Bixin and crude extract were examined in vitro in human lung cancer, cervical cancer, and breast cancer cells [40] Anti-proliferative activity appeared promising on both the isolated pigment and the crude extract.
In a randomized, controlled crossover study involving 12 healthy subjects, the effect of annatto intake associated with a single high-caloric meal (high fat and high carbohydrate) was evaluated [41]. Norbixin intake did not affect biochemical blood markers but reduced the postprandial levels of inflammatory cytokines and lipid oxidation 60–120 min after the meal. Bixin only partially prevented postprandial-induced lipid oxidation. The results indicated that the intake of norbixin might be an alternative to reduce the postprandial inflammatory and oxidative stress responses to high-caloric meals.
3.2. Paprika Capsanthin and Capsorubin
A possible problem with plant-derived colorants is that the flavor of the plant source may be carried over to the final product and may not be compatible with it [42]. This is not the case with paprika and saffron, however, both of which serve as spice and colorant.
Paprika is a deep red, pungent powder obtained from red pepper (Capsicum annuum) pods. It has a complex mixture of carotenoids, the most prominent of which are capsanthin and capsorubin (Figure 1) [19].
Paprika oleoresin is produced by solvent extraction of the ground powder [19]. Solutions in edible vegetable oil and water-miscible forms of oleoresin are also available in the market. Paprika is limited to products compatible with its flavors, such as meat products, sausages, smoked pork, sandwich spreads, soups, sauces, salad dressings, spice mixtures, cheeses, orange juice, snacks, confectionery, and baked products.
Paprika powders from Bulgaria, China, Hungary, Peru, Serbia, and Spain were examined to identify the most important differences in their major characteristics and to try to find chemical components that reveal their origin [43]. Carotenoids were found at the highest concentrations in samples from Peru and Spain and at the lowest in Serbian samples.
Compared with the traditional extraction methods, UAE and MAE can improve the color value of paprika pigment and shorten the extraction time [44]. More importantly, it can avoid the decomposition of active substances caused by long-time extraction under high temperatures and pressure.
Paprika oleoresin was obtained by accelerated solvent extraction (ASE), maceration extraction, and UAE [45]. ASE, also known as pressurized liquid extraction (PLE) or pressurized fluid extraction, is an automatic extraction technology performed at elevated temperature and pressure to achieve efficient extraction of compounds from solid or semisolid samples in a very short time. The color values, total carotenoid, and capsaicinoid content were significantly higher for ASE than the other two extraction methods.
Cinnamaldehyde and carotenoids in cinnamon and paprika oleoresins, respectively, exhibited pronounced antimicrobial and antioxidant potential [46]. The coencapsulation of the two oleoresins by spray chilling promoted greater stability and synergism between them. Cinnamon:paprika (1:1 and 2:1) mixtures showed a synergistic effect against Penicillium paneum and Aspergillus niger. The extracts also prevented the growth of microorganisms without direct contact with the agar. The concentration of carotenoids in the particles remained constant throughout the 49 days of storage at 5 and 25 °C.
De Aguiar et al. [47] reviewed the application of supercritical fluid technologies to Capsicum peppers and derived products, including oleoresin. Trends in encapsulation technologies applied to Capsicum and derivatives were also discussed. However, the compounds of interest were capsaicin and phenolic compounds, not carotenoids.
To offer consumers healthier meat products, paprika oleoresin was used to replace or reduce the nitrite level [48]. Approximately 3/4 of the initial nitrite level could be replaced with 0.1% paprika oleoresin solution.
3.3. Saffron (Crocin)
Saffron, the dried stigmas of Crocus sativus flowers, is considered the world’s most expensive spice. C. sativus has been in cultivation for many centuries. Presently, the major growing areas are Iran, India, Morocco, Spain, Turkey, Italy, Afghanistan, and New Zealand [49]. Iran produces almost 90% of the total world production. Saffron is used in the food industry for the manufacture of a wide range of products, including dairy, bakery, sauces, soups, chicken, rice, and beverages [19][42].
Kotheri et al. [49] called attention to the fact that only the stigma of the flower is used, the remaining floral parts going to waste. Utilization of this discarded material certainly merits research.
Saffron’s prominent constituents are crocin, picrocrocin, and safranal, responsible for its color, bitter taste, and aroma, respectively [50]. These compounds are the bio-oxidative cleavage products of zeaxanthin. The yellow crocin is a crocetin digentiobiose ester. It is a symmetrical apocarotenoid in which the two carboxylic groups of the C-20 carotenoid crocetin are esterified with the disaccharide gentiobiose (Figure 1), making the pigment water-soluble.
Crocin is a highly bioactive compound, but its use is limited by its instability to pH variations, light, heat, and oxidative stress, along with rapid absorption and low bioavailability [51]. Encapsulation of saffron extracts within polymeric matrices has been shown to improve their stability during storage [52] and in simulated gastric conditions [53].
Numerous health-promoting activities have been attributed to saffron/crocin, such as antioxidant, antitumor, anti-inflammatory, anticancer, antidiabetic, anxiolytic, neuroprotective, learning and memory-enhancing, antidepressant, antihyperlipidemic, antiatherosclerotic, anti-ischemia, antigenotoxic, hypoglycemic, hypotensive, antidegenerative [50][51][54][55][56][57][58][59][60][61][62]. Notably, intense research on the health effects of crocin has been undertaken, not in food research, but in pharmacological studies. The demand for saffron has been rising in the pharmaceutical industry [56]
Crocin and other crocetin glycosides are also found in the fruit of Gardenia jasminoides and Gardenia augusta [63]. Gardenia yellow is a colorant produced by water or ethanol extraction of these fruits and is approved for food use in Japan and China, but not in the USA or the EU.
