Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jacob Beiriger | -- | 3409 | 2023-11-13 14:41:50 | | | |
| 2 | Jessie Wu | + 4 word(s) | 3413 | 2023-11-14 03:06:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Beiriger, J.; Chauhan, K.; Khan, A.; Shahzad, T.; Parra, N.S.; Zhang, P.; Chen, S.; Nguyen, A.; Yan, B.; Bruckbauer, J.; et al. Advancements in Understanding NAFLD. Encyclopedia. Available online: https://encyclopedia.pub/entry/51484 (accessed on 08 February 2026).
Beiriger J, Chauhan K, Khan A, Shahzad T, Parra NS, Zhang P, et al. Advancements in Understanding NAFLD. Encyclopedia. Available at: https://encyclopedia.pub/entry/51484. Accessed February 08, 2026.
Beiriger, Jacob, Kashyap Chauhan, Adnan Khan, Taha Shahzad, Natalia Salinas Parra, Peter Zhang, Sarah Chen, Anh Nguyen, Brian Yan, John Bruckbauer, et al. "Advancements in Understanding NAFLD" Encyclopedia, https://encyclopedia.pub/entry/51484 (accessed February 08, 2026).
Beiriger, J., Chauhan, K., Khan, A., Shahzad, T., Parra, N.S., Zhang, P., Chen, S., Nguyen, A., Yan, B., Bruckbauer, J., & Halegoua-Demarzio, D. (2023, November 13). Advancements in Understanding NAFLD. In Encyclopedia. https://encyclopedia.pub/entry/51484
Beiriger, Jacob, et al. "Advancements in Understanding NAFLD." Encyclopedia. Web. 13 November, 2023.
Copy Citation
Non-alcoholic fatty liver disease (NAFLD), recently renamed by an international consensus panel as metabolic-associated fatty liver disease (MAFLD), affects up to 1 billion patients worldwide. This change in nomenclature is in keeping with more recent understanding of this disease and its inherent link to metabolic syndrome.
NAFLD
NASH
pathogenesis
genetic factors
dietary factors
environmental factors
therapeutic strategies
1. Epidemiology
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease with a prevalence of 25% worldwide. The highest rates are reported from South America (31%) and the Middle East (32%), followed by Asia (27%), the United States (24%), and Europe (23%). NAFLD is less common in Africa (14%). Among these patients in the US, the prevalence of NASH was 21% [1].
Research into NAFLD has rapidly expanded the understanding of its pathophysiology and defined useful diagnostic criteria. Diagnosis has shifted to focus on the metabolic criteria commonly associated with hepatic steatosis, such as T2DM and metabolic syndrome. These key comorbidities have led to the proposal to rename this condition to MAFLD [2]. The goal with re-defining fatty liver disease is to improve outcomes by identifying high-risk patients and intervening earlier in the metabolic dysfunction contributing to their disease [3]. Experts state that a focus on metabolic dysfunction better represents the pathophysiologic mechanism of the disease. Studies have shown that obesity, metabolic syndrome, and T2DM are associated with a greater risk of progression of NAFLD to NASH, HCC, or fibrosis [4]. NASH is predicted to become the most common indication for liver transplantation [5]. Therefore, the focus on metabolic criteria may better stratify patients who are at increased risk of disease progression. Compared to a diagnosis of exclusion in NAFLD, MAFLD’s positive inclusion of metabolic abnormalities is designed to risk-stratify patients at highest risk for progression to NASH, HCC, or fibrosis. Importantly, the presence of other hepatic disease does not exclude MAFLD diagnosis like the in the NAFLD definition. However, studies show that around 90% of patients meet the criteria of both NAFLD and MAFLD [6][7].
The new definition will alter the incidence and prevalence going forward. Given its novelty, prevalence data have been estimated from existing data. A recent meta-analysis by Chan et al. of more than 10 million patients globally found the prevalence of MAFLD to be 38% [3]. One study found the prevalence of MAFLD in North America to be 34.8%, another measured 39.1% [8][9]. As obesity and T2DM continue to increase around the world, the prevalence of MAFLD is likely to increase.
The definition of MAFLD includes patients who have two or more factors associated with metabolic dysfunction. Thus, the MAFLD definition encompasses lean and non-obese individuals with steatosis. The prevalence of MAFLD among these patients is estimated to be 5% and 12%, respectively [10].
The incidence of both NAFLD/MAFLD is difficult to determine due to a lack of cohesive screening guidelines and inaccurate tools. Ultrasound is commonly used in initial work-up, but it lacks the sensitivity to detect subtleties in early disease. The generally accepted value is that the incidence of NAFLD is around 2–6% [11].
