
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krishan Jain | -- | 3772 | 2023-11-13 14:47:42 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 3775 | 2023-11-14 01:37:55 | | |
Video Upload Options
Lung diseases rank third in terms of mortality and represent a significant economic burden globally. Scientists have been conducting research to better understand respiratory diseases and find treatments for them. An ideal in vitro model must mimic the in vivo organ structure, physiology, and pathology. Organoids are self-organizing, three-dimensional (3D) structures originating from adult stem cells, embryonic lung bud progenitors, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs). These 3D organoid cultures may provide a platform for exploring tissue development, the regulatory mechanisms related to the repair of lung epithelia, pathophysiological and immunomodulatory responses to different respiratory conditions, and screening compounds for new drugs. To create 3D lung organoids in vitro, both co-culture and feeder-free methods have been used. However, there exists substantial heterogeneity in the organoid culture methods, including the sources of type 2 alveolar cells (AT2) cells, media composition, and feeder cell origins.
1. Introduction
2. Sources of AT2 Cells
3. AT2 Organoid Culture Methods
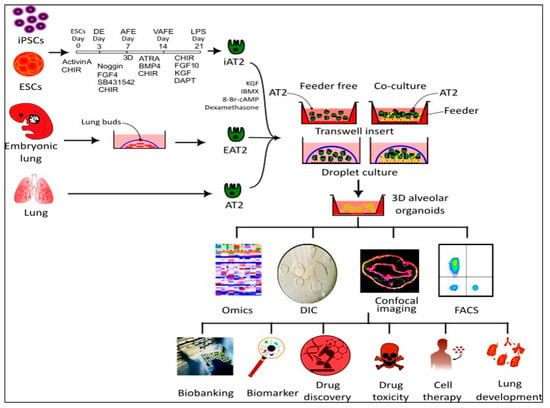
3.1. Co-Culture Method
3.1.1. EpCAM+ AT2 Co-Cultured with Fibroblast Cells
3.1.2. AT2 Co-Cultured with PDGFRA+ Cells
3.1.3. Human iPSC-Derived AT2
3.1.4. Mouse and Human ESC-Derived AT2 Cells
3.2. Feeder-Free Organoid Culture Systems
3.3. D Matrix Alternatives to Matrigel for Organoid Cultures
4. Applications
Lung cancer is the leading cause of cancer-related deaths globally. The absence of appropriate ex vivo models of the human alveolar epithelium has hindered our understanding of lung cancer pathogenesis and related therapy development. Early-stage diagnosis and treatment are essential for preventing cancer relapse and saving lives. Recently, Dost et al. [72] used both mouse AT2 and human iPSCAT2 organoid models to uncover the early consequences of oncogenic KRAS expression in vivo. Their work has provided novel tools for extensive data collection and studying the transcriptional and proteomic changes that distinguish normal epithelial progenitor cells from early-stage lung cancer. Their study revealed that reductions in AT2 lineage marker gene expression are an early consequence of oncogenic KRAS. Multiomics studies demonstrated that SPC-high cells in Kras activation and p53 loss (KP) lung tumor organoids exhibit higher tumorigenic capacity in the lung microenvironment compared to Hmga2-high cells [73].
HESC-derived organoids have been used to model human lung development. These organoids contained early-stage proximal and distal airway epithelial cells, including early-staged alveolar type 2 (AT2) cells (SPC+/SOX9+) and immature alveolar type 1 (AT1) cells (HOPX+/SOX9+) in vitro. However, when transplanted in vivo for the short term, these organoids differentiated into only a few distal progenitor epithelial cells (NKX2.1+, SOX9+, and P63+). In contrast, the long-term transplantation of these organoids resulted in the differentiation of lung distal bipotent progenitor cells (PDPN+/SPC+/SOX9+), AT2 cells (SPC+ SPB+), and immature AT1 cells (PDPN+, AQP5−). These long-term transplanted organoids also contained other cell types present in lung tissues, such as mesenchymal cells, vasculature, neuroendocrine-like cells, and nerve fiber structures [74].
In a recent study, mouse primary AT2 and human iPSCs-derived AT2 organoids were used to investigate the early stages of lung adenocarcinoma (LUAD) driven by KRAS mutation. The data from the alveolar organoids model may be useful in screening novel drug targets and developing new drug molecules to prevent lung cancer growth at the early stage.
