The substitution of synthetic food dyes with natural colorants continues to be assiduously pursued. The current list of natural carotenoid colorants consists of plant-derived annatto (bixin and norbixin), paprika (capsanthin and capsorubin), saffron (crocin), tomato and gac fruit lycopene, marigold lutein, and red palm oil (α- and β-carotene), along with microalgal Dunaliella β-carotene and Haematococcus astaxanthin and fungal Blakeslea trispora β-carotene and lycopene. Potential microalgal sources are being sought, especially in relation to lutein, for which commercial plant sources are lacking.
- plant-derived colorants
- microalgal carotenoids
- microencapsulation
- nanoencapsulation
- green extraction
- by-product valorization
1. Introduction
2. The Carotenoid Chromophore
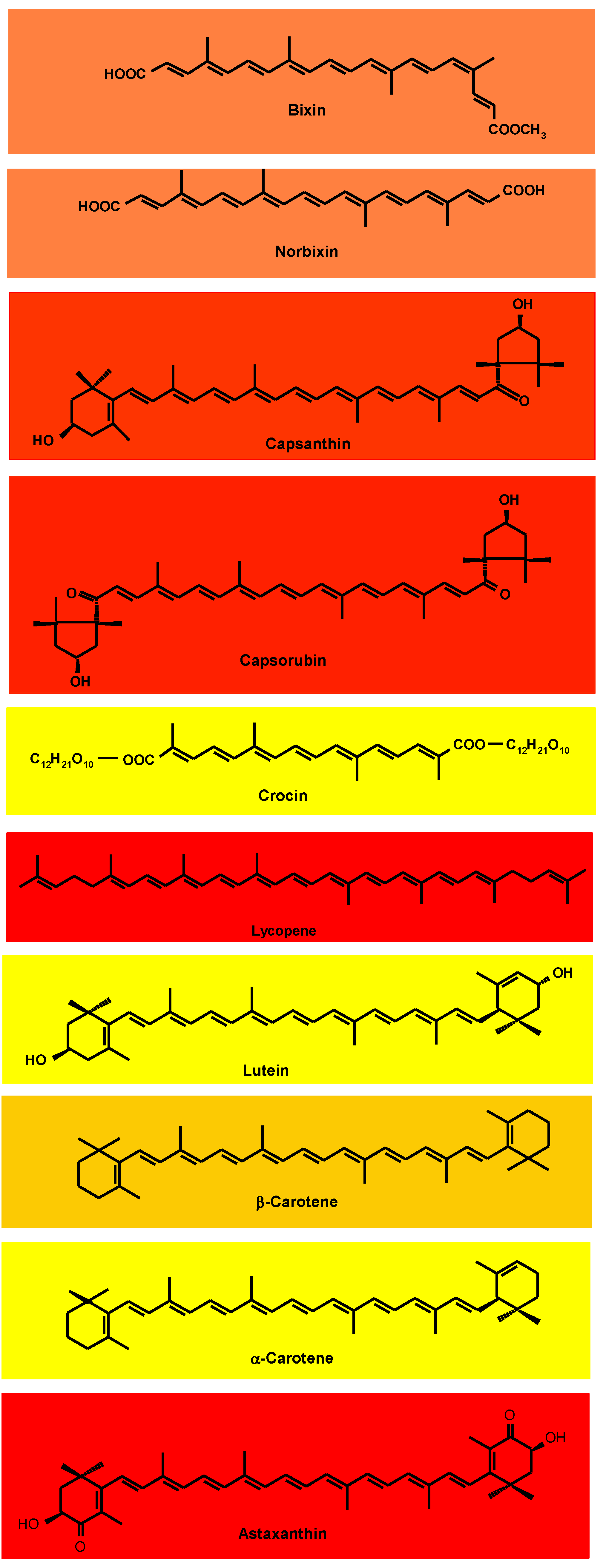
3. Carotenoid Colorants Derived from Higher Plants

3.1. Annatto Bixin and Norbixin
3.2. Paprika Capsanthin and Capsorubin
3.3. Saffron (Crocin)
3.4. Tomato and Gac Fruit Lycopene
3.5. Marigold Lutein
3.6. Red Palm Oil α- and β-Carotene
4. Microalgal Carotenoid Colorant
-
Selection of species with appropriate production time and yield of biomass and pigment;
-
Efficient culture system design and medium optimization (including the control of operating conditions like temperature, lighting, pH, aeration, agitation, and media components) to maximize biomass and pigment production at low cost.