2. Diagnosis
There is debate over whether screening for NAFLD is worthwhile and cost-effective [12]. Currently, the American Association for the Study of Liver Diseases (AASLD) does not recommend screening for NAFLD, even in high-risk populations, due to the uncertain long-term benefits and cost-effectiveness of screening [13]. It is well-established that patients with type 2 diabetes, obesity, dyslipidemia, and hypertension are at increased risk of cirrhosis, advanced fibrosis, and liver disease mortality [12][13]. Therefore, clinicians should have a higher degree of suspicion in patients with these comorbidities. The diagnosis of NAFLD is typically characterized by (1) the presence of chronically elevated liver enzymes and (2) imaging evidence of hepatic steatosis. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are commonly used to monitor liver function. An AST/ALT ratio of greater than 1 has been associated with a higher degree of fibrosis on liver biopsy [14]. However, elevated ALT alone is not correlated with a higher degree of fibrosis in NAFLD patients [15]. Although these serum biomarkers have high availability and applicability, they are not liver specific and can therefore be elevated in other comorbid conditions [16].
With regard to imaging, abdominal ultrasound is currently the first-line screening tool for NAFLD given its widespread availability and low cost [17]. Findings of steatosis on abdominal ultrasound include bright hepatic echoes, increased hepatorenal echogenicity, vascular blurring of the portal or hepatic vein, and subcutaneous tissue thickness [18]. Limitations of using ultrasound include inter- and intra-reader variability and anatomic constraints (bowel gas or a large body habitus) [19]. Liver biopsy is the gold standard for diagnosing NAFLD; however, it is costly, invasive, and susceptible to sampling bias [20]. Findings on liver biopsy of NAFLD include hepatic steatosis with or without hepatocyte ballooning, hepatic necrosis, Mallory bodies, and fibrosis [21]. Of note, the severity of fibrosis has the greatest association with liver-related morbidity and mortality when compared to other histological findings [22]. MRI proton density fat fraction (PDFF) and magnetic resonance spectroscopy (MRS) are useful for quantifying the triglyceride content in the liver [20]. However, MRI-PDFF and MRS are more costly and less available than abdominal ultrasound and are more often used in research settings rather than clinical practice [12]. Before diagnosing NAFLD, secondary causes of fatty liver disease such as viral hepatitis, alcoholic fatty liver disease, drug-induced liver disease, medical conditions (Wilson disease, hereditary hemochromatosis, celiac disease), metabolic diseases (glycogen storage diseases), and poor nutritional status must be excluded [23].
Once the diagnosis of NAFLD has been established, fibrosis scores are often calculated to assess the degree of NAFLD and guide management. The NAFLD fibrosis score (NFS), fibrosis-4 (FIB-4) index, and AST-to-platelet ratio can be calculated from routine laboratory results [12]. The NFS is based on a patient’s age, BMI, presence of diabetes, AST, ALT, platelet count, and albumin level, while the FIB-4 is based on the patient’s age, AST, ALT, and platelet count. Patients who have a FIB-4 ≤ 1.3 (age < 65 years) or FIB-4 ≤ 2.0 (age > 65 years) are at minimal risk for advanced fibrosis and are typically managed in the primary case setting (see figure below). Patients who have a FIB-4 of >1.3 (age < 65 years) or FIB-4 > 2 (age > 65 years) undergo elastography and are referred for specialist care (see figure below). NFS and FIB-4 scores have a negative predictive value > 90%, demonstrating their utility as an initial diagnostic test in assessing for NAFLD [16].
Ultrasound-based elastography, vibration-controlled transient elastography, point-shear wave elastography, two-dimensional shear wave elastography, or magnetic resonance elastography can be used to assess liver fibrosis in patients with intermediate-to-high risk of NAFLD based on their FIB-4 and NFS [12]. Transient elastography is most used given its availability.
3. Treatments
Weight loss is the first-line management of NAFLD and is recommended for patients with a BMI > 25. In addition to improving the quality of life in patients with NAFLD, weight loss has been shown to improve liver histology, hepatic steatosis, and inflammation [24][25][26][27]. Weight loss of 3–5% has been shown to be associated with decreased steatosis fibrosis regression, 5–7% with reduced inflammation, 7–10% with NASH resolution, and ≥10% with fibrosis regression [28].
Lifestyle interventions such as diet modification and exercise are attempted first. In patients with NASH or advanced fibrosis who do not sufficiently meet weight loss goals in lifestyle interventions, other strategies like bariatric surgery may be pursued. Surgical intervention with bariatric surgery can be considered in patients with a BMI > 40 or a BMI >35 with one obesity-related comorbidity [29]. Bariatric surgery has been shown to improve steatosis, reduce inflammation, and improve fibrosis score [30][31][32]. Surgical intervention has also shown benefits in patients with NASH. Lassailly et al. studied the effect of bariatric surgery in patients with NASH and found that NASH had disappeared in 85% of patients one year postoperatively [33]. However, worsening fibrosis has also been reported following bariatric surgery [31].
