Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jacob Beiriger and Version 2 by Jessie Wu.
Non-alcoholic fatty liver disease (NAFLD), recently renamed by an international consensus panel as metabolic-associated fatty liver disease (MAFLD), affects up to 1 billion patients worldwide. This change in nomenclature is in keeping with more recent understanding of this disease and its inherent link to metabolic syndrome.
- NAFLD
- NASH
- pathogenesis
- genetic factors
- dietary factors
- environmental factors
- therapeutic strategies
1. Epidemiology
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease with a prevalence of 25% worldwide. The highest rates are reported from South America (31%) and the Middle East (32%), followed by Asia (27%), the United States (24%), and Europe (23%). NAFLD is less common in Africa (14%). Among these patients in the US, the prevalence of NASH was 21% [1][2].
Research into NAFLD has rapidly expanded the understanding of its pathophysiology and defined useful diagnostic criteria. Diagnosis has shifted to focus on the metabolic criteria commonly associated with hepatic steatosis, such as T2DM and metabolic syndrome. These key comorbidities have led to the proposal to rename this condition to MAFLD [2][4]. The goal with re-defining fatty liver disease is to improve outcomes by identifying high-risk patients and intervening earlier in the metabolic dysfunction contributing to their disease [3][5]. Experts state that a focus on metabolic dysfunction better represents the pathophysiologic mechanism of the disease. Studies have shown that obesity, metabolic syndrome, and T2DM are associated with a greater risk of progression of NAFLD to NASH, HCC, or fibrosis [4][6]. NASH is predicted to become the most common indication for liver transplantation [5][7]. Therefore, the focus on metabolic criteria may better stratify patients who are at increased risk of disease progression. Compared to a diagnosis of exclusion in NAFLD, MAFLD’s positive inclusion of metabolic abnormalities is designed to risk-stratify patients at highest risk for progression to NASH, HCC, or fibrosis. Importantly, the presence of other hepatic disease does not exclude MAFLD diagnosis like the in the NAFLD definition. However, studies show that around 90% of patients meet the criteria of both NAFLD and MAFLD [6][7][8,9].
The new definition will alter the incidence and prevalence going forward. Given its novelty, prevalence data have been estimated from existing data. A recent meta-analysis by Chan et al. of more than 10 million patients globally found the prevalence of MAFLD to be 38% [3][5]. One study found the prevalence of MAFLD in North America to be 34.8%, another measured 39.1% [8][9][10,11]. As obesity and T2DM continue to increase around the world, the prevalence of MAFLD is likely to increase.
The definition of MAFLD includes patients who have two or more factors associated with metabolic dysfunction. Thus, the MAFLD definition encompasses lean and non-obese individuals with steatosis. The prevalence of MAFLD among these patients is estimated to be 5% and 12%, respectively [10][12].
The incidence of both NAFLD/MAFLD is difficult to determine due to a lack of cohesive screening guidelines and inaccurate tools. Ultrasound is commonly used in initial work-up, but it lacks the sensitivity to detect subtleties in early disease. The generally accepted value is that the incidence of NAFLD is around 2–6% [11][13].