-
Efficient and affordable downstream processes (biomass harvesting, cell wall disruption, pigment extraction, purification, and storage).
4.1. β-Carotene
4.2. Astaxanthin
4.3. Lutein
This entry is adapted from the peer-reviewed paper 10.3390/foods12224080
References
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15.
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205.
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280.
- Jurić, S.; Jurić, M.; Król-Kilińska, Z.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790.
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182.
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7.
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640.
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26.
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65.
- Rodriguez-Amaya, D.B. Current knowledge on the health benefits of carotenoids: Focus on the scientific evidence. In Global Perspectives on Astaxanthin. From Industrial Production to Food, Health, and Pharmaceutical Applications; Ravishankar, G.A., Ambati, R.R., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 693–717.
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and cognitive outcomes: A meta-analysis of randomized intervention trials. Antioxidants 2021, 10, 223.
- Li, J.; Abdel-Aal, E.-S.-M. Dietary lutein and cognitive function in adults: A meta-analysis of randomized controlled trials. Molecules 2021, 26, 5794.
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and zeaxanthin influence brain function in older adults: A randomized controlled trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90.
- Lin, S.; Shen, Y. Dietary carotenoids intake and depressive symptoms in US adults, NHANES 2015–2016. J. Affec. Disord. 2021, 282, 41–45.
- Yu, Q.; Xue, F.; Li, Z.; Li, X.; Ai, L.; Jin, M.; Xie, M.; Yu, Y. Dietary intake of carotenoids and risk of depressive symptoms: A systematic review and meta-analysis. Antioxidants 2022, 11, 2205.
- Kim, S.J.; Anh, N.H.; Diem, N.C.; Park, S.; Cho, Y.H.; Long, N.P.; Hwang, I.G.; Lim, J.; Kwon, S.W. Effects of β-cryptoxanthin on improvement in osteoporosis risk: A systematic review and meta-analysis of observational studies. Foods 2021, 10, 296.
- Regu, G.M.; Kim, H.; Kim, Y.J.; Paek, J.E.; Lee, G.; Chang, N.; Kwon, O. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30–75 years using data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008–2011). Nutrients 2017, 9, 1025.
- Xu, J.; Song, C.; Song, X.; Zhang, X.; Li, X. Carotenoids and risk of fracture: A meta-analysis of observational studies. Oncotarget 2017, 8, 2391–2399.
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology, and Technology; IFT Press-Wiley: Oxford, UK, 2016.
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigment. Food Chem. 2007, 101, 1145–1150.
- Meléndez-Martínez, A.J.; Esquivel, P.; Rodriguez-Amaya, D.B. Comprehensive review on carotenoid composition: Transformations during processing and storage of foods. Food Res. Int. 2023, 169, 112773.
- Rodriguez-Amaya, D.B.; Carle, R. Alterations of natural pigments. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 265–327.
- Bogacz-Radomska, L.; Harasym, J. β-Carotene-properties and production methods. Food Qual. Saf. 2018, 2, 69–74.
- Raddatz-Mota, D.; Pérez-Flores, L.J.; Carrari, F.; Mendoza-Espinoza, J.A.; de León-Sánchez, F.D.; Pinzón-López, L.L.; Godóy-Hernández, G.; Rivera-Cabrera, F. Achiote (Bixa orellana L.): A natural source of pigment and vitamin E. J. Food Sci. Technol. 2017, 54, 1729–1741.