Although no drug treatment has been approved by the United States Food and Drug Administration (FDA) for the direct treatment of NAFLD, drug therapies targeting weight loss can be considered. Weight loss pharmacological therapy may be initiated in patients with a BMI ≥ 30 or ≥27 with metabolic comorbidities such as diabetes or hypertension [34]. Current drug therapy options include glucagon-like peptide-1 (GLP-1) receptor agonists, lipase inhibitors, sympathomimetics, or combination drugs. Among these, GLP-1 agonists are the best-studied and evidence consistently demonstrates a positive effect of the drugs on NAFLD. Semaglutide and liraglutide are the two GLP-1 receptor agonists that have been approved for the treatment of obesity in the United States and are considered the first-line drug therapy. GLP-1 receptor agonists increase glucose-dependent insulin secretion, inhibit glucagon secretion, and slow gastric emptying [35]. They also act in multiple regions of the brain, where appetite and caloric intake are regulated [35]. Of the two, semaglutide is preferred given its superior dosing schedule of once weekly compared to once daily with liraglutide, as well as its greater efficacy in weight loss [36]. Multiple studies have shown that GLP-1 agonists can decrease liver fat content, improve ALT, and even slow the progression of fibrosis [37][38][39][40]. The effects of GLP-1 agonists on liver fat are primarily rooted in their ability to enhance insulin sensitivity and reduce hepatic glucose production, decreasing de novo lipogenesis and increased fatty acid oxidation within the liver. These mechanisms are particularly relevant as they address fundamental drivers of hepatic fat accumulation and inflammation, offering an avenue to mitigate progression. The leading GLP-1 agonist, semaglutide, has shown much promise in the treatment of NASH and its effects on decreasing markers of NASH and liver enzymes beyond its weight loss effects are discussed below. Liraglutide, another GLP-1 agonist that has also been known to cause weight loss, has also been associated with increased rates of NASH resolution and decreased enhanced liver fibrosis (ELF) score when compared to placebo [41]. While weight loss is desired given its theorized and observed benefits in NASH, quantification of this fat loss is important when thinking about weight loss as a treatment specifically for NASH. Several trials such as that by Vilar-Gomez et al. have demonstrated that a weight loss of at least 10% of initial body weight is associated with the highest rates of NAFLD activity score reduction, NASH resolution, and even fibrosis regression. The importance of weight loss from this trial is summarized in Figure 1 below [24].
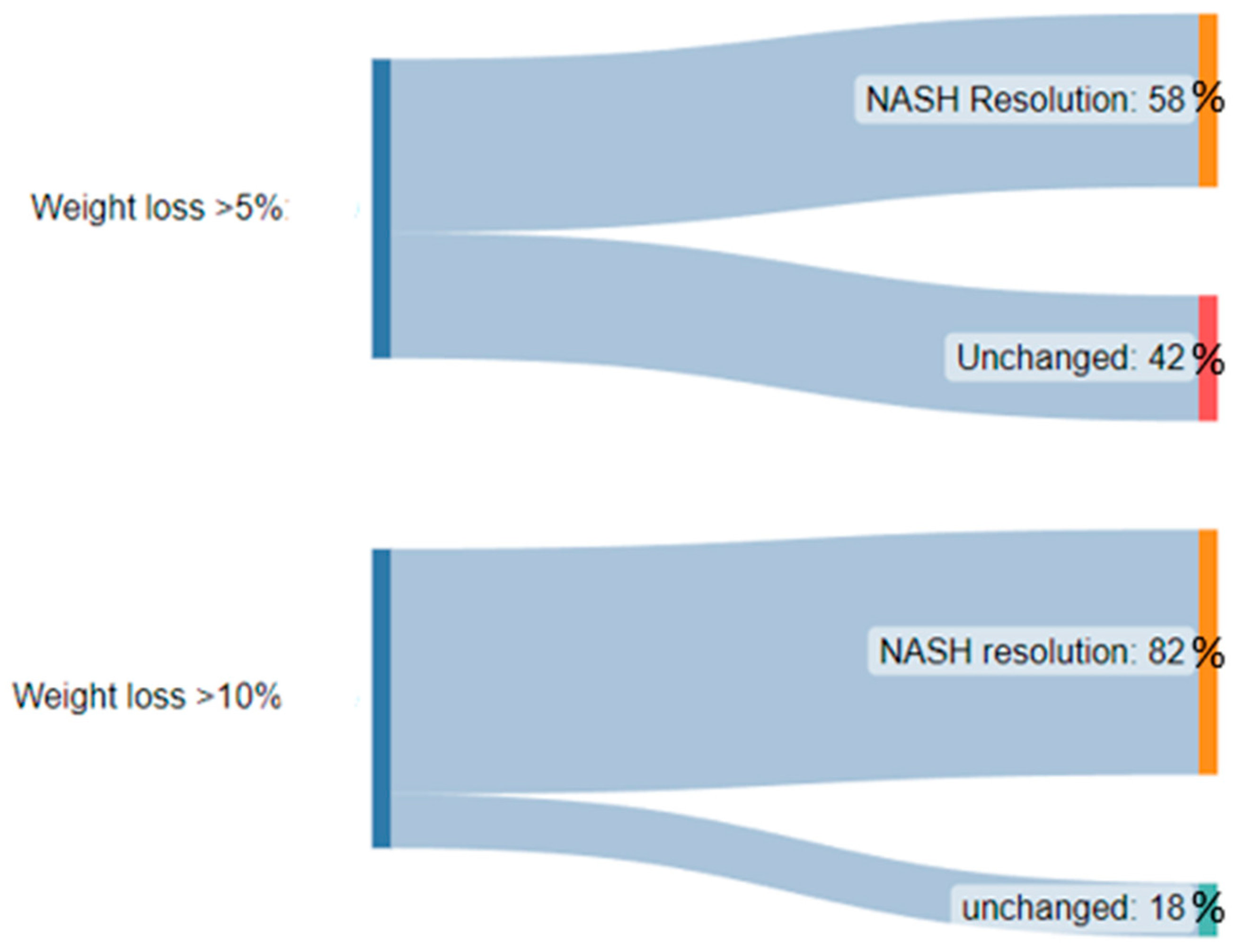
Figure 1. A diagram demonstrating the significantly higher proportion of patients with NASH resolution after weight loss of >10% body weight when compared to >5% body weight.