3.4. Tomato and Gac Fruit Lycopene
The widely reported health benefits of lycopene and lycopene-rich tomatoes, especially in relation to prostate cancer, had drawn worldwide attention. Hence, tomato lycopene extract and tomato lycopene concentrate as oleoresin, powder, and water-dispersible preparations became commercially available.
Interest in lycopene heightened with the comprehensive studies on inverse associations between tomato or lycopene intake or serum lycopene level and the risk of prostate cancer [64][65][66][67][68]. Lycopene was also linked with a reduction in the risk of developing other types of cancer. In a large prospective analysis with 20 y of follow-up, women with high plasma carotenoids were found to be at reduced breast cancer risk, particularly for more aggressive and ultimately fatal disease [69]. Meta-analyses also associated lycopene with a lower risk of esophageal cancer [70] and oral and pharyngeal cancer [71].
There is also good evidence from meta-analyses for an association between intake and/or blood concentration of lycopene with a reduced risk for stroke and cardiovascular diseases [72][73][74], A comprehensive meta-analysis suggested that high-intake or high-serum concentration of lycopene was linked with significant reductions in the risk of stroke (26%), mortality (37%) and cardiovascular diseases (14%) [75]. Moreover, supplementation with tomato products and lycopene had positive effects on blood lipids, blood pressure, and endothelial function [76].
The Asian gac fruit (Momordica cochinchinensis), has been shown to have an impressively high content of lycopene, 408 μg/g fresh weight (FW) according to Vuong et al. [77]. Traditionally used in Asia to provide red color for cuisines and enhance vision health, it is now commercially available as gac powder and gac oil, manufactured as natural colorants and medicinal supplements [78].
3.5. Marigold Lutein
Marigold (Tagetes erecta) flower is the commercial source of lutein. Originally cultivated in Mexico and other warmer areas of America, marigold is now naturalized in other tropical and subtropical regions [79].
Marigold lutein has been used as an additive in poultry feed to improve the pigmentation of the bird’s fat, skin, and egg yolk [16]. The main coloring component is all-E-lutein esterified with fatty acids.
Lutein is highly beneficial in terms of human health. According to most epidemiological and clinical trials, lutein and zeaxanthin have a role in the prevention of certain eye diseases such as age-related macular degeneration, cataracts, and retinitis pigmentosa [80].
A meta-analysis of six longitudinal cohort studies showed that dietary intake of lutein and zeaxanthin was significantly related to reduced risk of late age-related macular degeneration, but not to reduced risk of early age-related macular degeneration [81]. Statistically inverse association was observed between intake of these carotenoids and neovascular age-related macular degeneration. Another meta-analysis showed significant benefits of lutein and zeaxanthin supplementation to visual acuity and contrast sensitivity for macular degeneration patients, positively associated with elevation of macular pigment optical density [82].
Blood levels of lutein and zeaxanthin were inversely associated with age-related cataracts [83] and nuclear cataracts [84]. Likewise, dietary intakes of these carotenoids had a significant inverse association with nuclear cataracts and posterior subcapsular cataracts, but not with cortical cataracts [85].
Meta-analyses have also shown that higher intake and/or blood concentration of lutein/zeaxanthin were associated with lower risk of some types of cancer, such as esophageal cancer [70] and non-Hodgkin lymphoma [86]. Moreover, these carotenoids have also been linked with cardiometabolic health [87] and better cognitive function [10][11][88].
3.6. Red Palm Oil α- and β-Carotene
For a long time, orange carrot was the popular source of the provitamin A carotenoids β-carotene and α-carotene. A more concentrated source of these two carotenoids is red palm oil, which has been investigated and proposed for food-based intervention for vitamin A deficiency amelioration [89].
Oil palm (Elaeis guineenses) has been cultivated in Asia and Central America. It is the most important economic crop in Malaysia; other leading palm oil-producing countries are Indonesia and Thailand [90].
Red palm oil can be obtained from the mild processing of crude palm oil while refined, bleached, and deodorized palm oil is obtained by physical refining or chemical refining of the crude palm oil [90]. The bleaching process that uses bleaching clay removes the color pigments and residual soaps from the oil. During the deodorization step of physical refining, edible oils are subjected to high temperatures (250–270 °C) and low pressures (3–5 torr) to remove free fatty acids and volatile compounds that affect the oil’s odor and flavor. Palm carotenoids are removed during the refining process to obtain clear oil which is better for consumer acceptance and industry purposes.
Refined, bleached, and deodorized palm oil is the major processed product, used in more than 150 countries around the world [91]. Red palm oil has become well accepted in Africa, Indonesia, India, China, and Malaysia. It is not widely available in the international markets. The minimally processed palm oil has been introduced to Western consumers only recently, receiving mixed reactions. Some people find the red–orange hue unappetizing, while others view the color as a welcome reminder of the oil’s high carotene content [92].
Red palm oil can be added to food products such as cooking oil, shortening, spreads, salad dressings, and margarine. [93]. One product that has the potential to be developed based on red palm oil in Indonesia is margarine because it can act as a source of fat and also provides natural coloring and high nutrition from phytonutrient components, especially its carotene content. In Brazil and African countries, red palm oil has been used for years for culinary purposes.
Red palm oil is rich in phytonutrients such as tocotrienols, tocopherols, carotenoids, and phytosterols. Aside from the provitamin A activity, other health benefits attributed to red palm oil include improvement of ocular complications; cardioprotective effects in ischemic heart disease; antiatherogenic, antihemorrhagic, antihypertensive, and anticancer properties; support of normal reproduction for both males and females; improved management of diabetes and chemotherapy; improved management of hypobaric conditions; and protection against infection [91]. However, human studies to support these claims are lacking.
