Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Everton Freitas de Morais | -- | 1848 | 2023-11-14 18:24:36 | | | |
| 2 | Jessie Wu | + 6 word(s) | 1854 | 2023-11-15 03:35:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Morais, E.F.; De Farias Morais, H.G.; De Almeida Freitas, R.; Coletta, R.D. Histopathological Parameters for Salivary Gland Adenoid Cystic Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/51553 (accessed on 08 February 2026).
De Morais EF, De Farias Morais HG, De Almeida Freitas R, Coletta RD. Histopathological Parameters for Salivary Gland Adenoid Cystic Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/51553. Accessed February 08, 2026.
De Morais, Everton Freitas, Hannah Gil De Farias Morais, Roseana De Almeida Freitas, Ricardo D. Coletta. "Histopathological Parameters for Salivary Gland Adenoid Cystic Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/51553 (accessed February 08, 2026).
De Morais, E.F., De Farias Morais, H.G., De Almeida Freitas, R., & Coletta, R.D. (2023, November 14). Histopathological Parameters for Salivary Gland Adenoid Cystic Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/51553
De Morais, Everton Freitas, et al. "Histopathological Parameters for Salivary Gland Adenoid Cystic Carcinoma." Encyclopedia. Web. 14 November, 2023.
Copy Citation
Adenoid cystic carcinoma (ACC) is a rare salivary gland tumor that accounts for approximately 1% of all head and neck cancers. Despite its initial indolent behavior, long-term survival is poor due to locoregional recurrence in approximately 40% and distant metastasis in up to 60% of patients who undergo radical treatment. The histological parameters of ACC and the combination of these parameters in histopathological grading systems provide valuable prognostic information about the clinical course of the disease.
malignant salivary gland tumors
prognosis
histopathological features
histopathological grading systems
1. Histopathological Pattern and Adenoid Cystic Carcinoma Prognosis
Adenoid cystic carcinoma (ACC) is characterized by a dual cell population consisting of basaloid cells with myoepithelial/basal differentiation and luminal/epithelial cells, which may histologically acquire specific architectural patterns that determine three pathological subtypes [1][2]. The cribriform pattern is characterized by islands of small or cuboidal basaloid epithelial cells that contain a basophilic nucleus and scarce cytoplasm with multiple pseudocystic spaces filled with basophilic material compatible