Unlike the GLP-1 agonists, other medications traditionally used in weight loss like orlistat, sympathomimetics, and combination drugs have not been studied as extensively in the setting of NAFLD. Orlistat is a gastric and pancreatic lipase inhibitor used primarily for weight loss, but its adverse effects on the gastrointestinal system such as oily stools, diarrhea, and abdominal pain have limited its use [42]. Randomized controlled trials conducted by Harrison et al. and Zelber-Sagi et al. showed that using orlistat in the treatment of NAFLD was associated with an improvement in liver histopathology [43][44]. Studies have also shown that orlistat may be beneficial in improving liver fat content and reducing inflammatory enzymes [43][45][46][47]. Unfortunately, few studies exist that investigate the effect of sympathomimetic drugs such as phentermine, diethylpropion, benzphetamine, and phendimetrazine, as well as combination drugs such as phentermine–topiramate and bupropion–naltrexone on NAFLD.
Vitamin E is considered in patients with NASH and fibrosis stage ≥ 2 without diabetes mellitus. In a large, randomized trial conducted by Sanyal et al. studying the efficacy of vitamin E (800 international units daily) and pioglitazone versus placebo, patients treated with vitamin E were more likely to have improvement in their global histology score compared with patients who received placebo [48]. However, the use of vitamin E has been associated with an increased risk of hemorrhagic stroke and prostate carcinoma [49][50]. Studies have also found high-dose vitamin E supplementation (≥400 international units per day) to be inconsistently associated with an increase in all-cause mortality [51].
Pioglitazone, a thiazolidinedione, can be used in patients with NASH and diabetes mellitus. Pioglitazone has been shown to improve fibrosis, inflammation, and steatosis [52]. Because the use of pioglitazone is associated with an increased risk of weight gain, heart failure, and fractures, careful consideration of its risk-to-benefit ratio must be made prior to administration [53][54].
4. Emerging Therapeutic Options
Recent randomized control trials and laboratory studies on animal models have identified several potential therapies for NAFLD. These emerging therapies are mainly pharmacologic in nature, with antioxidant, lipid-lowering, nuclear-transcription-regulating, cytokine-targeting, hormone-mimetic, GLP-1 agonism, or other metabolic-profile-altering properties [55]. Of these many agents, several therapeutic options that have reached phase 2 (lanifibranor) or phase 3 clinical trials (obeticholic acid, elafibranor, cenicriviroc, arachidyl amido cholanoic acid, and resmetirom to name a few). Failed trials are attributed to underpowered sample sizes or too short a duration while others resulted in inconclusive results and poorly tolerated adverse effects.
4.1. Semaglutide
Semaglutide is a GLP-1 receptor antagonist that has shown some of the highest promise as a potential therapeutic agent for NASH in recent years. A pivotal study performed by Newsome et al. investigating the safety and efficacy of semaglutide administration in patients with NASH showed a statistically significant increase in patients with NASH resolution compared to placebo [56]. A recent network meta-analysis of 27 randomized controlled trials studying the efficacy of off-label therapy for NAFLD showed that semaglutide use led to a significantly higher decrease in AST and ALT levels versus placebo and that semaglutide was superior to liraglutide in decreasing AST and ALT in patients with NAFLD [41]. As discussed, weight gain is central to the development of NASH and thus weight loss is paramount in the ability to reverse NASH. The weight loss effects of semaglutide are well-known and sought after in patients with both diabetes and obesity. Interestingly, in the trial by Newsome et al., most of the NASH resolution was attributable to weight loss, while the authors describe a small proportion of patients (approximately 25%) with NASH resolution beyond that which would be expected from weight loss alone. This is congruent with some preclinical studies such as those by Rakipovski et al. demonstrating that semaglutide reduces inflammation in the liver by mechanisms independent of weight loss [57]. This apparent pleiotropic effect of semaglutide on NASH in addition to its established safety and availability for weight loss make this agent one of the most promising options for the treatment of NASH at present. Other agents have also shown some promise, however, they have been marred by undesirable side effects, lack of availability, or lack of real-world efficacy beyond clinical trials. Some of these are discussed below.