- Mercer, D.G.; Rodriguez-Amaya, D.B. Reactions and interactions of some food additives. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 579–635.
- Møller, A.H.; Jahangiri, A.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Effect of light, pH, metal ions and antioxidants on the colour stability of norbixin in aqueous solution. Int. J. Food Sci. Technol. 2018, 54, 1625–1632.
- Møller, A.H.; Jahangiri, A.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Mechanism behind the degradation of aqueous norbixin upon storage in light and dark environment. Food Chem. 2020, 310, 125967.
- Chuyen, H.V.; Eun, J.B. Effects of different extraction methods on the extraction yield, degradation of bixin and formation of harmful volatile compounds in the extracts from annatto seeds. Food Res. 2021, 5, 42–48.
- Jayakumar, J.; Sudha, P.; Rajkumar, P.; Pandiselvam, R.; Gurusamy, K.; Kumaran, K.; Subramanian, P. Comparative study on the effect of solvents on extraction of bixin from annatto seed (Bixa orellana L.) and optimization of process parameters using Box–Behnken design. Biomass Conv. Bioref. 2023.
- Bachtler, S.; Bart, H.-J. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process. 2021, 125, 1–13.
- Yolmeh, M.; Habibi Najafi, M.B.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food Chem. 2014, 155, 319–324.
- Alcázar-Alay, S.C.; Osorio-Tobón, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent extraction: Process integration and economic evaluation. Food Res. Int. 2017, 99 Pt 1, 393–402.
- Møller, A.H.; Wijaya, W.; Jahangiri, A.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Van der Meeren, P.; Dalsgaard, T.K. Norbixin binding to whey protein isolate - alginate electrostatic complexes increases its solubility and stability. Food Hydrocoll. 2020, 101, 105559.
- Liu, H.; Zhang, J.; Xiong, Y.; Peng, S.; McClements, D.J.; Zou, L.; Liang, R.; Liu, W. Improving norbixin dispersibility and stability by liposomal encapsulation using the pH-driven method. J. Sci. Food Agric. 2022, 102, 2070–2079.
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R.; Salehi, F. Modeling of antibacterial activity of annatto dye on Escherichia coli in mayonnaise. Food Biosci. 2014, 8, 8–13.
- Handayani, I.; Haryanti, P.; Sulistyo, S.B. Color and antibacterial activity of annatto extracts at various pH of distilled water solvent and extraction temperature. Food Res. 2021, 5, 247–253.
- Shakeri, A.; Soheili, V.; Karimi, M.; Hosseininia, S.A.; Bazzaz, B.S.F. Biological activities of three natural plant pigments and their health benefits. J Food Meas Charact. 2018, 12, 356–361.
- Habibi Najafi, M.B.; Fatemizadeh, S.S.; Boroojerdi, S.R.; Hosseini, F.; Karazhyan, R. In vitro evaluation of antimold activity of annatto natural dye and its effects on microbial, physicochemical, and sensory properties of bread. J. Food Prot. 2018, 81, 1598–1604.
- Beni, A.A.; Rodrigues, R.F.; Conte, L.; Costa, I.F.; Delalibera, E.A.; Roehrs, M.; Rampelotto, C.; Emanuelli, T.; Somacal, S. Dietary supplementation with annatto food-coloring extracts increases the resistance of human erythrocytes to hemolysis. Nutr. Res. 2020, 76, 71–81.
- Kusmita, L.; Franyoto, Y.D.; Mutmainah, M.; Puspitaningrum, I.; Nurcahyanti, A.D.R. Bixa orellana L. carotenoids: Antiproliferative activity on human lung cancer, breast cancer, and cervical cancer cells in vitro. Nat. Prod. Res. 2022, 36, 6421–6427.
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto carotenoids attenuate oxidative stress and inflammatory response after high-calorie meal in healthy subjects. Food Res. Int. 2017, 100 Pt 1, 771–779.