Considering the large-scale production of refined, bleached, deodorized palm oil, during which enormous amounts of β-carotene and α-carotene are wasted, sporadically through the years, proposals to separate these valuable carotenoids from the oil before processing have surfaced. An example is the recent work of Hoe et al. [94]. Liquid-liquid extraction was used to directly extract palm carotenes from crude palm oil without disrupting the subsequent production of refined palm oil. Dimethyl sulfoxide and dichloromethane were adopted as solvent systems because of their excellent performance in palm carotene extraction. After optimization, the optimal percentage recovery of palm carotene was 41%. The total recovery of palm carotene increased up to 68% by using a multistage extraction approach.
4. Microalgal Carotenoid Colorant
Microbial fermentation for the production of natural colorants has been intensely investigated in recent years [6][7][95][96][97][98][99][100][101]. Several advantages have been cited, such as controlled cultivation, faster growth, higher yields, easier extraction, lower-cost raw materials, no seasonal variations, higher renewability than plant and animal sources, and strain improvement techniques to increase natural pigment. Microbial colorants are often considered better alternative to synthetic food colors compared to higher plant pigments However, in spite of the considerable potential and wide research interest, few carotenoids from microorganisms have reached commercial production: β-carotene by the microalga Dunaliella salina, astaxanthin by the microalga Haematococcus pluvialis, and β-carotene and lycopene by the fungus Blakeslea trispora. According to Begum et al. [102], β-carotene from other microalgae especially Cyanobacteria is being produced in large scale in India.
H. pluvialis is the widely used scientific name for this microalga. However, Nakata and Ota [103] asserted that the correct name is H. lacustris. Ren et al. [104] observed slight phylogenetic distance and genome structural differences between the two Haematococcus chloroplast genomes and retained the two names, recommending further studies.
Aside from D. salina and H. fluvialis, Saini et al. [100] stated that commercial production using carotenoid-rich microalgae, such as Chlorella zofingiensis (canthaxanthin), Scenedesmus spp. (lutein), Botryococcusbraunii (echinenone), and Phaeodactylum tricornutum (fucoxanthin), has been established. These colorants, however, are not in the approved lists of FDA, EFSA, and CODEX [105][106][107].
Although in-depth research is still needed to overcome technological bottlenecks, it is widely acknowledged that microalgae can become a prominent and popular source of commercial food pigments in the coming future [108]. Saini et al. [100] highlighted the potential of microalgae, in native or engineered strains, including the metabolic strategies that are used or can be used to produce higher amounts of biopigments.
Microalgal production of carotenoids has many benefits [7][102][108][109][110][111][112][113]. It is less labor-intensive and only a small area of non-arable land is needed. The growth rate is 5–10 times that of higher plants. It can be carried out year-round and can adapt to a wide range of conditions and climates. Wastewater can be used as a growing medium. Microalgal cultivation may clean the environment through CO2 sequestration and wastewater treatment. The large-scale production of carotenoids from microalgae is still limited, however, not yet considered sufficiently cost-effective to compete with chemical synthesis and extraction from plant sources [113][114].
The entire process consists of cell cultivation, biomass harvesting, cell disruption, pigment extraction, purification, and storage. Reviewing the vast literature, the following requirements become evident for the microalgal production of carotenoids to reach the industrial scale:
-
Selection of species with appropriate production time and yield of biomass and pigment;
-
Efficient culture system design and medium optimization (including the control of operating conditions like temperature, lighting, pH, aeration, agitation, and media components) to maximize biomass and pigment production at low cost.
-
Efficient and affordable downstream processes (biomass harvesting, cell wall disruption, pigment extraction, purification, and storage).
Microalgae can be cultivated in open (lakes and ponds) or closed systems (photobioreactors) [115]. The raceway ponds are the cheapest to construct and maintain, consume less energy, and are therefore the most commercially employed. Open ponds, however, have the following disadvantages: nonuniform light intensity, higher evaporation losses, greater requirement for water, reduced temperature control, poor mass transfer rates, diffusion of CO2 to the atmosphere, and high risk of contamination [116]. Although much more expensive, photobioreactors are subject to minimal risk of contamination, have better control of culture conditions, require less light and area, and can result in higher biomass and pigment yield [109]. Pigment production in microalgae is affected by various factors such as nutrient availability, salinity, pH, temperature, light wavelength and intensity, photoperiods, pesticides, and heavy metals [102].
Downstream processing, especially cell harvesting and disruption, is difficult. Because of their tiny cell size, microalgal harvesting remains an arduous step and encompasses about 20–30% of the total cost needed for the entire process [117]. It can be performed by filtration, flocculation, centrifugation, sedimentation, and a combination of these strategies [118]. Centrifugation, the most widely employed, is fast, efficient, and suitable for most strains [113]. However, the capital and maintenance costs and energy consumption are too high. Filtration is time and energy-consuming for small-size microalgae. Gravity sedimentation is inexpensive but requires a long time for small, uniformly suspended cells when no additional flocculants are present. Chemical harvesting methods require lower capital investment and consume much less energy but are not as efficient as mechanical methods. Flocculation has received much attention because of the possibility of treating large-scale microalgal suspensions at a lower cost. Technologies are rapidly evolving, including advanced settling tanks, membrane filters, oscillating filters, dissolved air flotation systems, hydrocyclones, electrocoagulation, flow-through centrifuges, and even scalable fractionation [7].
Many microalgal species have rigid cell walls that impede full recovery of the pigments. The efficiency of cell disruption depends on the microalgae species, particularly the cell membrane composition and morphology [119][120]. Mechanical or non-mechanical methods can be used. Mechanical methods consist of bead milling, pressing, high-pressure homogenization, microwave treatment, ultrasonication, autoclaving, and lyophilization [112]. They are best for industry, but energy consumption is high [121]. Non-mechanical methods involve the use of acids or alkalis, osmotic shock, and enzymatic processes. These methods consume less energy and disrupt cell membranes uniformly, but take more time, may affect product quality, and are more difficult to control.