Feeder-free organoid-derived AT2 cells have shown great potential for cellular therapy in lung regeneration. In a recent study, mesenchyme-free AT2 organoids were transplanted into the lungs of mice injured by influenza. The transplanted organoids retained their AT2 fate; however, in some cases, they adopted a dysplastic fate. These dysplastic organoids did not appear to improve the oxygen-exchange capability of the injured lungs in recipient mice. Further investigations have been requested to understand the molecular changes that occur in AT2 organoids after transplantation in the influenza-injured microenvironment in order to optimize organoid transplants [34].
5. Conclusions
References
- Chong, L.; Ahmadvand, N.; Noori, A.; Lv, Y.; Chen, C.; Bellusci, S.; Zhang, J.S. Injury activated alveolar progenitors (IAAPs): The underdog of lung repair. Cell Mol. Life Sci. 2023, 80, 145.
- Brownfield, D.G.; de Arce, A.D.; Ghelfi, E.; Gillich, A.; Desai, T.J.; Krasnow, M.A. Alveolar cell fate selection and lifelong maintenance of AT2 cells by FGF signaling. Nat. Commun. 2022, 13, 7137.
- Konishi, S.; Tata, A.; Tata, P.R. Defined conditions for long-term expansion of murine and human alveolar epithelial stem cells in three-dimensional cultures. STAR Protoc. 2022, 3, 101447.
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036.
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074.
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076.
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54.
- Franks, T.J.; Chong, P.Y.; Chui, P.; Galvin, J.R.; Lourens, R.M.; Reid, A.H.; Selbs, E.; McEvoy, C.P.; Hayden, C.D.; Fukuoka, J.; et al. Lung pathology of severe acute respiratory syndrome (SARS): A study of 8 autopsy cases from Singapore. Hum. Pathol. 2003, 34, 743–748.
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell 2020, 27, 890–904.e898.
- Baldassi, D.; Gabold, B.; Merkel, O.M. Air-Liquid Interface Cultures of the Healthy and Diseased Human Respiratory Tract: Promises, Challenges, and Future Directions. Adv. NanoBiomed Res. 2021, 1, 2000111.
- Miller, A.J.; Spence, J.R. In Vitro Models to Study Human Lung Development, Disease and Homeostasis. Physiology 2017, 32, 246–260.
- Yamamoto, Y.; Korogi, Y.; Hirai, T.; Gotoh, S. A method of generating alveolar organoids using human pluripotent stem cells. Methods Cell Biol. 2020, 159, 115–141.
- Fang, X.; Song, Y.; Hirsch, J.; Galietta, L.J.; Pedemonte, N.; Zemans, R.L.; Dolganov, G.; Verkman, A.S.; Matthay, M.A. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L242–L249.
- Cheek, J.M.; Kim, K.J.; Crandall, E.D. Tight monolayers of rat alveolar epithelial cells: Bioelectric properties and active sodium transport. Am. J. Physiol. 1989, 256, C688–C693.
- Demaio, L.; Tseng, W.; Balverde, Z.; Alvarez, J.R.; Kim, K.J.; Kelley, D.G.; Senior, R.M.; Crandall, E.D.; Borok, Z. Characterization of mouse alveolar epithelial cell monolayers. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L1051–L1058.
- Ali, G.; Zhang, M.; Zhao, R.; Jain, K.G.; Chang, J.; Komatsu, S.; Zhou, B.; Liang, J.; Matthay, M.A.; Ji, H.L. Fibrinolytic niche is required for alveolar type 2 cell-mediated alveologenesis via a uPA-A6-CD44(+)-ENaC signal cascade. Signal Transduct. Target. Ther. 2021, 6, 97.
- Ali, G.; Zhang, M.; Chang, J.; Zhao, R.; Jin, Y.; Zhang, J.; Ji, H.-L. PAI-1 regulates AT2-mediated re-alveolarization and ion permeability. Stem Cell Res. Ther. 2023, 14, 185.
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94.
- Pei, R.; Feng, J.; Zhang, Y.; Sun, H.; Li, L.; Yang, X.; He, J.; Xiao, S.; Xiong, J.; Lin, Y.; et al. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection. Protein Cell 2021, 12, 717–733.