- Kläui, H.; Bauernfeind, J.C. Carotenoids as food colors. In Carotenoids as Colorants and Vitamin A Precursors; Bauernfeind, J.C., Ed.; Academic Press: New York, NY, USA, 1981; pp. 47–317.
- Molnár, H.; Kónya, E.; Zalán, Z.; Bata-Vidács, I.; Tömösközi-Farkas, R.; Székács, A.; Adányi, N. Chemical characteristics of spice paprika of different origins. Food Control 2018, 83, 54–60.
- Pang, M.; Lium, Q.; Yu, Y.L.; Cai, S.L. Ultrasonic-microwave synergistic extraction of paprika pigment. E3S Web Conf. 2019, 78, 02009.
- Huang, P.; Yu, Q.; Feng, X.; Ma, C.; Kan, J. Optimization of accelerated solvent extraction of paprika oleoresin and its effect on capsaicinoid and carotenoid composition. J. Food Compost. Anal. 2022, 110, 104589.
- Procopio, F.R.; Ferraz, M.C.; do Prado-Silva, L.; Paulino, B.N.; Sant’Ana, A.S.; Pastore, G.M.; Sobral, P.J.A.; Hubinger, M.D. Antifungal synergistic effect of paprika and cinnamon oleoresins and their coencapsulation by spray chilling technique to produce a carotenoid-cinnamaldehyde-rich food powder. Food Bioprocess Technol. 2022, 15, 2826–2838.
- De Aguiar, A.C.; Vigano, J.; Anthero, A.G.S.; Dias, A.L.B.; Hubinger, M.D.; Martínez, J. Supercritical fluids and fluid mixtures to obtain high-value compounds from Capsicum peppers. Food Chem. X 2022, 13, 100228.
- Kim, G.H.; Chin, K.B. Characteristics of low-nitrite pork emulsified-sausages with paprika oleoresin solution during refrigerated storage. J. Anim. Sci. Technol. 2021, 63, 394–404.
- Kothari, D.; Thakur, R.; Kumar, R. Safron (Crocus sativus L.): Gold of the spices—A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677.
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; safron. Trends Food Sci. Technol. 2016, 58, 69–78.
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017, 99, 401–408.
- Chranioti, C.; Nikoloudaki, A.; Tzia, C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydr. Polym. 2015, 127, 252–263.
- Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969.
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M.; et al. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706.
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80.
- Ali, A.; Yu, L.; Kousar, S.; Khalid, W.; Maqbool, Z.; Aziz, A.; Arshad, M.S.; Aadil, R.M.; Trif, M.; Riaz, S.; et al. Crocin: Functional characteristics, extraction, food applications and efficacy against brain related disorders. Front Nutr. 2022, 9, 1009807.
- Bukhari, S.I.; Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745.
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249.
- Ghaffari, S.; Roshanravan, N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 2019, 109, 21–27.
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules 2020, 26, 86.
- Rahiman, N.; Akaberi, M.; Sahebkar, A.; Emami, S.A.; Tayarani-Najaran, Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc. Res. 2018, 18, 82–89.
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp. Ther. Med. 2023, 25, 235.
- Zhou, W.E.; Zhang, Y.; Li, Y.; Ling, Y.; Li, H.N.; Li, S.H.; Jiang, S.J.; Ren, Z.Q.; Huang, Z.Q.; Zhang, F. Determination of gardenia yellow colorants in soft drink, pastry, instant noodles with ultrasound-assisted extraction by high performance liquid chromatography-electrospray ionization tandem mass spectrum. J Chromatogr. A 2016, 1446, 59–69.
- Giovannucci, E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp. Biol. Med. 2002, 227, 852–859.
- Giovannucci, E. Lycopene and prostate cancer risk. Methodological considerations in the epidemiologic literature. Pure Appl. Chem. 2002, 74, 1427–1434.
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and risk of prostate cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1260.
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose-response meta-analysis of observational studies. PLoS ONE 2015, 10, e0137427.
- Rowles, J.L., 3rd; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377.
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 1197–1205.
- Ge, X.-X.; Xing, M.-Y.; Yu, L.-F.; Shen, P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 1911–1918.