with glycosaminoglycans (Figure 1A,B). In the tubular pattern, the ductal structures are lined with a single layer of epithelial cells or one or more layers of basaloid cells. An amorphous eosinophilic material may be present in the center of the tubules and the stroma in this pattern is often hyalinized (Figure 1C,D). In the solid pattern, tumor islands are filled with luminal and non-luminal cells dispersed in a dense fibrous connective stroma; areas with ductal or pseudocystic spaces are rare [3][4][5] (Figure 1E,F). Most cases of ACC exhibit more than one pathological pattern [6][7].
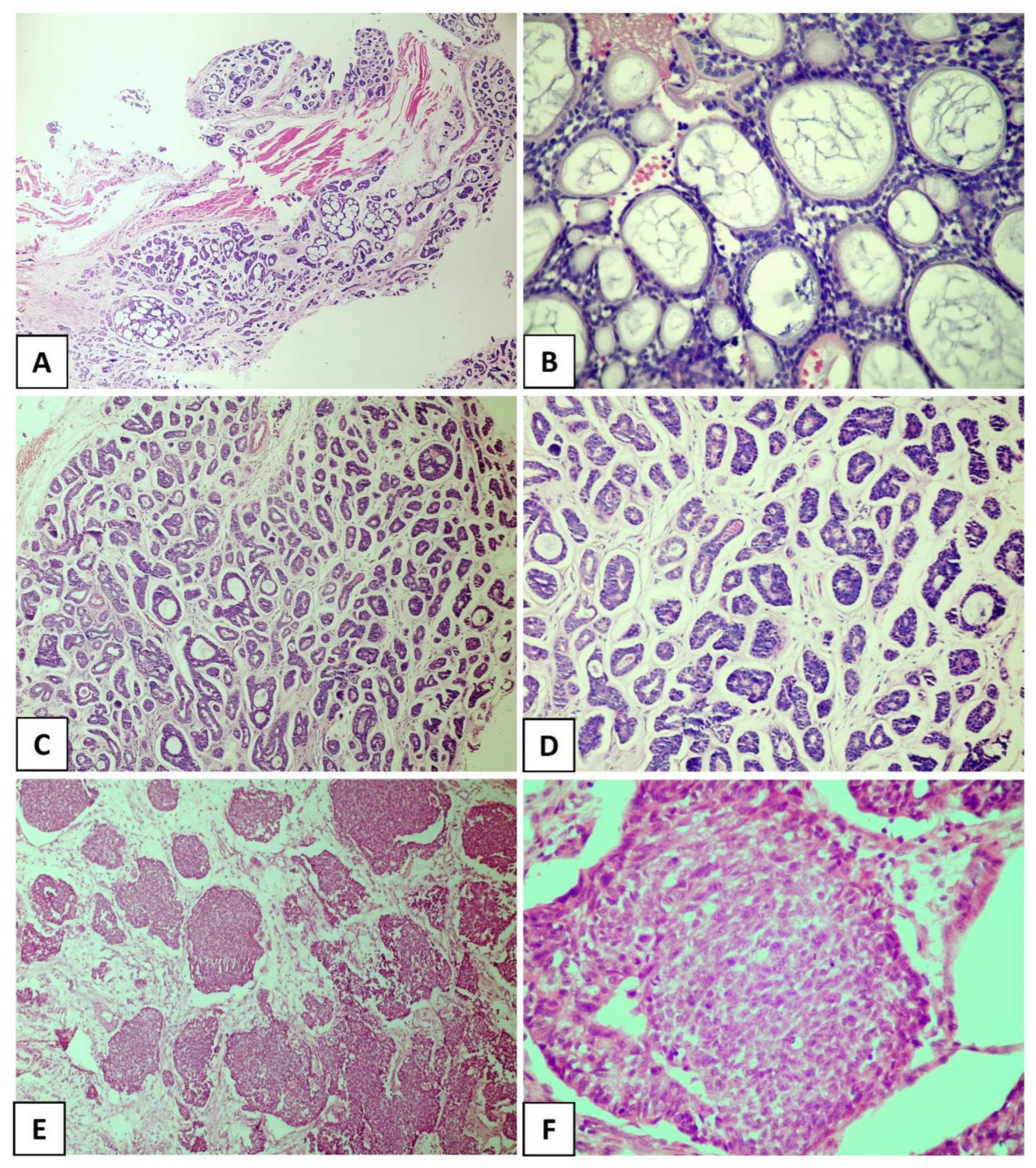
Figure 1. Histological subtypes of the salivary gland adenoid cystic carcinoma. (A,B) Cribriform pattern, (C,D) tubular pattern, and (E,F) solid pattern. ((A) In original magnification of 40×, (C–E) in original magnification of 100×, and (B,F) in original magnification of 200×).
For decades, studies have tried to identify histopathological factors that correlate with the prognosis of ACC [8][9][10][11][12]. The solid proliferation of tumor cells (predominance of solid areas) was notoriously one of the first proposed prognostic factors and has been considered a useful histological parameter for assessing survival rates in patients with ACC [8]. Indeed, the solid subtype is considered the most aggressive variant of ACC, which has a poor prognosis [8][13][14][15]. This importance has been confirmed by all histological grading systems for ACC available so far, which are based on the presence and/or proportion of the solid component in the tumor [8][9][10][11][12]. Furthermore, according to the current WHO classification for salivary gland tumors, ACC in which the solid component accounts for more than one-third of the tumor exhibits a poor clinical course [16].
Compared to the cribriform and tubular subtypes, the solid pattern is characterized by high loss of heterozygosity, a larger number of chromosomal and somatic mutations, and high expression of p53 [17][18][19][20][21]. Clinicopathological studies indicate a higher risk of lymph node metastases in solid ACC [8] and a direct association of this pattern with poor overall survival [22]. In addition to these results, de Morais et al. [15] found that the presence of the solid pattern contributed to the negative impact on long-term disease-specific survival (10 years) of cases evaluated in both univariate and multivariate analyses, corroborating the importance of the solid pattern for the prognostic evaluation of ACC.
