
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaoyan Chen | -- | 3586 | 2023-11-12 11:35:45 | | | |
| 2 | Lindsay Dong | + 240 word(s) | 3826 | 2023-11-13 04:02:15 | | |
Video Upload Options
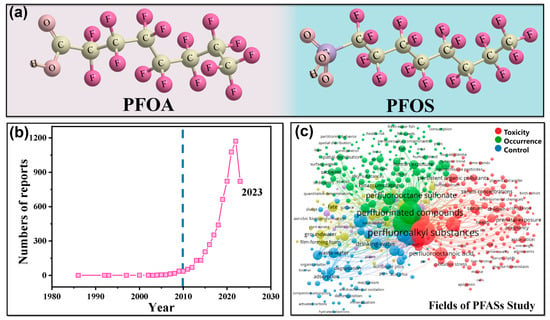
Per- and polyfluoroalkyl substances (PFASs) are an emerging group of persistent organic pollutants in aquatic environments with high levels of toxicity and bioaccumulation. The risks posed by PFASs to the environment and health have attracted increasing attention. To remove them from water, advanced oxidation processes (AOPs), with the merits of high efficiency and low cost, are mainly used. Photo/electrocatalytic heterogeneous AOPs, with the assistance of nanostructured catalysts and external energy in the form of light/electricity, have emerged as one of the most powerful techniques, overcoming the difficulty associated with defluorination and achieving the effective and complete degradation of PFASs in water. The structures of photo/electrocatalysts play a critical role in the production of reactive oxygen species, the electron transfer process, and the degradation pathway and its efficiency.
1. Introduction
2. Fundamentals of PFAS Degradation by AOPs
2.1. General Pathways and ROS for PFAS Degradation
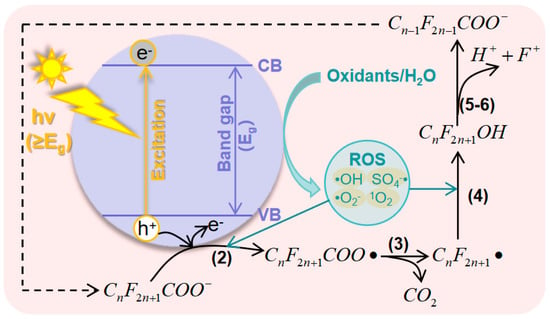
2.2. Principles of Photocatalytic AOPs for PFAS
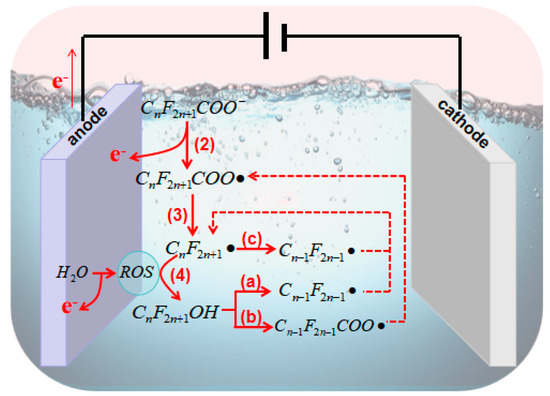
2.3. Principles of Electrocatalytic AOPs for PFASs
3. Photocatalysts in AOPs for PFAS Degradation
3.1. Metal Oxide-Based Materials
3.2. Bi-Based Materials
3.3. Other Compounds and Composites
4. Electrocatalysts in AOPs for PFAS Degradation
4.1. BDD-Based Materials
4.2. Metal Oxide-Based Materials
4.3. Other Compounds and Composites
5. Conclusions
- (1)
-
For catalysts in the photocatalytic oxidation of PFAS systems, current research primarily focuses on improving catalyst activity by addressing the rapid recombination of photogenerated e−−h+. Methods include introducing surface defects or oxygen vacancies, metal-doping, heterojunction construction, and crystal facet regulation [28][61][63][64][94][95][96][97]. Furthermore, material composites and morphology regulation have been utilized to enhance reaction probabilities between PFASs and active groups, effectively enhancing the efficiency of PFAS degradation [17][28][98][99].