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid intake from natural sources and head and neck cancer: A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1003–1011.
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091.
- Li, X.; Xu, J. Dietary and circulating lycopene and stroke risk: A meta-analysis of prospective studies. Sci. Rep. 2014, 4, 5031.
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61, 9.
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158.
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108.
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica cochinchinensis Spreng. (gac) fruit carotenoids reevaluated. J. Food Compos. Anal. 2006, 19, 664–668.
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2014, 50, 567–577.
- Khalil, M.; Raila, J.; Ali, M.; Islan, R.N.S.; Schenk, R. Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under processing conditions. J. Funct. Foods 2012, 4, 602–610.
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12.
- Ma, L.; Dou, H.L.; Wu, Y.Q.; Huang, Y.M.; Huang, Y.B.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359.
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 56, 252–258.
- Cui, Y.H.; Jing, C.X.; Pan, H.W. Association of blood antioxidants and vitamins with risk of age-related cataract: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2013, 98, 778–786.
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between lutein and zeaxanthin status and the risk of cataract: A meta-analysis. Nutrients 2014, 6, 452–465.
- Ma, L.; Hao, Z.X.; Liu, R.R.; Yu, R.B.; Shi, Q.; Pan, J.P. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 63–70.
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid intake and risk of non-Hodgkin lymphoma: A systematic review and dose-response meta-analysis of observational studies. Ann. Hematol. 2017, 96, 957–965.
- Leermakers, E.T.M.; Darweesh, S.K.L.; Baena, C.P.; Moreira, E.M.; van Lent, D.M.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481–494.
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: Findings from the Irish Longitudinal Study on Aging. J. Gerontol. 2017, 72, 1431–1436.
- Dong, S.; Xia, H.; Wang, F.; Sun, G. The effect of red palm oil on vitamin A deficiency: A meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1281.
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red palm oil: A review on processing, health benefits and its application in food. J. Oleo Sci. 2021, 70, 1201–1210.
- Loganathan, R.; Subramaniam, K.M.; Radhakrishnan, A.K.; Choo, Y.-M.; Teng, K.-T. Health-promoting effects of red palm oil: Evidence from animal and human studies. Nutr. Rev. 2017, 75, 98–113.
- Cassidy, L. Red palm oil. Inform 2017, 28, 6–10.
- Purnama, K.O.; Setyaningsih, D.; Hambali, E.; Taniwiryono, D. Processing, characteristics, and potential application of red palm oil—A review. Int. J. Oil Palm 2020, 3, 40–55.
- Hoe, B.C.; Chan, E.S.; Nagasundara Ramanan, R.; Ooi, C.W. Direct recovery of palm carotene by liquid-liquid extraction. J. Food Eng. 2022, 313, 110755.
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12.
- Das, A.; Yoon, S.H.; Lee, S.H.; Kim, J.Y.; Oh, D.K.; Kim, S.W. An update on microbial carotenoid production: Application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512.
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398.
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2022, 62, 6932–6946.
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76.
- Saini, R.K.; Keum, Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674.
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678.
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222.
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot. (Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348.
- Chekanov, K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar. Drugs 2023, 21, 108.
- U.S. Food & Drug Administration. Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2023.
- European Commission. Food Additives Database; European Commission: Brussels, Belgium, 2023.
- FAO. Combined Compendium of Food Additive Specifications; FAO: Rome, Italy, 2023.
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food Industry. Mar. Drugs 2023, 21, 82.
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417.
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412.
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428.
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.J.; Murray, P.M.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014. 64, 332–344.
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902.
- Rodriguez-Amaya, D.B.; Maldonade, I.R. Potentials and challenges in the production of microalgal pigments with reference to carotenoids, chlorophylls, and phycobiliproteins. In Handbook of Algal Technology and Phytochemicals Volume 1 Food, Health and Neutraceutical Applications; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 109–118.
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406.
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353.
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein production from microalgae: A review. Bioresour. Technol. 2023, 376, 128875.