Extraction with organic solvents (e.g., acetone, methanol, ethanol, hexane, dodecane) at a higher temperature and pressure, is popular and has been standardized to meet commercial specifications [113]. This conventional method, however, is known to have inherent limitations: use of large volumes of often toxic solvents, long extraction times, low efficiency and selectivity, and disposal of potentially hazardous solvents to the environment [122][123]. Innovative techniques have been introduced, such as SC-CO2 extraction [124][125] and MAE, UAE, EAE, and PLE [126][127]. These techniques have several advantages: extraction of biologically active compounds without degradation or loss of activity [122], use of green solvents (environmentally safe and non-toxic solvents), higher extraction yield, and shorter process time. Poojary et al. [123] reviewed the various methods, pointing out the differences in yield, selectivity, and economic and environmental sustainability.
An option to avoid extraction of carotenoids is to use the whole microalgal biomass if the final application allows (e.g., animal feed) [7]. In addition to carotenoids, microalgal cells can contain other beneficial compounds. The whole biomass would provide additional nutritional benefits even though the carotenoid effect could be diluted by the extra biomass.
4.1. β-Carotene
The main carotenoids of microalgae are astaxanthin, β-carotene, lutein, lycopene, zeaxanthin, violaxanthin, and fucoxanthin [110]. The first three are the most studied and are discussed in greater detail in this research.
It is well known that Dunaliella salina, the commonly cultivated microalga for β-carotene, has several advantages for commercial production. It lacks a cell wall and produces high levels of β-carotene (10–12% on dry cell weight) [128]. Being halotolerant, it can be cultivated in an open saline mass culture relatively free of competing microorganisms and predators. It is suited for cultivation in coastal areas where seawater is rich in salt and nutrients. Production follows a two-stage strategy. In the first stage, adequate conditions for D. salina growth are provided. After cell concentration reaches a certain level, stress conditions (e.g., nutrient limitation, intense light, and low water activity) are applied to accumulate more carotenoids [110].
4.2. Astaxanthin
The cost and complexity of synthesizing optically active astaxanthin encouraged the production of microalgal astaxanthin. H. pluvialis, the commercial source of microalgal astaxanthin, accumulates up to 3.8% astaxanthin on a dry weight basis [113].
Astaxanthin production by this microalga is much more problematic than the production of Dunaliella β-carotene. Since Haematococus is a freshwater alga, it is susceptible to contamination with other organisms, making open-air culture extremely difficult. Since the optimal conditions for cell growth differ from those of astaxanthin biosynthesis, a two-stage process is usually adopted [129][130]. The first stage is performed photoautotrophically under controlled culture conditions suitable for microalgal growth, in either tubular, bubble column, or airlift photobioreactors. The following stage, which is less prone to contamination, is performed in open cultivation ponds, subject to environmental and nutrient stress to stimulate carotenoid accumulation.
Although already employed commercially, H. pluvialis has slow growth, low biomass yield, high light requirement, and vulnerability to contamination. Numerous studies have been carried out to boost Haematococcus astaxanthin production, mainly modifying the design/configuration of the culture system and optimizing the culture conditions, e.g., [131][132][133][134]. Aside from technological improvement, stress must be induced to increase carotenoid concentration, such as nitrogen deprivation, strong light intensity, salt stress, and phosphate deficiency.
H, pluvialis develops a thick and rigid three-layered cell wall during astaxanthin accumulation under stress, which forms a strong barrier to astaxanthin extraction. The methods applied to break the cell wall and extract astaxanthin from Chlorella and Haematococcus were discussed by Kim et al. [135] comparing efficiency, energy consumption, type and dosage of solvent, biomass concentration, toxicity, scalability, and synergistic combinations. Kim et al. [136] discussed further the various physical, chemical, and biological cell disruption methods and compared the theoretical mechanisms, biomass status (wet, dry, and live), cell-disruption efficacy, astaxanthin extractability, cost, scalability, synergistic combinations, and impact on the stress-sensitive astaxanthin content.
The extraction performance of astaxanthin and lutein from H. pluvialis in the red phase (the phase characterized by the transition of the color from green to red) was assessed using bench-scale SC-CO2 installation [124]. The cell wall of H. pluvialis red biomass was disrupted by mechanical (ball milling) pre-treatment. Maximum recovery of astaxanthin and lutein were 99% and 52%, respectively, at 50 °C and 550 bars.
To determine the processes that yield maximum astaxanthin recovery from H. pluvialis, bead milling, high-pressure homogenization, and no disruption of H. pluvialis biomass were coupled with spray drying, vacuum drying, and freeze-drying in all possible combinations [137]. Eventually, astaxanthin was extracted using SC-CO2. All combinations of milling or high-pressure homogenization and lyophilization or spray-drying resulted in similar recoveries. Evaluating the results in an economic context, bead milling, and spray-drying prior to supercritical CO2 extraction were recommended to achieve the maximum astaxanthin recoveries.
In a large-scale investigation of astaxanthin production by H. pluvialis in two European cities, it was concluded that for Europe, natural astaxanthin was not a competitive alternative to the synthetic form for aquaculture [138]. However, astaxanthin production by H. pluvialis in sites characterized by high solar radiation and high temperatures was considered an attractive venture. Hague et al. [139] reported intensified astaxanthin production using bioethanol wastewater streams as potential ‘green’ media to culture H. pluvialis.
Chlorella zofingiensis is considered a good alternative source of astaxanthin for commercial production [140][141]. It has a high growth rate and high cell density that can be achieved through heterotrophic glucose-fed cultivation.
Reviewing the diversity and distribution of carotenogenic microalgae in Europe, Chekanov [104] cited Acetabularia acetabulum, Botryoccus braunii, Bracteacoccus bullatus, B. giganteus, B. minor, Chlainomonas rubra, Chloromonas nivalis, C. hindakii, and C. krienitzii as potential sources of astaxanthin.