4.2. Obeticholic Acid
Obeticholic acid (OCA) is a synthetic bile acid derivative of chenodeoxycholic acid that acts as a farnesoid X receptor (FXR) agonist [58]. FXR is a nuclear receptor found intracellularly in the liver, intestines, kidneys, and adrenal glands, where it plays a role in glucose and lipid metabolism [55]. Upon stimulation by increasing bile acid levels, FXR suppresses the transcription of the CYP7A1 gene, thereby inhibiting 7α-hydroxylase, downregulating bile acid synthesis, and upregulating cholesterol synthesis [59].
Early studies and clinical trials that demonstrated increased insulin sensitivity; decreased hepatic inflammatory and fibrotic markers; increases in low-density lipoprotein cholesterol (LDL-c) that could be mitigated with concomitant statin therapy; and minimal adverse events have prompted further phase 2 and 3 clinical trials [60][61].
The FLINT trial was a phase 2b clinical trial that involved 283 patients with non-cirrhotic NASH [62]. The study compared OCA to a placebo, finding a significant improvement in liver histology in the OCA group compared to the placebo. Pruritus was identified as an adverse effect of OCA therapy [62]. OCA also significantly increased LDL and cholesterol levels and decreased HDL levels, requiring concomitant statin treatment, compared to the placebo [62].
An ongoing phase 3 clinical trial is the REGENERATE trial, which aims to compare the effects of 10 mg OCA and 25 mg OCA to a placebo on histological improvement and liver-related outcomes in patients with fibrotic, non-cirrhotic NASH [63][64]. The study involves 2480 participants. A December 2019 interim analysis of the ongoing trial reported a significant fibrosis improvement without exacerbation of NASH in the 25 mg OCA group compared to the placebo but no significant NASH resolution in either OCA group compared to the placebo [64]. Mild-to-moderate pruritus was noted as an adverse event in OCA therapy, corroborating findings of previous randomized control trials [64].
4.3. Lanifibranor
Lanifibranor is an antifibrotic indole sulfonamide α/γ/δ PPAR agonist that is novel for its ability to activate all three PPAR isoforms [65]. Early animal studies modeling the effects of lanifibranor on NASH found that lanifibranor normalized insulin sensitivity, decreased histologic characteristics of NASH including hepatic steatosis, inflammation, and ballooning, and inhibited the activity of hepatic stellate cells responsible for fibrinogenesis [66]. The pan-PPAR activity of lanifibranor is thought to mediate therapeutic improvements in pathways involving glucose metabolism and insulin sensitization, fatty acid and triglyceride metabolism, and energy homeostasis [67].
A recent phase 2b randomized control trial compared the efficacy of 1200 mg or 800 mg lanifibranor to a placebo in 247 patients with biopsy-proven, highly active, non-cirrhotic NASH over 24 weeks [68]. Of the 247 participants, 42% had type 2 diabetes and 26% had moderate-to-severe fibrosis [68]. The study demonstrated a significantly higher percentage of at least a 2-point decrease in steatosis, activity, fibrosis (SAF-A) score in the 1200 mg (but not the 800 mg) lanifibranor experimental group compared to the placebo. Also, compared to the placebo, both the 1200 mg and 800 mg groups had higher rates of NASH resolution with and without improvement in fibrosis stage [68]. The lanifibranor groups also demonstrated decreased liver enzyme levels, improvements in measures of glycemic control (such as reductions in glycated hemoglobin), increased HDL cholesterol levels, increased dose-dependent adiponectin levels, and decreases in serum triglycerides [68]. Adverse effects of lanifibranor therapy included gastrointestinal events such as nausea and diarrhea, peripheral edema, anemia, and weight gain [68]. Further research on the efficacy and adverse effect profile of lanifibranor awaits phase 3 clinical trials.
4.4. Arachidyl Amido Cholanoic Acid
Arachidyl amido cholanoic acid (Aramchol) is an inhibitor of stearoyl-CoA desaturase 1 (SCD-1). SCD-1 is the rate-determining enzyme in the conversion of saturated fatty acids to monounsaturated fatty acids [69]. Animal studies have shown that the inhibition or deficiency of SCD-1 reduced carbohydrate-induced adiposity, hepatic steatosis, fibrosis, and insulin resistance [70][71][72].