Some findings that have been described as markers of poor prognosis (Figure 2A,D) are more common in the solid pattern of ACC compared to the cribriform and tubular subtypes, particularly tumor necrosis and high-grade transformation [4][23][24][25]. Xu et al. [4] explored the presence of necrosis as a prognostic factor for ACC in a large retrospective cohort of 135 patients and found that its presence is a predictor of poor recurrence-free survival and disease-free survival. In that study, the presence of necrosis was associated with a high mitotic index and severe nuclear atypia [4]. High-grade transformation, i.e., the presence of a distinct population of anaplastic cells [24], has been histologically defined when the tumor exhibits intense nuclear atypia, a high mitotic index, prominent necrosis, and loss of biphasic ductal-myoepithelial differentiation, features indicative of a poor prognosis [4][23][25]. In a recent study conducted by Zhu et al. [26], high-grade transformation was the only histological parameter associated with shortened overall survival and disease-specific survival in multivariate analysis. It is important to highlight that numerous studies have identified particular mutations in solid tumors. Ferrarotto et al. [27] detected a high frequency of NOTCH1 mutations among solid ACCs with liver and bone metastasis, as well as a poorer prognosis in these cases. Liu et al. [28] reported that loss of PTEN expression was more frequently seen in ACC compared to other salivary gland malignancies, especially in the poorly differentiated, high-grade subtype of solid ACC.
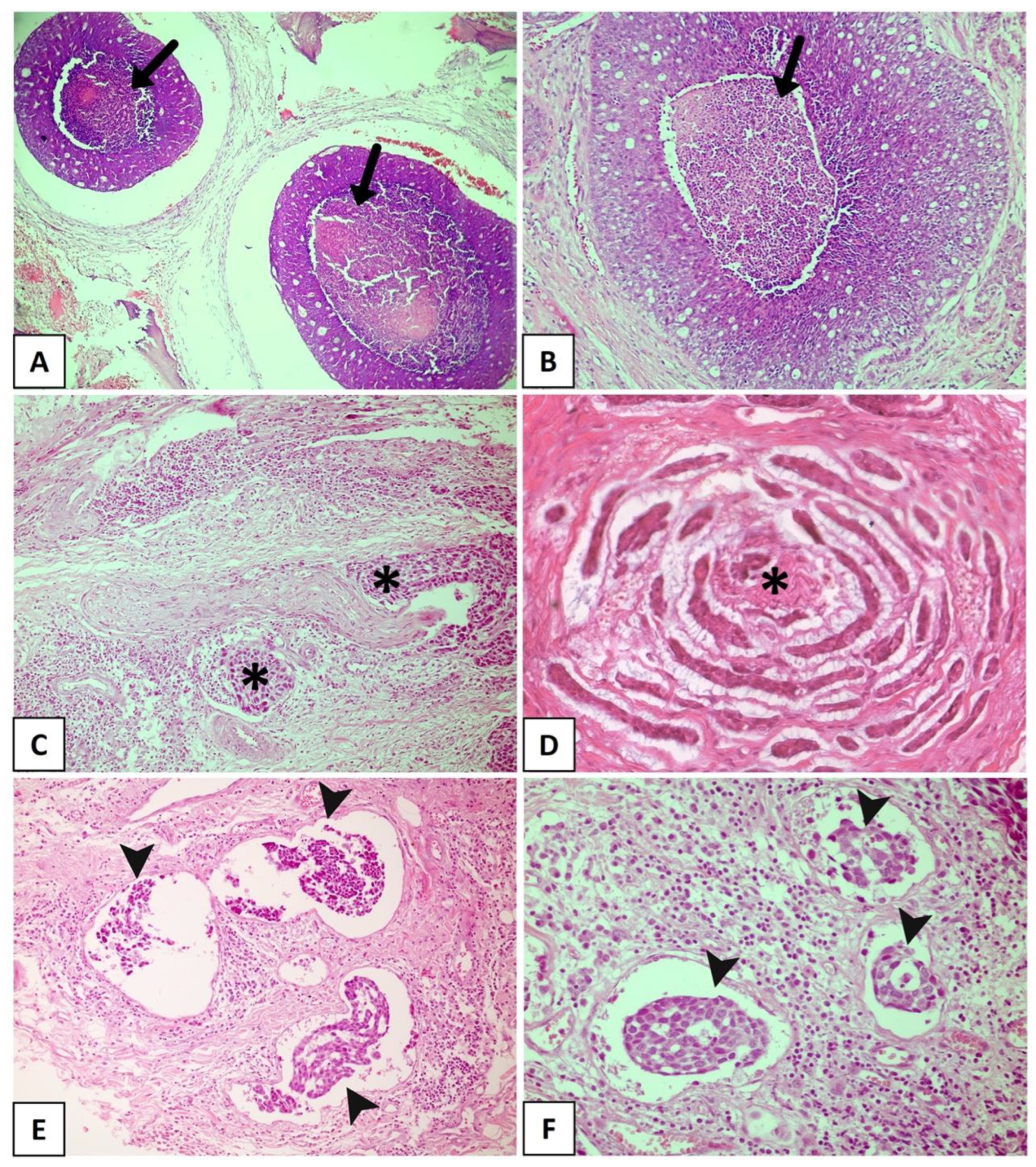
Figure 2. Histopathological features with promising prognostic potential for adenoid cystic carcinoma. (A,B) The black arrow showing extensive areas of necrosis. Solid subtype with perineural invasion in cords (C) and small islands (D) (asterisks). (E,F) Solid tumor with vascular invasion (arrowhead). (Figure (A) in original magnification of 40×, (B,C,E,F) in original magnification of 100×, and (D) in original magnification of 200×).