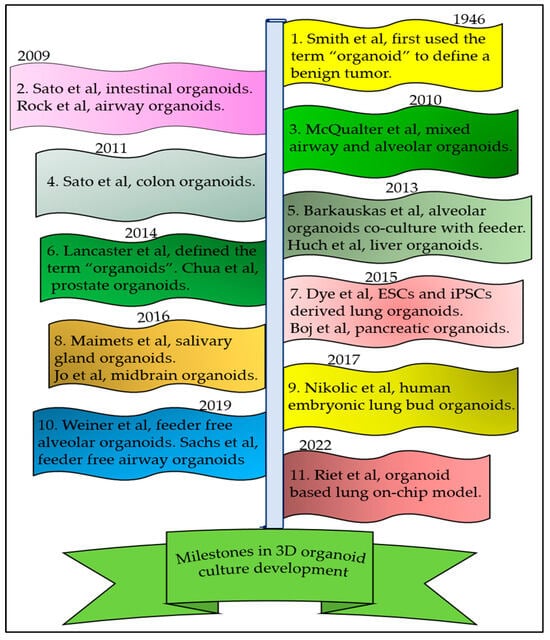
- Smith, E.; Cochrane, W.J. CYSTIC ORGANOID TERATOMA: (Report of a Case). Can. Med. Assoc. J. 1946, 55, 151–152.
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125.
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265.
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597.
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772.
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250.
- Chua, C.W.; Shibata, M.; Lei, M.; Toivanen, R.; Barlow, L.J.; Bergren, S.K.; Badani, K.K.; McKiernan, J.M.; Benson, M.C.; Hibshoosh, H.; et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 2014, 16, 951–961.
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338.
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257.
- Maimets, M.; Rocchi, C.; Bron, R.; Pringle, S.; Kuipers, J.; Giepmans, B.N.; Vries, R.G.; Clevers, H.; de Haan, G.; van Os, R.; et al. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016, 6, 150–162.
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775.
- McQualter, J.L.; Yuen, K.; Williams, B.; Bertoncello, I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA 2010, 107, 1414–1419.
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.H.; Tsai, Y.-H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098.
- Nikolić, M.Z.; Caritg, O.; Jeng, Q.; Johnson, J.-A.; Sun, D.; Howell, K.J.; Brady, J.L.; Laresgoiti, U.; Allen, G.; Butler, R.; et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. eLife 2017, 6, e26575.
- Weiner, A.I.; Jackson, S.R.; Zhao, G.; Quansah, K.K.; Farshchian, J.N.; Neupauer, K.M.; Littauer, E.Q.; Paris, A.J.; Liberti, D.C.; Scott Worthen, G.; et al. Mesenchyme-free expansion and transplantation of adult alveolar progenitor cells: Steps toward cell-based regenerative therapies. NPJ Regen. Med. 2019, 4, 17.
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term expanding human airway organoids for disease modeling. Embo J. 2019, 38, e100300.
- van Riet, S.; van Schadewijk, A.; Khedoe, P.; Limpens, R.; Bárcena, M.; Stolk, J.; Hiemstra, P.S.; van der Does, A.M. Organoid-based expansion of patient-derived primary alveolar type 2 cells for establishment of alveolus epithelial Lung-Chip cultures. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 322, L526–L538.
- Lim, K.; Donovan, A.P.A.; Tang, W.; Sun, D.; He, P.; Pett, J.P.; Teichmann, S.A.; Marioni, J.C.; Meyer, K.B.; Brand, A.H.; et al. Organoid modeling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell 2023, 30, 20–37.e29.
- Nadkarni, R.R.; Abed, S.; Draper, J.S. Stem Cells in Pulmonary Disease and Regeneration. Chest 2018, 153, 994–1003.
- Aichler, M.; Kunzke, T.; Buck, A.; Sun, N.; Ackermann, M.; Jonigk, D.; Gaumann, A.; Walch, A. Molecular similarities and differences from human pulmonary fibrosis and corresponding mouse model: MALDI imaging mass spectrometry in comparative medicine. Lab. Investig. 2018, 98, 141–149.
- Pan, H.; Deutsch, G.H.; Wert, S.E. Comprehensive anatomic ontologies for lung development: A comparison of alveolar formation and maturation within mouse and human lung. J. Biomed. Semant. 2019, 10, 18.
- Huang, X.; Fan, W.; Sun, J.; Yang, J.; Zhang, Y.; Wang, Q.; Li, P.; Zhang, Y.; Zhang, S.; Li, H.; et al. SARS-CoV-2 induces cardiomyocyte apoptosis and inflammation but can be ameliorated by ACE inhibitor Captopril. Antivir. Res. 2023, 215, 105636.