A phase 2 clinical trial of 60 patients with biopsy-confirmed NASH in Israel compared 100 mg or 300 mg Aramchol to a placebo to assess for changes in hepatic steatosis [73]. The 300 mg Aramchol group experienced a significant reduction in hepatic steatosis, and the study found Aramchol to be well-tolerated without significant adverse effects [73].
The ARREST trial, a phase 2b clinical trial, administered 400 mg or 600 mg Aramchol or a placebo to 247 participants with prediabetes or type 2 diabetes and NASH to assess for significant decreases in hepatic triglycerides as its primary endpoint and for improved hepatic histology and aminotransferase as the secondary endpoint [74]. The group receiving 400 mg Aramchol demonstrated a significant decrease in hepatic steatosis compared to the placebo group. The 600 mg Aramchol group decreased hepatic steatosis but insignificantly compared to the placebo [74]. Aramchol therapy also decreased alanine aminotransferase (ALT), aspartate aminotransferase (AST), and hemoglobin A1c (HbA1c) levels [74]. The study also concluded that Aramchol was well-tolerated and safe, though a higher incidence of urinary tract infections was noted in both Aramchol groups compared to the group receiving the placebo [74].
The ARMOR trial is an ongoing, phase 3/4 trial consisting of an open-label part and two double-blind parts that has enrolled 2000 participants [75]. The primary endpoints of the open-label part include improvement in fibrosis and resolution of NASH, and the endpoints of the double-blind parts include improvement in fibrosis, resolution of NASH, and assessment of long-term clinical outcomes [75].
4.5. Resmetirom
Resmetirom is a thyroid hormone receptor-β (THR-β) agonist. Thyroid hormones are thought to mediate hepatic triglyceride metabolism by promoting hepatic autophagy to aid the transport of fatty acids to the mitochondria for oxidative metabolism [76][77]. Disruptions in lipid metabolism pathways, such as those mediated by thyroid hormones, are thought to contribute to NAFLD [78]. There are several thyroid hormone receptor (THR) isoforms, but THR-β is the most commonly found THR in the liver [78]. Thus, targeting this receptor decreases the risk of adverse effects due to the stimulation of other THR isoforms found in heart and bone tissue [79]. TH analogues have been shown to reduce hepatic steatosis and lipid peroxidation [78]. In animal studies, resmetirom was found to be well-tolerated and caused statistically significant reductions in LDL-c and triglyceride levels [80]. An early randomized controlled trial supported findings from animal models, showing significant reductions compared to the placebo in LDL-c, non-HDL cholesterol, apolipoprotein B, and triglycerides without causing significant adverse events [81].
In a phase 2 clinical trial, 125 patients with biopsy-confirmed NASH and a hepatic fat fraction greater than or equal to 10% were administered 80 mg resmetirom or a placebo for 36 weeks [82]. The primary endpoint was a change in hepatic steatosis measured by MRI proton density fat fraction (MRI-PDFF) in the resmetirom group at 12 weeks [82]. At weeks 12 and 36, the resmetirom group demonstrated a significant reduction in hepatic steatosis compared to the placebo group. Resmetirom was mostly well-tolerated, but transient mild diarrhea and nausea were significant adverse effects [82].
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Mak, L.Y.; Yuen, M.F.; Seto, W.K. Letter regarding “A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement”. J. Hepatol. 2020, 73, 1573–1574.
- Chan, K.E.; Koh, T.J.L.; Tang, A.S.P.; Quek, J.; Yong, J.N.; Tay, P.; Tan, D.J.H.; Lim, W.H.; Lin, S.Y.; Huang, D.; et al. Global Prevalence and Clinical Characteristics of Metabolic-associated Fatty Liver Disease: A Meta-Analysis and Systematic Review of 10 739 607 Individuals. J. Clin. Endocrinol. Metab. 2022, 107, 2691–2700.
- Xian, Y.X.; Weng, J.P.; Xu, F. MAFLD vs. NAFLD: Shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin. Med. J. 2020, 134, 8–19.
- Shaker, M. Liver transplantation for nonalcoholic fatty liver disease: New challenges and new opportunities. World J. Gastroenterol. 2014, 20, 5320–5330.
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020, 40, 2082–2089.
- Wong, V.W.-S.; Wong, G.L.-H.; Woo, J.; Abrigo, J.M.; Chan, C.K.-M.; Shu, S.S.-T.; Leung, J.K.-Y.; Chim, A.M.-L.; Kong, A.P.-S.; Lui, G.C.-Y.; et al. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 2161–2171.e5.
- Wong, R.J.; Cheung, R. Trends in the Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in the United States, 2011–2018. Clin. Gastroenterol. Hepatol. 2022, 20, e610–e613.
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021, 41, 1290–1293.
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752.
- Lin, H.; Zhang, X.; Li, G.; Wong, G.L.-H.; Wong, V.W.-S. Epidemiology and Clinical Outcomes of Metabolic (Dysfunction)-associated Fatty Liver Disease. J. Clin. Transl. Hepatol. 2021, 9, 972–982.