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362.
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88.
- Günerken, E.; D’Hondt, E.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260.
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841.
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176.
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016, 14, 214.
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334.
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent advances in supercritical CO2 extraction of pigments, lipids and bioactive compounds from microalgae. Molecules 2023, 28, 1410.
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103.
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213.
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756.
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283.
- Wichuk, K.; Brynjólfsson, S.; Fu, W. Biotechnological production of value-added carotenoids from microalgae: Emerging technology and prospects. Bioengineered 2014, 5, 204–208.
- Poonkum, W.; Powtongsook, S.; Pavasant, P. Astaxanthin induction in microalga H. pluvialis with flat panel airlift photobioreactors under indoor and outdoor conditions. Prep. Biochem. Biotechnol. 2015, 45, 1–17.
- Yang, Z.; Cheng, J.; Li, K.; Zhou, J.; Cen, K. Optimizing gas transfer to improve growth rate of Haematococcus pluvialis in a raceway pond with chute and oscillating baffles. Bioresour. Technol. 2016, 214, 276–283.
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335.
- Zhang, Z.; Huang, J.J.; Sun, D.; Lee, Y.; Chen, F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour. Technol. 2017, 224, 515–522.
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310.
- Kim, B.; Youn Lee, S.; Lakshmi Narasimhan, A.; Kim, S.; Oh, Y.K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124.
- Koopmann, I.K.; Möller, S.; Elle, C.; Hindersin, S.; Kramer, A.; Labes, A. Optimization of astaxanthin recovery in the downstream process of Haematococcus pluvialis. Foods 2022, 11, 1352.
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190.
- Haque, F.; Dutta, A.; Thimmanagarib, M.; Chiang, Y.W. Intensified green production of astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 1–11.
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910.
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515.
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20.
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479.
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282.
- Dineshkumar, R.; Subramanian, G.; Dash, S.K.; Sen, R. Development of an optimal light-feeding strategy coupled with semi-continuous reactor operation for simultaneous improvement of microalgal photosynthetic efficiency, lutein production and CO2 sequestration. Biochem. Eng. J. 2016, 113, 47–56.
- Chen, C.Y.; Lu, I.C.; Nagarajan, D.; Chang, C.H.; Ng, I.S.; Lee, D.J.; Chang, J.S. A highly efficient two-stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB-1-M12. Bioresour. Technol. 2018, 253, 141–147.
- Jeon, J.Y.; Kwon, J.S.; Kang, S.T.; Kim, B.R.; Jung, Y.; Han, J.G.; Park, J.H.; Hwang, J.K. Optimization of culture media for large-scale lutein production by heterotrophic Chlorella vulgaris. Biotechnol. Prog. 2014, 30, 736–743.
- Flórez-Miranda, L.; Cañizares-Villanueva, R.O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Flores-Ortíz, C.M. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74.
- Mulders, K.J.; Lamers, P.P.; Martens, D.E. Wijffels. R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242.
- Zheng, H.S.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795.
- Přibyl, P.; Pilný, J.; Cepák, V.; Petr Kaštánek, P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Res. 2016, 16, 69–75.
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Rep. (Amst.) 2016, 10, 117–125.
- Chiu, P.H.; Soong, K.; Chen, C.-N. Cultivation of two thermotolerant microalgae under tropical conditions: Influences of carbon sources and light duration on biomass and lutein productivity in four seasons. Bioresour. Technol. 2016, 212, 190–198.
- Hong, S.J.; Yim, K.J.; Ryu, Y.J.; Lee, C.G.; Jang, H.J.; Jung, J.Y.; Kim, Z.H. Improvement of lutein and zeaxanthin production in Mychonastes sp. 247 by optimizing light intensity and culture salinity conditions. J. Microbiol. Biotechnol. 2023, 33, 260–267.
- Heo, J.; Shin, D.S.; Cho, K.; Cho, D.H.; Lee, Y.J.; Kim, H.S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7.