2. Individual Histological Features and Adenoid Cystic Carcinoma Prognosis
Several morphological characteristics were identified as potential prognostic markers for patients with ACC.
PNI, which is nowadays considered a consequence of interactions among tumor cells, neural cells, and the perineural niche [15], is found in 48–82% of head and neck ACCs (Figure 2C). It has been widely accepted as the fourth metastatic pathway in ACC, together with the other three well-known routes: direct invasion of surrounding tissues, lymphatic metastasis, and hematogenous metastasis [29][30][31][32]. PNI has been widely reported as an independent negative prognostic factor in head and neck ACC and is significantly associated with decreased overall survival, disease-specific survival, locoregional control, and distant metastasis [33][34][35][36]. Fang et al. [29] reinforced the importance of a careful assessment of PNI in ACC, considering the degree of impairment (invaded layers of the nerve sheath), number of neural invasions, margins of involved nerves (compromised neural circumference), and sites of tumor dissemination (large or small nerves). A meta-analysis conducted by Ju et al. [37] indicated that PNI is strongly associated with poor overall and disease-free survival in ACC. Fordice [38] showed that survival significantly decreases when a large nerve is involved. Invasion involving the perineurium or endoneurium increases the risk of recurrence when compared to tumors whose growth occurs only adjacent to the nerves [39]. In a multicenter cohort of 239 patients with ACC and neural invasion, Amit et al. [40] found intraneural invasion to be an independent negative prognostic marker for overall survival and disease-specific survival. Thus, considering these findings, intraneural invasion is more strongly correlated with a poor prognosis than PNI and is a reliable prognostic predictor of ACC. Considering the association between PNI and ACC prognosis, a comprehensive investigation into the underlying mechanisms related to this process is crucial. This exploration is essential to determine potential predictors of this condition and to identify specific markers that can accurately predict tumor prognosis. Such discoveries could facilitate the development of targeted drugs and effectively reduce the risk of ACC recurrence. However, several studies have highlighted the close correlation between distinct PNI characteristics and factors such as ACC metastasis, locoregional recurrence of the tumor, and overall quality of life of patients [41].
LVI is another histological finding associated with poor clinical behavior in different malignant neoplasms [34][42][43][44][45][46][47][48]. In ACC (Figure 2E,F), LVI is reported in a large number of cases, ranging from 5% to 70%, and is a known indicator of high recurrence risk and poor prognosis [49][50]. Tang et al. [51] revealed LVI to be an independent prognostic determinant, whereas Oplatek et al. [44] found a significant association with recurrences. In a systematic review and meta-analysis, Martins-Andrade et al. [33] found LVI to be a significant prognostic parameter for head and neck ACC, which was associated with lower overall survival rates and a higher risk of lymph node metastases. The prognostic relevance of LVI as a risk factor for recurrence has been documented in the literature [44][51]. Jeong et al. [48] identified the presence of LVI in surgical pathology specimens to be associated with a reduction in 5-year overall survival in recurrent and metastatic ACC, with multivariate analysis confirming its role as an independent risk factor. Although these results indicate an aggressive evolution associated with LVI, most published studies only described its presence or absence and considered LVI to be a secondary finding. Indeed, few studies investigated the true importance of LVI and its impact on survival in ACC as a primary endpoint. Furthermore, the profile of tumor emboli is not well described, with the types of vessels that are being invaded, whether blood or lymphatic, and the morphological characteristics of the invaded vessel being unknown [33][52]. Therefore, large studies investigating the prognostic potential of LVI in ACC are necessary.