Ambati et al. [113] discussed the in vitro and in vivo trials for the biological activities of astaxanthin: antioxidant effects, anti-lipid peroxidation activity, anti-inflammation, anti-diabetic activity, cardiovascular disease prevention, anticancer activity, immuno-modulation, and neuroprotection. More recent reviews on the health benefits of this highly regarded carotenoid have been published, e.g., [142][143].
4.3. Lutein
The potential of microalgae as a lutein source is being intensely investigated. As can be discerned in Section 3, sources of this highly important health-promoting carotenoid are lacking, The commercial source of lutein is the marigold flower. Lutein production from marigold petals, however, has several disadvantages, such as low biomass and low lutein content, high labor demand for harvesting and separation of the petals, season dependence, and arable land occupation. Microalgae have emerged as promising alternatives to marigold. They have a much higher growth rate and can be harvested throughout the year [117][144]. The entire microalgal biomass can be processed for lutein extraction. Microalgae have much higher lutein content compared with marigold and other terrestrial plants. Microalgae processing from harvesting to carotenoid extraction is relatively simple compared with processing marigold flowers, requiring less labor.
Lin et al. [111] compared the different stages of lutein production from marigold flowers and from microalgae. Microalgae had faster growth rates and 3–4 times higher lutein yield. Marigolds needed more land and water but required less nutrients (N, P, K) and less energy.
In spite of the many advantages cited for lutein production by microalgae, a microalgal lutein product has not reached the market. The technical obstacles cited for lutein production by microalgae are lutein values not high enough to be economically feasible on an industrial scale, high harvesting cost, and high energy demand for cell disruption and extraction. Rapid cultivation of algal strains with high lutein content and efficient downstream processing at affordable costs are needed. Lutein productivity can be achieved by selecting adequate species, obtaining high lutein-yielding mutants, and optimizing culture conditions [144][145].
Optimization of culture systems and conditions for enhanced lutein production has been widely pursued, e.g., [144][145][146][147]. Efficient strategies for improving lutein productivity include fed-batch culture, two-stage cultivation, and in situ lutein accumulation [117]. A biomass production process including two stages, heterotrophy/photoinduction, was developed to improve biomass and lutein production by the green microalgae Tetradesmus incrassatulus (formerly Scenedesmus incrassatulus) [148].
The most investigated factors that affect lutein production are algal species, temperature, light, photoperiod, pH, nutrient availability, and salinity [110][117]. Unlike astaxanthin and β-carotene, which are secondary carotenoids, lutein is a primary carotenoid required for the structure and function of the light-harvesting complexes in photosynthesis. Stress conditions have been repeatedly shown to enhance astaxanthin and β-carotene production but not necessarily lutein accumulation, Augmenting lutein production by stress conditions is difficult [149].
Recognizing that the choice of a high-yielding strain, along with an effective photobioreactor design will enable maximal lutein production from microalgae in an economically viable manner, Zheng et al. [150] summarized the recent research progress in lutein production from microalgae, including competent microalgal strains, cultivation systems, and subsequent biomass treatment technologies.
When cultivated in an outdoor thin-layer photobioreactor, the greatest increase in carotenoid accumulation occurred under conditions of nitrogen sufficiency and high light. The results suggest that in Tetradesmus sp., light is a critical factor in the accumulation of carotenoids (mostly lutein) and nitrogen availability plays only a minor role [151].
Phototrophic cultivation of microalgae has been considered as a strategy to optimize lutein production due to increased mixing efficiency, higher gas retention time, and shorter cost-effective light path [152].
In thermotolerant microalgae Desmodesmus sp. F2 and Coelastrella sp. F50, cultivated under outdoor tropical conditions, lutein content did not change significantly in microalgae grown with different carbon sources or in different seasons [153]. The major factor influencing productivity was the duration of effective irradiance.
The systematic development of an optimal light-feeding strategy coupled with a semi-continuous mode of reactor operation resulted in greater lutein productivity, photosynthetic efficiency, and CO2 fixation rate of Mychonastes homosphaera (formerly Chlorella minutissima). Moreover, in this process of optimization and integration, light-energy consumption was significantly reduced by 32%, in comparison with constant illumination [145].
Light-related (e.g., quality, source, and intensity) strategies have been very effective in promoting the productivity of microalgal biomass and the accumulation of lutein. Chiu et al. [153] observed that in Tetradesmus sp., light was a critical factor in the accumulation of carotenoids; nitrogen availability played only a minor role. Optimization of light intensity was also utilized as a means of improving lutein productivity in Mychonastes sp. [154] and Parachlorella sp. JD-076 [155].
References
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15.
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205.
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280.
- Jurić, S.; Jurić, M.; Król-Kilińska, Z.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790.
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182.
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7.
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640.
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26.
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65.
- Rodriguez-Amaya, D.B. Current knowledge on the health benefits of carotenoids: Focus on the scientific evidence. In Global Perspectives on Astaxanthin. From Industrial Production to Food, Health, and Pharmaceutical Applications; Ravishankar, G.A., Ambati, R.R., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 693–717.
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and cognitive outcomes: A meta-analysis of randomized intervention trials. Antioxidants 2021, 10, 223.
- Li, J.; Abdel-Aal, E.-S.-M. Dietary lutein and cognitive function in adults: A meta-analysis of randomized controlled trials. Molecules 2021, 26, 5794.
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and zeaxanthin influence brain function in older adults: A randomized controlled trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90.
- Lin, S.; Shen, Y. Dietary carotenoids intake and depressive symptoms in US adults, NHANES 2015–2016. J. Affec. Disord. 2021, 282, 41–45.
- Yu, Q.; Xue, F.; Li, Z.; Li, X.; Ai, L.; Jin, M.; Xie, M.; Yu, Y. Dietary intake of carotenoids and risk of depressive symptoms: A systematic review and meta-analysis. Antioxidants 2022, 11, 2205.