- Warren, H.S.; Tompkins, R.G.; Moldawer, L.L.; Seok, J.; Xu, W.; Mindrinos, M.N.; Maier, R.V.; Xiao, W.; Davis, R.W. Mice are not men. Proc. Natl. Acad. Sci. USA 2015, 112, E345.
- Martić-Kehl, M.I.; Schibli, R.; Schubiger, P.A. Can animal data predict human outcome? Problems and pitfalls of translational animal research. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1492–1496.
- Yuan, T.; Volckaert, T.; Chanda, D.; Thannickal, V.J.; De Langhe, S.P. Fgf10 Signaling in Lung Development, Homeostasis, Disease, and Repair After Injury. Front. Genet. 2018, 9, 418.
- Abraham, V.; Chou, M.L.; DeBolt, K.M.; Koval, M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am. J. Physiol. 1999, 276, L825–L834.
- Weller, N.K.; Karnovsky, M.J. Improved isolation of rat lung alveolar type II cells. More representative recovery and retention of cell polarity. Am. J. Pathol. 1986, 122, 92–100.
- Choi, J.; Park, J.E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.K.; Han, N.; Lee, J.H. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell 2020, 27, 366–382.e367.
- Gonzalez, R.F.; Allen, L.; Gonzales, L.; Ballard, P.L.; Dobbs, L.G. HTII-280, a Biomarker Specific to the Apical Plasma Membrane of Human Lung Alveolar Type II Cells. J. Histochem. Cytochem. 2010, 58, 891–901.
- Hasegawa, K.; Sato, A.; Tanimura, K.; Uemasu, K.; Hamakawa, Y.; Fuseya, Y.; Sato, S.; Muro, S.; Hirai, T. Fraction of MHCII and EpCAM expression characterizes distal lung epithelial cells for alveolar type 2 cell isolation. Respir. Res. 2017, 18, 150.
- Toulmin, S.A.; Bhadiadra, C.; Paris, A.J.; Lin, J.H.; Katzen, J.; Basil, M.C.; Morrisey, E.E.; Worthen, G.S.; Eisenlohr, L.C. Type II alveolar cell MHCII improves respiratory viral disease outcomes while exhibiting limited antigen presentation. Nat. Commun. 2021, 12, 3993.
- Zhao, S.; Wu, X.; Tan, Z.; Ren, Y.; Li, L.; Ou, J.; Lin, Y.; Song, H.; Feng, L.; Seto, D.; et al. Generation of Human Embryonic Stem Cell-Derived Lung Organoids for Modeling Infection and Replication Differences between Human Adenovirus Types 3 and 55 and Evaluating Potential Antiviral Drugs. J. Virol. 2023, 97, e0020923.
- Hoffman, E.T.; Uriarte, J.J.; Uhl, F.E.; Eckstrom, K.; Tanneberger, A.E.; Becker, C.; Moulin, C.; Asarian, L.; Ikonomou, L.; Kotton, D.N.; et al. Human alveolar hydrogels promote morphological and transcriptional differentiation in iPSC-derived alveolar type 2 epithelial cells. Sci. Rep. 2023, 13, 12057.
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123.
- Jain, K.G.; Zhao, R.; Liu, Y.; Guo, X.; Yi, G.; Ji, H.L. Wnt5a/β-catenin axis is involved in the downregulation of AT2 lineage by PAI-1. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 323, L515–L524.
- Suezawa, T.; Kanagaki, S.; Korogi, Y.; Nakao, K.; Hirai, T.; Murakami, K.; Hagiwara, M.; Gotoh, S. Modeling of lung phenotype of Hermansky-Pudlak syndrome type I using patient-specific iPSCs. Respir. Res. 2021, 22, 284.
- Lee, J.H.; Bhang, D.H.; Beede, A.; Huang, T.L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.H.; Ryeom, S.; Kim, C.F. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014, 156, 440–455.
- Tamai, K.; Sakai, K.; Yamaki, H.; Moriguchi, K.; Igura, K.; Maehana, S.; Suezawa, T.; Takehara, K.; Hagiwara, M.; Hirai, T.; et al. iPSC-derived mesenchymal cells that support alveolar organoid development. Cell Rep. Methods 2022, 2, 100314.