The presence of positive surgical margins has also been proposed as a prognostic factor for ACC [33]. Amit et al. [53] analyzed the role of surgical margins in 507 cases of head and neck ACC in an international multicenter study and found a high rate of positive surgical margins (50% of the analyzed cases). The authors concluded that positive margins are predictors of poor survival in head and neck ACC, while negative margins, corresponding to tumor-free margins <5 mm, are associated with a favorable outcome. Although de Morais et al. [15] found a statistically significant association between positive margins and a poor prognosis at 5 and 10 years in univariate analysis, in multivariate analysis, the status of the surgical margin itself did not have an independent prognostic value. Furthermore, there are no data in the literature that confirm the prognostic role of positive surgical margins in recurrent and metastatic ACC [22][50]. It is worthwhile noting that the ability to achieve large tumor-free margins depends on a number of factors, including tumor location and size, histopathological pattern, and previous treatment [29]. In many cases, surgery is limited by the proximity of vital structures [15], as occurs in some cases of head and neck ACC that have an anatomical propensity for intracranial extension involving vital structures, posing a challenge to achieve negative surgical margins [29]. Studies have shown that patients with ACC arising at sites close to the base of the skull (nasopharynx, nasal cavity, and paranasal sinuses) have a significantly higher risk of local recurrence [53][54].
In a recent study, Xuan et al. [55] proposed an evaluation based on the dominant cell type. The authors applied immunohistochemistry against p63 and CD117 and classified ACCs into epithelial (E-ACC), myoepithelial (M-ACC), and conventional (C-ACC) subtypes. The results showed that the E-ACC subtype was an independent negative prognostic factor for overall survival and recurrence-free survival. According to the authors, the main advantages of this analysis for ACC prognostic prediction are the reduction in subjectivity associated with traditional histological grading, as well as the practicality and simplicity of the method [55].
With respect to prognostic studies, researchers must consider the inconsistencies of the results reported in the literature, which may be due to many factors such as differences in patient treatment protocols among cohorts, the duration of postoperative follow-up, and the differences in the proportion of patients in different stages of the disease [55][56]. However, despite all these drawbacks, the findings highlight the importance of extensive histological evaluation of the primary tumor to identify the elements that imply greater tumor aggressiveness [50], without disregarding the fact that the intrinsic characteristics of the primary tumor may also influence the outcome of recurrent and metastatic disease [57].
References
- Nishida, H.; Kusaba, T.; Kawamura, K.; Oyama, Y.; Daa, T. Histopathological aspects of the prognostic factors for salivary gland cancers. Cancers 2023, 15, 1236.
- Dewenter, I.; Otto, S.; Kakoschke, T.K.; Smolka, W.; Obermeier, K.T. Recent advances, systemic therapy, and molecular targets in adenoid cystic carcinoma of the head and neck. J. Clin. Med. 2023, 12, 1463.
- Hellquist, H.; Skalova, A. Histopathology of the Salivary Glands; Springer: Berlin/Heidelberg, Germany, 2014.
- Xu, B.; Drill, E.; Ho, A.; Ho, A.; Dunn, L.; Prieto-Granada, C.N.; Chan, T.; Ganly, I.; Ghossein, R.; Katabi, N. Predictors of outcome in adenoid cystic carcinoma of salivary glands: A clinicopathologic study with correlation between MYB fusion and protein expression. Am. J. Surg. Pathol. 2017, 41, 1422–1432.
- Iyer, J.; Hariharan, A.; Cao, U.M.N.; Mai, C.T.T.; Wang, A.; Khayambashi, P.; Nguyen, B.H.; Safi, L.; Tran, S.D. An overview on the histogenesis and morphogenesis of salivary gland neoplasms and evolving diagnostic approaches. Cancers 2021, 13, 3910.
- Gondivkar, S.M.; Gadbail, A.R.; Chole, R.; Parikh, R.V. Adenoid cystic carcinoma: A rare clinical entity and literature review. Oral Oncol. 2011, 47, 231–236.
- Alali, F.; Kochaji, N. Proliferative activity of myoepithelial cells in normal salivary glands and adenoid cystic carcinomas based on double immunohistochemical labeling. Asian Pac. J. Cancer Prev. 2018, 19, 1965–1970.
- van Weert, S.; van der Waal, I.; Witte, B.I.; Leemans, C.R.; Bloemena, E. Histopathological grading of adenoid cystic carcinoma of the head and neck: Analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015, 51, 71–76.
- Morita, N.; Murase, T.; Ueda, K.; Nagao, T.; Kusafuka, K.; Nakaguro, M.; Urano, M.; Taguchi, K.I.; Yamamoto, H.; Kano, S.; et al. Pathological evaluation of tumor grade for salivary adenoid cystic carcinoma: A proposal of an objective grading system. Cancer Sci. 2021, 112, 1184–1195.
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am. J. Surg. 1974, 128, 512–520.
- Perzin, K.H.; Gullane, P.; Clairmont, A.C. Adenoid cystic carcinomas arising in salivary glands; a correlation of histologic features and clinical course. Cancer 1978, 42, 265–282.