- Kim, S.J.; Anh, N.H.; Diem, N.C.; Park, S.; Cho, Y.H.; Long, N.P.; Hwang, I.G.; Lim, J.; Kwon, S.W. Effects of β-cryptoxanthin on improvement in osteoporosis risk: A systematic review and meta-analysis of observational studies. Foods 2021, 10, 296.
- Regu, G.M.; Kim, H.; Kim, Y.J.; Paek, J.E.; Lee, G.; Chang, N.; Kwon, O. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30–75 years using data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008–2011). Nutrients 2017, 9, 1025.
- Xu, J.; Song, C.; Song, X.; Zhang, X.; Li, X. Carotenoids and risk of fracture: A meta-analysis of observational studies. Oncotarget 2017, 8, 2391–2399.
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology, and Technology; IFT Press-Wiley: Oxford, UK, 2016.
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigment. Food Chem. 2007, 101, 1145–1150.
- Meléndez-Martínez, A.J.; Esquivel, P.; Rodriguez-Amaya, D.B. Comprehensive review on carotenoid composition: Transformations during processing and storage of foods. Food Res. Int. 2023, 169, 112773.
- Rodriguez-Amaya, D.B.; Carle, R. Alterations of natural pigments. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 265–327.
- Bogacz-Radomska, L.; Harasym, J. β-Carotene-properties and production methods. Food Qual. Saf. 2018, 2, 69–74.
- Raddatz-Mota, D.; Pérez-Flores, L.J.; Carrari, F.; Mendoza-Espinoza, J.A.; de León-Sánchez, F.D.; Pinzón-López, L.L.; Godóy-Hernández, G.; Rivera-Cabrera, F. Achiote (Bixa orellana L.): A natural source of pigment and vitamin E. J. Food Sci. Technol. 2017, 54, 1729–1741.
- Mercer, D.G.; Rodriguez-Amaya, D.B. Reactions and interactions of some food additives. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 579–635.
- Møller, A.H.; Jahangiri, A.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Effect of light, pH, metal ions and antioxidants on the colour stability of norbixin in aqueous solution. Int. J. Food Sci. Technol. 2018, 54, 1625–1632.
- Møller, A.H.; Jahangiri, A.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Mechanism behind the degradation of aqueous norbixin upon storage in light and dark environment. Food Chem. 2020, 310, 125967.
- Chuyen, H.V.; Eun, J.B. Effects of different extraction methods on the extraction yield, degradation of bixin and formation of harmful volatile compounds in the extracts from annatto seeds. Food Res. 2021, 5, 42–48.
- Jayakumar, J.; Sudha, P.; Rajkumar, P.; Pandiselvam, R.; Gurusamy, K.; Kumaran, K.; Subramanian, P. Comparative study on the effect of solvents on extraction of bixin from annatto seed (Bixa orellana L.) and optimization of process parameters using Box–Behnken design. Biomass Conv. Bioref. 2023.
- Bachtler, S.; Bart, H.-J. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process. 2021, 125, 1–13.
- Yolmeh, M.; Habibi Najafi, M.B.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food Chem. 2014, 155, 319–324.
- Alcázar-Alay, S.C.; Osorio-Tobón, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent extraction: Process integration and economic evaluation. Food Res. Int. 2017, 99 Pt 1, 393–402.
- Møller, A.H.; Wijaya, W.; Jahangiri, A.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Van der Meeren, P.; Dalsgaard, T.K. Norbixin binding to whey protein isolate - alginate electrostatic complexes increases its solubility and stability. Food Hydrocoll. 2020, 101, 105559.
- Liu, H.; Zhang, J.; Xiong, Y.; Peng, S.; McClements, D.J.; Zou, L.; Liang, R.; Liu, W. Improving norbixin dispersibility and stability by liposomal encapsulation using the pH-driven method. J. Sci. Food Agric. 2022, 102, 2070–2079.
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R.; Salehi, F. Modeling of antibacterial activity of annatto dye on Escherichia coli in mayonnaise. Food Biosci. 2014, 8, 8–13.
- Handayani, I.; Haryanti, P.; Sulistyo, S.B. Color and antibacterial activity of annatto extracts at various pH of distilled water solvent and extraction temperature. Food Res. 2021, 5, 247–253.
- Shakeri, A.; Soheili, V.; Karimi, M.; Hosseininia, S.A.; Bazzaz, B.S.F. Biological activities of three natural plant pigments and their health benefits. J Food Meas Charact. 2018, 12, 356–361.
- Habibi Najafi, M.B.; Fatemizadeh, S.S.; Boroojerdi, S.R.; Hosseini, F.; Karazhyan, R. In vitro evaluation of antimold activity of annatto natural dye and its effects on microbial, physicochemical, and sensory properties of bread. J. Food Prot. 2018, 81, 1598–1604.
- Beni, A.A.; Rodrigues, R.F.; Conte, L.; Costa, I.F.; Delalibera, E.A.; Roehrs, M.; Rampelotto, C.; Emanuelli, T.; Somacal, S. Dietary supplementation with annatto food-coloring extracts increases the resistance of human erythrocytes to hemolysis. Nutr. Res. 2020, 76, 71–81.
- Kusmita, L.; Franyoto, Y.D.; Mutmainah, M.; Puspitaningrum, I.; Nurcahyanti, A.D.R. Bixa orellana L. carotenoids: Antiproliferative activity on human lung cancer, breast cancer, and cervical cancer cells in vitro. Nat. Prod. Res. 2022, 36, 6421–6427.
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto carotenoids attenuate oxidative stress and inflammatory response after high-calorie meal in healthy subjects. Food Res. Int. 2017, 100 Pt 1, 771–779.
- Kläui, H.; Bauernfeind, J.C. Carotenoids as food colors. In Carotenoids as Colorants and Vitamin A Precursors; Bauernfeind, J.C., Ed.; Academic Press: New York, NY, USA, 1981; pp. 47–317.