- Nyeng, P.; Norgaard, G.A.; Kobberup, S.; Jensen, J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev. Biol. 2008, 8, 2.
- Ohmichi, H.; Koshimizu, U.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development 1998, 125, 1315–1324.
- McQualter, J.L.; McCarty, R.C.; Van der Velden, J.; O’Donoghue, R.J.J.; Asselin-Labat, M.-L.; Bozinovski, S.; Bertoncello, I. TGF-β signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell Res. 2013, 11, 1222–1233.
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Gotoh, S.; Ito, I.; Nagasaki, T.; Yamamoto, Y.; Konishi, S.; Korogi, Y.; Matsumoto, H.; Muro, S.; Hirai, T.; Funato, M.; et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 2014, 3, 394–403.
- Hawkins, F.; Kramer, P.; Jacob, A.; Driver, I.; Thomas, D.C.; McCauley, K.B.; Skvir, N.; Crane, A.M.; Kurmann, A.A.; Hollenberg, A.N.; et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Investig. 2017, 127, 2277–2294.
- Jacob, A.; Vedaie, M.; Roberts, D.A.; Thomas, D.C.; Villacorta-Martin, C.; Alysandratos, K.D.; Hawkins, F.; Kotton, D.N. Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3303–3332.
- Ciechanowicz, A.K.; Sielatycka, K.; Cymer, M.; Skoda, M.; Suszyńska, M.; Bujko, K.; Ratajczak, M.Z.; Krause, D.S.; Kucia, M. Bone Marrow-Derived VSELs Engraft as Lung Epithelial Progenitor Cells after Bleomycin-Induced Lung Injury. Cells 2021, 10, 1570.
- Chen, Y.-W.; Huang, S.X.; de Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017, 19, 542–549.
- Rock, J.R.; Barkauskas, C.E.; Cronce, M.J.; Xue, Y.; Harris, J.R.; Liang, J.; Noble, P.W.; Hogan, B.L.M. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA 2011, 108, E1475–E1483.
- Tanjore, H.; Xu, X.C.; Polosukhin, V.V.; Degryse, A.L.; Li, B.; Han, W.; Sherrill, T.P.; Plieth, D.; Neilson, E.G.; Blackwell, T.S.; et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2009, 180, 657–665.
- Alysandratos, K.-D.; Garcia-de-Alba, C.; Yao, C.; Pessina, P.; Huang, J.; Villacorta-Martin, C.; Hix, O.T.; Minakin, K.; Burgess, C.L.; Bawa, P.; et al. Culture impact on the transcriptomic programs of primary and iPSC-derived human alveolar type 2 cells. JCI Insight 2023, 8, e158937.
- Yamamoto, Y.; Gotoh, S.; Korogi, Y.; Seki, M.; Konishi, S.; Ikeo, S.; Sone, N.; Nagasaki, T.; Matsumoto, H.; Muro, S.; et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods 2017, 14, 1097–1106.
- Loebel, C.; Weiner, A.I.; Eiken, M.K.; Katzen, J.B.; Morley, M.P.; Bala, V.; Cardenas-Diaz, F.L.; Davidson, M.D.; Shiraishi, K.; Basil, M.C.; et al. Microstructured Hydrogels to Guide Self-Assembly and Function of Lung Alveolospheres. Adv. Mater. 2022, 34, e2202992.
- Dost, A.F.M.; Moye, A.L.; Vedaie, M.; Tran, L.M.; Fung, E.; Heinze, D.; Villacorta-Martin, C.; Huang, J.; Hekman, R.; Kwan, J.H.; et al. Organoids Model Transcriptional Hallmarks of Oncogenic KRAS Activation in Lung Epithelial Progenitor Cells. Cell Stem Cell 2020, 27, 663–678.e668.
- Kim, C.; Li, J.; Dang, S.; Schurmann, P.; Dost, A.; Moye, A.; Paschini, M.; Bhetariya, P.; Bronson, R.; Sui, S.H. Organoid modeling reveals the tumorigenic potential of the alveolar progenitor cell state. Res. Sq. 2023; preprint.
- Chen, Y.; Feng, J.; Zhao, S.; Han, L.; Yang, H.; Lin, Y.; Rong, Z. Long-Term Engraftment Promotes Differentiation of Alveolar Epithelial Cells from Human Embryonic Stem Cell Derived Lung Organoids. Stem Cells Dev. 2018, 27, 1339–1349.