- Szanto, P.A.; Luna, M.A.; Tortoledo, M.E.; White, R.A. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984, 54, 1062–1069.
- Mays, A.C.; Hanna, E.Y.; Ferrarotto, R.; Phan, J.; Bell, D.; Silver, N.; Mulcahy, C.F.; Roberts, D.; Abdelmeguid, A.S.A.; Fuller, C.D.; et al. Prognostic factors and survival in adenoid cystic carcinoma of the sinonasal cavity. Head Neck 2018, 40, 2596–2605.
- Takebayashi, S.; Shinohara, S.; Tamaki, H.; Tateya, I.; Kitamura, M.; Mizuta, M.; Tanaka, S.; Kojima, T.; Asato, R.; Maetani, T.; et al. Adenoid cystic carcinoma of the head and neck: A retrospective multicenter study. Acta Otolaryngol. 2018, 138, 73–79.
- de Morais, E.F.; da Silva, L.P.; Moreira, D.G.L.; Mafra, R.P.; Rolim, L.S.A.; de Moura Santos, E.; de Souza, L.B.; de Almeida Freitas, R. Prognostic factors and survival in adenoid cystic carcinoma of the head and neck: A retrospective clinical and histopathological analysis of patients seen at a cancer center. Head Neck Pathol. 2021, 15, 416–424.
- WHO Classification of Tumours Editorial Board. Head and neck tumours. Lyon (France). In International Agency for Research on Cancer, 5th ed.; WHO Classification of Tumours Series; WHO: Geneva, Switzerland, 2022; Volume 9.
- Yamamoto, Y.; Virmani, A.K.; Wistuba, I.I.; McIntire, D.; Vuitch, F.; Albores-Saavedra, J.; Gazdar, A.F. Loss of heterozygosity and microsatellite alterations in p53 and RB genes in adenoid cystic carcinoma of the salivary glands. Hum. Pathol. 1996, 27, 1204–1210.
- Zhu, Q.R.; White, F.H.; Tipoe, G.L. P53 oncoprotein accumulation in adenoid cystic carcinoma of parotid and palatine salivary glands. Pathology 1997, 29, 154–158.
- Yamamoto, Y.; Wistuba, I.I.; Kishimoto, Y.; Virmani, A.K.; Vuitch, F.; Albores-Saavedra, J.; Gazdar, A.F. DNA analysis at p53 locus in adenoid cystic carcinoma: Comparison of molecular study and p53 immunostaining. Pathol. Int. 1998, 48, 273–280.
- Vékony, H.; Ylstra, B.; Wilting, S.M.; Meijer, G.A.; van de Wiel, M.A.; Leemans, C.R.; van der Waal, I.; Bloemena, E. DNA copy number gains at loci of growth factors and their receptors in salivary gland adenoid cystic carcinoma. Clin. Cancer Res. 2007, 13, 3133–3139.
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798.
- van Weert, S.; Reinhard, R.; Bloemena, E.; Buter, J.; Witte, B.I.; Vergeer, M.R.; Leemans, C.R. Differences in patterns of survival in metastatic adenoid cystic carcinoma of the head and neck. Head Neck 2017, 39, 456–463.
- Seethala, R.R.; Hunt, J.L.; Baloch, Z.W.; Livolsi, V.A.; Leon Barnes, E. Adenoid cystic carcinoma with high-grade transformation: A report of 11 cases and a review of the literature. Am. J. Surg. Pathol. 2007, 31, 1683–1694.
- Hellquist, H.; Skálová, A.; Barnes, L.; Cardesa, A.; Thompson, L.D.; Triantafyllou, A.; Williams, M.D.; Devaney, K.O.; Gnepp, D.R.; Bishop, J.A.; et al. Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: A collective international review. Adv. Ther. 2016, 33, 357–368.
- Skalova, A.; Leivo, I.; Hellquist, H.; Agaimy, A.; Simpson, R.H.W.; Stenman, G.; Vander Poorten, V.; Bishop, J.A.; Franchi, A.; Hernandez-Prera, J.C.; et al. High-grade transformation/dedifferentiation in salivary gland carcinomas: Occurrence across subtypes and clinical Significance. Adv. Anat. Pathol. 2021, 28, 107–118.
- Zhu, Y.; Zhu, X.; Xue, X.; Zhang, Y.; Hu, C.; Liu, W.; Lu, H. Exploration of high-grade transformation and postoperative radiotherapy on prognostic analysis for primary adenoid cystic carcinoma of the head and neck. Front. Oncol. 2021, 11, 647172.