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224.
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357.
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362.
- Wong, V.W.; Wong, G.L.; Tsang, S.W.; Hui, A.Y.; Chan, A.; Choi, P.C.; Chim, A.M.; Chu, S.; Chan, F.K.; Sung, J.J.; et al. Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment. Pharmacol. Ther. 2009, 29, 387–396.
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281 e4.
- Papatheodoridi, M.; Cholongitas, E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr. Pharm. Des. 2018, 24, 4574–4586.
- Khov, N.; Sharma, A.; Riley, T.R. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 6821–6825.
- Kinner, S.; Reeder, S.B.; Yokoo, T. Quantitative Imaging Biomarkers of NAFLD. Dig. Dis. Sci. 2016, 61, 1337–1347.
- Loomba, R. Role of imaging-based biomarkers in NAFLD: Recent advances in clinical application and future research directions. J. Hepatol. 2018, 68, 296–304.
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542.
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association between Fibrosis Stage and Outcomes of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625 e12.
- Tsai, E.; Lee, T.P. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography. Clin. Liver Dis. 2018, 22, 73–92.
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gómez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5.
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005, 54, 603–608.
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129.
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166.
- Hannah, W.N.; Harrison, S.A., Jr. Effect of Weight Loss, Diet, Exercise, and Bariatric Surgery on Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2016, 20, 339–350.
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138.
- Bower, G.; Toma, T.; Harling, L.; Jiao, L.R.; Efthimiou, E.; Darzi, A.; Athanasiou, T.; Ashrafian, H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: A Systematic Review of Liver Biochemistry and Histology. Obes. Surg. 2015, 25, 2280–2289.
- Chavez-Tapia, N.C.; Tellez-Avila, F.I.; Barrientos-Gutierrez, T.; Mendez-Sanchez, N.; Lizardi-Cervera, J.; Uribe, M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst. Rev. 2010, 2010, CD007340.
- Mathurin, P.; Hollebecque, A.; Arnalsteen, L.; Buob, D.; Leteurtre, E.; Caiazzo, R.; Pigeyre, M.; Verkindt, H.; Dharancy, S.; Louvet, A.; et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 2009, 137, 532–540.
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379–388.
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Guidelines, R.O.T.A.O.C.P. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 842–884. Available online: https://www.aace.com/publications/guidelines (accessed on 10 May 2023).
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756.
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150.
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690.
- Petit, J.-M.; Cercueil, J.-P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of Liraglutide Therapy on Liver Fat Content in Patients with Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415.
- Bi, Y.; Zhang, B.; Xu, W.; Yang, H.; Feng, W.; Li, C.; Tong, G.; Li, M.; Wang, X.; Shen, S.; et al. Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol. 2014, 51, 865–873.
- Khoo, J.; Hsiang, J.; Taneja, R.; Law, N.M.; Ang, T.L. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: A pilot randomized trial. Diabetes Obes. Metab. 2017, 19, 1814–1817.
- Luo, Q.; Wei, R.; Cai, Y.; Zhao, Q.; Liu, Y.; Liu, W.J. Efficacy of Off-Label Therapy for Non-alcoholic Fatty Liver Disease in Improving Non-invasive and Invasive Biomarkers: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Med. 2022, 9, 793203.
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65.
- Zelber–Sagi, S.; Kessler, A.; Brazowsky, E.; Webb, M.; Lurie, Y.; Santo, M.; Leshno, M.; Blendis, L.; Halpern, Z.; Oren, R. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2006, 4, 639–644.
- Harrison, S.A.; Fecht, W.; Brunt, E.M.; Neuschwander-Tetri, B.A. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology 2009, 49, 80–86.
- Assy, N.; Hussein, O.; Abassi, Z. Weight loss induced by orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with non-alcoholic steatohepatitis. Gut 2007, 56, 443–444.
- Harrison, S.A.; Fincke, C.; Helinski, D.; Torgerson, S.; Hayashi, P. A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Aliment. Pharmacol. Ther. 2004, 20, 623–628.
- Belkaid, P.M.; Harrat, Z.; Hamrioui, B.; Thellier, M.; Datry, A.; Danis, M. A simple media for isolation and culture of leishmania. Bull. Soc. Pathol. Exot. 1996, 89, 276–277.
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685.
- Klein, E.A.; Thompson, I.; Tangen, C.M.; Lucia, M.S.; Goodman, P.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; Karp, D.D.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556.
- Schürks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ 2010, 341, c5702.
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46.
- Boettcher, E.; Csako, G.; Pucino, F.; Wesley, R.; Loomba, R. Meta-analysis: Pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012, 35, 66–75.
- Yau, H.; Rivera, K.; Lomonaco, R.; Cusi, K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr. Diab Rep. 2013, 13, 329–341.
- Bril, F.; Cusi, K. Management of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Call to Action. Diabetes Care 2017, 40, 419–430.