2. Diagnosis
There is debate over whether screening for NAFLD is worthwhile and cost-effective [12][69]. Currently, the American Association for the Study of Liver Diseases (AASLD) does not recommend screening for NAFLD, even in high-risk populations, due to the uncertain long-term benefits and cost-effectiveness of screening [13][70]. It is well-established that patients with type 2 diabetes, obesity, dyslipidemia, and hypertension are at increased risk of cirrhosis, advanced fibrosis, and liver disease mortality [12][13][69,70]. Therefore, clinicians should have a higher degree of suspicion in patients with these comorbidities. The diagnosis of NAFLD is typically characterized by (1) the presence of chronically elevated liver enzymes and (2) imaging evidence of hepatic steatosis. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are commonly used to monitor liver function. An AST/ALT ratio of greater than 1 has been associated with a higher degree of fibrosis on liver biopsy [14][71]. However, elevated ALT alone is not correlated with a higher degree of fibrosis in NAFLD patients [15][72]. Although these serum biomarkers have high availability and applicability, they are not liver specific and can therefore be elevated in other comorbid conditions [16][73]. With regard to imaging, abdominal ultrasound is currently the first-line screening tool for NAFLD given its widespread availability and low cost [17][74]. Findings of steatosis on abdominal ultrasound include bright hepatic echoes, increased hepatorenal echogenicity, vascular blurring of the portal or hepatic vein, and subcutaneous tissue thickness [18][75]. Limitations of using ultrasound include inter- and intra-reader variability and anatomic constraints (bowel gas or a large body habitus) [19][76]. Liver biopsy is the gold standard for diagnosing NAFLD; however, it is costly, invasive, and susceptible to sampling bias [20][77]. Findings on liver biopsy of NAFLD include hepatic steatosis with or without hepatocyte ballooning, hepatic necrosis, Mallory bodies, and fibrosis [21][78]. Of note, the severity of fibrosis has the greatest association with liver-related morbidity and mortality when compared to other histological findings [22][79]. MRI proton density fat fraction (PDFF) and magnetic resonance spectroscopy (MRS) are useful for quantifying the triglyceride content in the liver [20][77]. However, MRI-PDFF and MRS are more costly and less available than abdominal ultrasound and are more often used in research settings rather than clinical practice [12][69]. Before diagnosing NAFLD, secondary causes of fatty liver disease such as viral hepatitis, alcoholic fatty liver disease, drug-induced liver disease, medical conditions (Wilson disease, hereditary hemochromatosis, celiac disease), metabolic diseases (glycogen storage diseases), and poor nutritional status must be excluded [23][80]. Once the diagnosis of NAFLD has been established, fibrosis scores are often calculated to assess the degree of NAFLD and guide management. The NAFLD fibrosis score (NFS), fibrosis-4 (FIB-4) index, and AST-to-platelet ratio can be calculated from routine laboratory results [12][69]. The NFS is based on a patient’s age, BMI, presence of diabetes, AST, ALT, platelet count, and albumin level, while the FIB-4 is based on the patient’s age, AST, ALT, and platelet count. Patients who have a FIB-4 ≤ 1.3 (age < 65 years) or FIB-4 ≤ 2.0 (age > 65 years) are at minimal risk for advanced fibrosis and are typically managed in the primary case setting (see figure below). Patients who have a FIB-4 of >1.3 (age < 65 years) or FIB-4 > 2 (age > 65 years) undergo elastography and are referred for specialist care (see figure below). NFS and FIB-4 scores have a negative predictive value > 90%, demonstrating their utility as an initial diagnostic test in assessing for NAFLD [16][73]. Ultrasound-based elastography, vibration-controlled transient elastography, point-shear wave elastography, two-dimensional shear wave elastography, or magnetic resonance elastography can be used to assess liver fibrosis in patients with intermediate-to-high risk of NAFLD based on their FIB-4 and NFS [12][69]. Transient elastography is most used given its availability.3. Treatments