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J. Clin. Oncol. 2017, 35, 352–360.
- Liu, H.; Du, L.; Wang, R.; Wei, C.; Liu, B.; Zhu, L.; Liu, P.; Liu, Q.; Li, J.; Lu, S.-L.; et al. High frequency of loss of PTEN expression in human solid salivary adenoid cystic carcinoma and its implication for targeted therapy. Oncotarget 2015, 6, 11477–11491.
- Fang, Y.; Peng, Z.; Wang, Y.; Gao, K.; Liu, Y.; Fan, R.; Zhang, H.; Xie, Z.; Jiang, W. Current opinions on diagnosis and treatment of adenoid cystic carcinoma. Oral Oncol. 2022, 130, 105945.
- Akbaba, S.; Bostel, T.; Lang, K.; Bahadir, S.; Lipman, D.; Schmidberger, H.; Matthias, C.; Rotter, N.; Knopf, A.; Freudlsperger, C.; et al. Large German multicenter experience on the treatment outcome of 207 patients with adenoid cystic carcinoma of the major salivary glands. Front. Oncol. 2020, 10, 593379.
- Kawakita, D.; Murase, T.; Ueda, K.; Kano, S.; Tada, Y.; Tsukahara, K.; Okami, K.; Onitsuka, T.; Fujimoto, Y.; Matoba, T.; et al. The impact of clinicopathological factors on clinical outcomes in patients with salivary gland adenoid cystic carcinoma: A multi-institutional analysis in Japan. Int. J. Clin. Oncol. 2020, 25, 1774–1785.
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408.
- Martins-Andrade, B.; Dos Santos Costa, S.F.; Sant’ana, M.S.P.; Altemani, A.; Vargas, P.A.; Fregnani, E.R.; Abreu, L.G.; Batista, A.C.; Fonseca, F.P. Prognostic importance of the lymphovascular invasion in head and neck adenoid cystic carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019, 93, 52–58.
- Lim, W.S.; Oh, J.S.; Roh, J.L.; Kim, J.S.; Kim, S.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prediction of distant metastasis and survival in adenoid cystic carcinoma using quantitative 18F-FDG PET/CT measurements. Oral Oncol. 2018, 77, 98–104.
- Zhang, C.Y.; Xia, R.H.; Han, J.; Wang, B.S.; Tian, W.D.; Zhong, L.P.; Tian, Z.; Wang, L.Z.; Hu, Y.H.; Li, J. Adenoid cystic carcinoma of the head and neck: Clinicopathologic analysis of 218 cases in a Chinese population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 368–375.
- Chang, C.F.; Hsieh, M.Y.; Chen, M.K.; Chou, M.C. Adenoid cystic carcinoma of head and neck: A retrospective clinical analysis of a single institution. Auris Nasus Larynx. 2018, 45, 831–837.
- Ju, J.; Li, Y.; Chai, J.; Ma, C.; Ni, Q.; Shen, Z.; Wei, J.; Sun, M. The role of perineural invasion on head and neck adenoid cystic carcinoma prognosis: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 691–701.
- Fordice, J.; Kershaw, C.; El-Naggar, A.; Goepfert, H. Adenoid cystic carcinoma of the head and neck: Predictors of morbidity and mortality. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 149.
- Teymoortash, A.; Zieger, L.; Hoch, S.; Pagenstecher, A.; Hofer, M.J. Distinct microscopic features of perineural invasion in adenoid cystic carcinoma of the head and neck. Histopathology 2014, 64, 1037–1039.
- Amit, M.; Binenbaum, Y.; Trejo-Leider, L.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Miles, B.; Yang, X.; Lei, D.; et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 2015, 37, 1038–1045.
- Liu, X.; Yang, X.; Zhan, C.; Zhang, Y.; Hou, J.; Yin, X. Perineural invasion in adenoid cystic carcinoma of the salivary glands: Where we are and where we need to go. Front. Oncol. 2020, 10, 1493.
- Ouyang, D.Q.; Liang, L.Z.; Zheng, G.S.; Ke, Z.F.; Weng, D.S.; Yang, W.F.; Su, Y.X.; Liao, G.Q. Risk factors and prognosis for salivary gland adenoid cystic carcinoma in southern China: A 25-year retrospective study. Medicine 2017, 96, e5964.
- Ouyang, D.Q.; Liang, L.Z.; Ke, Z.F.; Zheng, G.S.; Weng, D.S.; Yang, W.F.; Su, Y.X.; Liao, G.Q. Association between high expression of phosphorylated Akt and mammalian target of rapamycin and improved survival in salivary gland adenoid cystic carcinoma. Head Neck 2017, 39, 1145–1154.