- Molnár, H.; Kónya, E.; Zalán, Z.; Bata-Vidács, I.; Tömösközi-Farkas, R.; Székács, A.; Adányi, N. Chemical characteristics of spice paprika of different origins. Food Control 2018, 83, 54–60.
- Pang, M.; Lium, Q.; Yu, Y.L.; Cai, S.L. Ultrasonic-microwave synergistic extraction of paprika pigment. E3S Web Conf. 2019, 78, 02009.
- Huang, P.; Yu, Q.; Feng, X.; Ma, C.; Kan, J. Optimization of accelerated solvent extraction of paprika oleoresin and its effect on capsaicinoid and carotenoid composition. J. Food Compost. Anal. 2022, 110, 104589.
- Procopio, F.R.; Ferraz, M.C.; do Prado-Silva, L.; Paulino, B.N.; Sant’Ana, A.S.; Pastore, G.M.; Sobral, P.J.A.; Hubinger, M.D. Antifungal synergistic effect of paprika and cinnamon oleoresins and their coencapsulation by spray chilling technique to produce a carotenoid-cinnamaldehyde-rich food powder. Food Bioprocess Technol. 2022, 15, 2826–2838.
- De Aguiar, A.C.; Vigano, J.; Anthero, A.G.S.; Dias, A.L.B.; Hubinger, M.D.; Martínez, J. Supercritical fluids and fluid mixtures to obtain high-value compounds from Capsicum peppers. Food Chem. X 2022, 13, 100228.
- Kim, G.H.; Chin, K.B. Characteristics of low-nitrite pork emulsified-sausages with paprika oleoresin solution during refrigerated storage. J. Anim. Sci. Technol. 2021, 63, 394–404.
- Kothari, D.; Thakur, R.; Kumar, R. Safron (Crocus sativus L.): Gold of the spices—A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677.
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; safron. Trends Food Sci. Technol. 2016, 58, 69–78.
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017, 99, 401–408.
- Chranioti, C.; Nikoloudaki, A.; Tzia, C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydr. Polym. 2015, 127, 252–263.
- Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969.
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M.; et al. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706.
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80.
- Ali, A.; Yu, L.; Kousar, S.; Khalid, W.; Maqbool, Z.; Aziz, A.; Arshad, M.S.; Aadil, R.M.; Trif, M.; Riaz, S.; et al. Crocin: Functional characteristics, extraction, food applications and efficacy against brain related disorders. Front Nutr. 2022, 9, 1009807.
- Bukhari, S.I.; Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745.
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249.
- Ghaffari, S.; Roshanravan, N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 2019, 109, 21–27.
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules 2020, 26, 86.
- Rahiman, N.; Akaberi, M.; Sahebkar, A.; Emami, S.A.; Tayarani-Najaran, Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc. Res. 2018, 18, 82–89.
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp. Ther. Med. 2023, 25, 235.
- Zhou, W.E.; Zhang, Y.; Li, Y.; Ling, Y.; Li, H.N.; Li, S.H.; Jiang, S.J.; Ren, Z.Q.; Huang, Z.Q.; Zhang, F. Determination of gardenia yellow colorants in soft drink, pastry, instant noodles with ultrasound-assisted extraction by high performance liquid chromatography-electrospray ionization tandem mass spectrum. J Chromatogr. A 2016, 1446, 59–69.
- Giovannucci, E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp. Biol. Med. 2002, 227, 852–859.
- Giovannucci, E. Lycopene and prostate cancer risk. Methodological considerations in the epidemiologic literature. Pure Appl. Chem. 2002, 74, 1427–1434.
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and risk of prostate cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1260.
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose-response meta-analysis of observational studies. PLoS ONE 2015, 10, e0137427.
- Rowles, J.L., 3rd; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377.
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 1197–1205.
- Ge, X.-X.; Xing, M.-Y.; Yu, L.-F.; Shen, P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 1911–1918.
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid intake from natural sources and head and neck cancer: A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1003–1011.
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091.
- Li, X.; Xu, J. Dietary and circulating lycopene and stroke risk: A meta-analysis of prospective studies. Sci. Rep. 2014, 4, 5031.
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61, 9.
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158.
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108.
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica cochinchinensis Spreng. (gac) fruit carotenoids reevaluated. J. Food Compos. Anal. 2006, 19, 664–668.
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2014, 50, 567–577.
- Khalil, M.; Raila, J.; Ali, M.; Islan, R.N.S.; Schenk, R. Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under processing conditions. J. Funct. Foods 2012, 4, 602–610.
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12.
- Ma, L.; Dou, H.L.; Wu, Y.Q.; Huang, Y.M.; Huang, Y.B.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359.
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 56, 252–258.
- Cui, Y.H.; Jing, C.X.; Pan, H.W. Association of blood antioxidants and vitamins with risk of age-related cataract: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2013, 98, 778–786.
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between lutein and zeaxanthin status and the risk of cataract: A meta-analysis. Nutrients 2014, 6, 452–465.
- Ma, L.; Hao, Z.X.; Liu, R.R.; Yu, R.B.; Shi, Q.; Pan, J.P. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 63–70.
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid intake and risk of non-Hodgkin lymphoma: A systematic review and dose-response meta-analysis of observational studies. Ann. Hematol. 2017, 96, 957–965.
- Leermakers, E.T.M.; Darweesh, S.K.L.; Baena, C.P.; Moreira, E.M.; van Lent, D.M.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481–494.
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: Findings from the Irish Longitudinal Study on Aging. J. Gerontol. 2017, 72, 1431–1436.
- Dong, S.; Xia, H.; Wang, F.; Sun, G. The effect of red palm oil on vitamin A deficiency: A meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1281.
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red palm oil: A review on processing, health benefits and its application in food. J. Oleo Sci. 2021, 70, 1201–1210.