Weight loss is the first-line management of NAFLD and is recommended for patients with a BMI > 25. In addition to improving the quality of life in patients with NAFLD, weight loss has been shown to improve liver histology, hepatic steatosis, and inflammation [24][25][26][27][81,82,83,84]. Weight loss of 3–5% has been shown to be associated with decreased steatosis fibrosis regression, 5–7% with reduced inflammation, 7–10% with NASH resolution, and ≥10% with fibrosis regression [28][85]. Lifestyle interventions such as diet modification and exercise are attempted first. In patients with NASH or advanced fibrosis who do not sufficiently meet weight loss goals in lifestyle interventions, other strategies like bariatric surgery may be pursued. Surgical intervention with bariatric surgery can be considered in patients with a BMI > 40 or a BMI >35 with one obesity-related comorbidity [29][86]. Bariatric surgery has been shown to improve steatosis, reduce inflammation, and improve fibrosis score [30][31][32][87,88,89]. Surgical intervention has also shown benefits in patients with NASH. Lassailly et al. studied the effect of bariatric surgery in patients with NASH and found that NASH had disappeared in 85% of patients one year postoperatively [33][90]. However, worsening fibrosis has also been reported following bariatric surgery [31][88]. Although no drug treatment has been approved by the United States Food and Drug Administration (FDA) for the direct treatment of NAFLD, drug therapies targeting weight loss can be considered. Weight loss pharmacological therapy may be initiated in patients with a BMI ≥ 30 or ≥27 with metabolic comorbidities such as diabetes or hypertension [34][91]. Current drug therapy options include glucagon-like peptide-1 (GLP-1) receptor agonists, lipase inhibitors, sympathomimetics, or combination drugs. Among these, GLP-1 agonists are the best-studied and evidence consistently demonstrates a positive effect of the drugs on NAFLD. Semaglutide and liraglutide are the two GLP-1 receptor agonists that have been approved for the treatment of obesity in the United States and are considered the first-line drug therapy. GLP-1 receptor agonists increase glucose-dependent insulin secretion, inhibit glucagon secretion, and slow gastric emptying [35][92]. They also act in multiple regions of the brain, where appetite and caloric intake are regulated [35][92]. Of the two, semaglutide is preferred given its superior dosing schedule of once weekly compared to once daily with liraglutide, as well as its greater efficacy in weight loss [36][93]. Multiple studies have shown that GLP-1 agonists can decrease liver fat content, improve ALT, and even slow the progression of fibrosis [37][38][39][40][94,95,96,97]. The effects of GLP-1 agonists on liver fat are primarily rooted in their ability to enhance insulin sensitivity and reduce hepatic glucose production, decreasing de novo lipogenesis and increased fatty acid oxidation within the liver. These mechanisms are particularly relevant as they address fundamental drivers of hepatic fat accumulation and inflammation, offering an avenue to mitigate progression. The leading GLP-1 agonist, semaglutide, has shown much promise in the treatment of NASH and its effects on decreasing markers of NASH and liver enzymes beyond its weight loss effects are discussed below. Liraglutide, another GLP-1 agonist that has also been known to cause weight loss, has also been associated with increased rates of NASH resolution and decreased enhanced liver fibrosis (ELF) score when compared to placebo [41][98]. While weight loss is desired given its theorized and observed benefits in NASH, quantification of this fat loss is important when thinking about weight loss as a treatment specifically for NASH. Several trials such as that by Vilar-Gomez et al. have demonstrated that a weight loss of at least 10% of initial body weight is associated with the highest rates of NAFLD activity score reduction, NASH resolution, and even fibrosis regression. The importance of weight loss from this trial is summarized in Figure 1 below [24][81].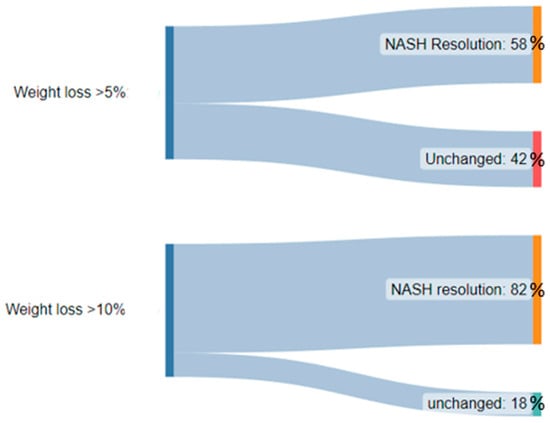
Figure 1.
A diagram demonstrating the significantly higher proportion of patients with NASH resolution after weight loss of >10% body weight when compared to >5% body weight.