- Oplatek, A.; Ozer, E.; Agrawal, A.; Bapna, S.; Schuller, D.E. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope 2010, 120, 65–70.
- Amit, M.; Binenbaum, Y.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Miles, B.; Yang, X.; Lei, D.; Bjøerndal, K.; et al. Analysis of failure in patients with adenoid cystic carcinoma of the head and neck. An international collaborative study: Failure Patterns of Adenoid Cystic Carcinoma in the Head and Neck. Head Neck 2014, 36, 998–1004.
- Marcinow, A.; Ozer, E.; Teknos, T.; Wei, L.; Hurtuk, A.; Old, M.; Agrawal, A.; Carrau, R.; Iwenofu, O.H. Clinicopathologic predictors of recurrence and overall survival in adenoid cystic carcinoma of the head and neck: A single institutional experience at a tertiary care center. Head Neck 2014, 36, 1705–1711.
- Cavalieri, S.; Mariani, L.; Vander Poorten, V.; Van Breda, L.; Cau, M.C.; Lo Vullo, S.; Alfieri, S.; Resteghini, C.; Bergamini, C.; Orlandi, E.; et al. Prognostic nomogram in patients with metastatic adenoid cystic carcinoma of the salivary glands. Eur. J. Cancer 2020, 136, 35–42.
- Jeong, I.S.; Roh, J.L.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for survival and distant metastasis in 125 patients with head and neck adenoid cystic carcinoma undergoing primary surgery. J. Cancer Res. Clin. Oncol. 2020, 146, 1343–1350.
- Gao, M.; Hao, Y.; Huang, M.X.; Ma, D.Q.; Luo, H.Y.; Gao, Y.; Peng, X.; Yu, G.Y. Clinicopathological study of distant metastases of salivary adenoid cystic carcinoma. Int. J. Oral Maxillofac. Surg. 2013, 42, 923–928.
- Lombardi, D.; Tomasoni, M.; Lorini, L.; Gurizzan, C.; Tomasini, D.; Ardighieri, L.; Battocchio, S.; Bozzola, A.; Mattavelli, D.; Paderno, A.; et al. Baseline prognostic factors affecting survival in recurrent and/or metastatic salivary gland adenoid cystic carcinoma. Oral Oncol. 2022, 126, 105764.
- Tang, Q.L.; Fan, S.; Li, H.G.; Chen, W.L.; Shen, X.M.; Yuan, X.P.; Chang, S.H.; Song, Y. Expression of Cyr61 in primary salivary adenoid cystic carcinoma and its relation to Ki-67 and prognosis. Oral Oncol. 2011, 47, 365–370.
- Alsarraj, M.; Alshehri, S.M.; Qattan, A.; Mofti, A.; Wazqer, L.; Bukhari, S.; Shamsaldin, A.; Rajab, R. Lymph node involvement and the clinical stage as predictors of the survival of patients with adenoid cystic carcinoma of the head and neck: A systematic review and meta-analysis. Cureus 2022, 14, e30780.
- Amit, M.; Na’ara, S.; Trejo-Leider, L.; Ramer, N.; Burstein, D.; Yue, M.; Miles, B.; Yang, X.; Lei, D.; Bjoerndal, K.; et al. Defining the surgical margins of adenoid cystic carcinoma and their impact on outcome: An international collaborative study. Head Neck 2017, 39, 1008–1014.
- Garden, A.S.; Weber, R.S.; Morrison, W.H.; Ang, K.K.; Peters, L.J. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 619–626.
- Xuan, L.; Yuan, J.; Zhang, H.; Zhang, Y.; Liu, H. Dominant cell type analysis predicts head and neck adenoid cystic carcinoma outcomes. Ann. Diagn. Pathol. 2022, 56, 151867.
- Liu, S.; Yang, J.; Lu, H.; Wu, Y.; Yang, W.; Xu, W.; Zhang, C. Adenoid cystic carcinoma of submandibular gland: Emphasis on locoregional metastasis and prognosis. Oral Dis. 2022; epub ahead of print.
- Lorini, L.; Ardighieri, L.; Bozzola, A.; Romani, C.; Bignotti, E.; Buglione, M.; Guerini, A.; Lombardi, D.; Deganello, A.; Tomasoni, M.; et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021, 115, 105213.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
468
Revisions:
2 times
(View History)
Update Date:
15 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