- Loganathan, R.; Subramaniam, K.M.; Radhakrishnan, A.K.; Choo, Y.-M.; Teng, K.-T. Health-promoting effects of red palm oil: Evidence from animal and human studies. Nutr. Rev. 2017, 75, 98–113.
- Cassidy, L. Red palm oil. Inform 2017, 28, 6–10.
- Purnama, K.O.; Setyaningsih, D.; Hambali, E.; Taniwiryono, D. Processing, characteristics, and potential application of red palm oil—A review. Int. J. Oil Palm 2020, 3, 40–55.
- Hoe, B.C.; Chan, E.S.; Nagasundara Ramanan, R.; Ooi, C.W. Direct recovery of palm carotene by liquid-liquid extraction. J. Food Eng. 2022, 313, 110755.
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12.
- Das, A.; Yoon, S.H.; Lee, S.H.; Kim, J.Y.; Oh, D.K.; Kim, S.W. An update on microbial carotenoid production: Application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512.
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398.
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2022, 62, 6932–6946.
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76.
- Saini, R.K.; Keum, Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674.
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678.
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222.
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot. (Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348.
- Chekanov, K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar. Drugs 2023, 21, 108.
- U.S. Food & Drug Administration. Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2023.
- European Commission. Food Additives Database; European Commission: Brussels, Belgium, 2023.
- FAO. Combined Compendium of Food Additive Specifications; FAO: Rome, Italy, 2023.
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food Industry. Mar. Drugs 2023, 21, 82.
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417.
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412.
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428.
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.J.; Murray, P.M.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014. 64, 332–344.
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902.
- Rodriguez-Amaya, D.B.; Maldonade, I.R. Potentials and challenges in the production of microalgal pigments with reference to carotenoids, chlorophylls, and phycobiliproteins. In Handbook of Algal Technology and Phytochemicals Volume 1 Food, Health and Neutraceutical Applications; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 109–118.
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406.
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353.
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein production from microalgae: A review. Bioresour. Technol. 2023, 376, 128875.
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362.
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88.
- Günerken, E.; D’Hondt, E.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260.
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841.
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176.
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016, 14, 214.
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334.
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent advances in supercritical CO2 extraction of pigments, lipids and bioactive compounds from microalgae. Molecules 2023, 28, 1410.
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103.
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213.
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756.
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283.
- Wichuk, K.; Brynjólfsson, S.; Fu, W. Biotechnological production of value-added carotenoids from microalgae: Emerging technology and prospects. Bioengineered 2014, 5, 204–208.
- Poonkum, W.; Powtongsook, S.; Pavasant, P. Astaxanthin induction in microalga H. pluvialis with flat panel airlift photobioreactors under indoor and outdoor conditions. Prep. Biochem. Biotechnol. 2015, 45, 1–17.
- Yang, Z.; Cheng, J.; Li, K.; Zhou, J.; Cen, K. Optimizing gas transfer to improve growth rate of Haematococcus pluvialis in a raceway pond with chute and oscillating baffles. Bioresour. Technol. 2016, 214, 276–283.
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335.
- Zhang, Z.; Huang, J.J.; Sun, D.; Lee, Y.; Chen, F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour. Technol. 2017, 224, 515–522.
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310.
- Kim, B.; Youn Lee, S.; Lakshmi Narasimhan, A.; Kim, S.; Oh, Y.K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124.
- Koopmann, I.K.; Möller, S.; Elle, C.; Hindersin, S.; Kramer, A.; Labes, A. Optimization of astaxanthin recovery in the downstream process of Haematococcus pluvialis. Foods 2022, 11, 1352.
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190.
- Haque, F.; Dutta, A.; Thimmanagarib, M.; Chiang, Y.W. Intensified green production of astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 1–11.
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910.
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515.
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20.
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479.
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282.
- Dineshkumar, R.; Subramanian, G.; Dash, S.K.; Sen, R. Development of an optimal light-feeding strategy coupled with semi-continuous reactor operation for simultaneous improvement of microalgal photosynthetic efficiency, lutein production and CO2 sequestration. Biochem. Eng. J. 2016, 113, 47–56.
- Chen, C.Y.; Lu, I.C.; Nagarajan, D.; Chang, C.H.; Ng, I.S.; Lee, D.J.; Chang, J.S. A highly efficient two-stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB-1-M12. Bioresour. Technol. 2018, 253, 141–147.
- Jeon, J.Y.; Kwon, J.S.; Kang, S.T.; Kim, B.R.; Jung, Y.; Han, J.G.; Park, J.H.; Hwang, J.K. Optimization of culture media for large-scale lutein production by heterotrophic Chlorella vulgaris. Biotechnol. Prog. 2014, 30, 736–743.
- Flórez-Miranda, L.; Cañizares-Villanueva, R.O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Flores-Ortíz, C.M. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74.
- Mulders, K.J.; Lamers, P.P.; Martens, D.E. Wijffels. R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242.
- Zheng, H.S.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795.
- Přibyl, P.; Pilný, J.; Cepák, V.; Petr Kaštánek, P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Res. 2016, 16, 69–75.
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Rep. (Amst.) 2016, 10, 117–125.
- Chiu, P.H.; Soong, K.; Chen, C.-N. Cultivation of two thermotolerant microalgae under tropical conditions: Influences of carbon sources and light duration on biomass and lutein productivity in four seasons. Bioresour. Technol. 2016, 212, 190–198.
- Hong, S.J.; Yim, K.J.; Ryu, Y.J.; Lee, C.G.; Jang, H.J.; Jung, J.Y.; Kim, Z.H. Improvement of lutein and zeaxanthin production in Mychonastes sp. 247 by optimizing light intensity and culture salinity conditions. J. Microbiol. Biotechnol. 2023, 33, 260–267.
- Heo, J.; Shin, D.S.; Cho, K.; Cho, D.H.; Lee, Y.J.; Kim, H.S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
15 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