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Sinha, R.A. Current treatment paradigms emerging therapies for NAFLD/NASH. Front. Biosci. 2021, 26, 206–237.
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124.
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE(-/-) and LDLr(-/-) Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic. Transl. Sci. 2018, 3, 844–857.
- Ho, P.P.; Steinman, L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2016, 113, 1600–1605.
- Guirguis, E.; Grace, Y.; Bolson, A.; DellaVecchia, M.J.; Ruble, M. Emerging therapies for the treatment of nonalcoholic steatohepatitis: A systematic review. Pharmacotherapy 2021, 41, 315–328.
- Pockros, P.J.; Fuchs, M.; Freilich, B.; Schiff, E.; Kohli, A.; Lawitz, E.J.; Hellstern, P.A.; Owens-Grillo, J.; Van Biene, C.; Shringarpure, R.; et al. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019, 39, 2082–2093.
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574–582.e1.
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965.
- Ratziu, V.; Sanyal, A.J.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R.; et al. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803.
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196.
- Boubia, B.; Poupardin, O.; Barth, M.; Binet, J.; Peralba, P.; Mounier, L.; Mounier, L.; Jacquier, E.; Gauthier, E.; Lepais, V.; et al. Design, Synthesis, and Evaluation of a Novel Series of Indole Sulfonamide Peroxisome Proliferator Activated Receptor (PPAR) alpha/gamma/delta Triple Activators: Discovery of Lanifibranor, a New Antifibrotic Clinical Candidate. J. Med. Chem. 2018, 61, 2246–2265.
- Wettstein, G.; Luccarini, J.; Poekes, L.; Faye, P.; Kupkowski, F.; Adarbes, V.; Defrêne, E.; Estivalet, C.; Gawronski, X.; Jantzen, I.; et al. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol. Commun. 2017, 1, 524–537.
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240.
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558.
- Hodson, L. BA Fielding, Stearoyl-CoA desaturase: Rogue or innocent bystander? Prog. Lipid. Res. 2013, 52, 15–42.
- Walle, P.; Takkunen, M.; Männistö, V.; Vaittinen, M.; Lankinen, M.; Kärjä, V.; Käkelä, P.; Ågren, J.; Tiainen, M.; Schwab, U.; et al. Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism 2016, 65, 655–666.
- Issandou, M.; Bouillot, A.; Brusq, J.M.; Forest, M.C.; Grillot, D.; Guillard, R.; Martin, S.; Michiels, C.; Sulpice, T.; Daugan, A. Pharmacological inhibition of stearoyl-CoA desaturase 1 improves insulin sensitivity in insulin-resistant rat models. Eur. J. Pharmacol. 2009, 618, 28–36.
- Iruarrizaga-Lejarreta, M.; Varela-Rey, M.; Fernández-Ramos, D.; Martínez-Arranz, I.; Delgado, T.C.; Simon, J.; Juan, V.G.; Delacruz-Villar, L.; Azkargorta, M.; Lavin, J.L.; et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol. Commun. 2017, 1, 911–927.
- Safadi, R.; Konikoff, F.M.; Mahamid, M.; Zelber-Sagi, S.; Halpern, M.; Gilat, T.; Oren, R.; Hershkovitz, A.; Rosenthal-Galili, Z.; Zuckerman, E.; et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2085–2091.e1.
- Ratziu, V.; de Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, A.L.; Ben Bashat, D.; Lackner, K.; et al. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021, 27, 1825–1835.
- Rubin, V.R.; Bojanic, K.; Smolic, M.; Rubin, J.; Tabll, A.; Smolic, R. An Update on Efficacy and Safety of Emerging Hepatic Antifibrotic Agents. J. Clin. Transl. Hepatol. 2021, 9, 60–70.
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Reciprocal Crosstalk between Autophagic and Endocrine Signaling in Metabolic Homeostasis. Endocr. Rev. 2017, 38, 69–102.
- Sinha, R.A.; You, S.-H.; Zhou, J.; Siddique, M.M.; Bay, B.-H.; Zhu, X.; Privalsky, M.L.; Cheng, S.-Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438.
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173–1191.
- Sherman, S.I.; Ringel, M.D.; Smith, M.J.; Kopelen, H.A.; Zoghbi, W.A.; Ladenson, P.W. Augmented hepatic and skeletal thyromimetic effects of tiratricol in comparison with levothyroxine. J. Clin. Endocrinol. Metab. 1997, 82, 2153–2158.
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.-S.; et al. Discovery of 2--3,5-dio xo-2,3,4,5-tetrahydro triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J. Med. Chem. 2014, 57, 3912–3923.
- Taub, R.; Chiang, E.; Chabot-Blanchet, M.; Kelly, M.J.; Reeves, R.A.; Guertin, M.-C.; Tardif, J.-C. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-beta agonist. Atherosclerosis 2013, 230, 373–380.
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
564
Revisions:
2 times
(View History)
Update Date:
14 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