- (2)
-
For catalysts in the electrocatalytic oxidation of PFAS systems, current research mainly focuses on increasing the yield of active groups through various methods [60][94][100]. Additionally, enhancing the efficiency of electron transfer and mass transfer processes is achieved through metal loading and constructing nano 3D structures [20][83][89][100]. The stability of electrodes is also improved through the construction of intermediate layers [40][101].
References
- Sadia, M.; Nollen, I.; Helmus, R.; ter Laak, T.L.; Béen, F.; Praetorius, A.; van Wezel, A.P. Occurrence, Fate, and Related Health Risks of PFAS in Raw and Produced Drinking Water. Environ. Sci. Technol. 2023, 57, 3062–3074.
- Dong, C.; Huang, F.; Deng, H.; Schaffrath, C.; Spencer, J.B.; O’Hagan, D.; Naismith, J.H. Crystal structure and mechanism of a bacterial fluorinating enzyme. Nature 2004, 427, 561–565.
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381.
- Chow, S.J.; Croll, H.C.; Ojeda, N.; Klamerus, J.; Capelle, R.; Oppenheimer, J.; Jacangelo, J.G.; Schwab, K.J.; Prasse, C. Comparative investigation of PFAS adsorption onto activated carbon and anion exchange resins during long-term operation of a pilot treatment plant. Water Res. 2022, 226, 119198.
- Bolan, N.; Sarkar, B.; Vithanage, M.; Singh, G.; Tsang, D.C.W.; Mukhopadhyay, R.; Ramadass, K.; Vinu, A.; Sun, Y.; Ramanayaka, S.; et al. Distribution, behaviour, bioavailability and remediation of poly- and per-fluoroalkyl substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021, 155, 106600.
- Bruton, T.K.; Sedlak, D.L. Treatment of Aqueous Film-Forming Foam by Heat-Activated Persulfate Under Conditions Representative of In Situ Chemical Oxidation. Environ. Sci. Technol. 2017, 51, 13878–13885.
- Bao, Y.; Huang, J.; Cagnetta, G.; Yu, G. Removal of F-538 as PFOS alternative in chrome plating wastewater by UV/Sulfite reduction. Water Res. 2019, 163, 114907.
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766.
- Pierpaoli, M.; Szopińska, M.; Wilk, B.K.; Sobaszek, M.; Łuczkiewicz, A.; Bogdanowicz, R.; Fudala-Książek, S. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J. Hazard. Mater. 2021, 403, 123606.
- Wackett, L.P. Why Is the Biodegradation of Polyfluorinated Compounds So Rare? mSphere 2021, 6, 10–1128.
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352.
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909.
- Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Appl. Sci. 2021, 11, 8468.
- Broman, J.; Ceja, A.; Godoy, T.; Rivera, D.R.; Dionne, P.; Schipper, J.; Henkemeyer, S.; Cegielski, S.; Wong, G.; Kaur, A.; et al. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) via Lacasse Enzymatic Degradation and Electrochemical Advanced Oxidation. In Proceedings of the 31st Waste-Management Education Research Conference (WERC), Las Cruces, NM, USA, 11–14 April 2021.
- Xiao, F.; Sasi, P.C.; Alinezhad, A.; Sun, R.; Ali, M.A. Thermal Phase Transition and Rapid Degradation of Forever Chemicals (PFAS) in Spent Media Using Induction Heating. ACS ES&T Eng. 2023, 3, 1370–1380.
- Horikoshi, S.; Sato, S.; Abe, M.; Serpone, N. A novel liquid plasma AOP device integrating microwaves and ultrasounds and its evaluation in defluorinating perfluorooctanoic acid in aqueous media. Ultrason. Sonochem. 2011, 18, 938–942.
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Gar Alalm, M. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 127152.
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater. 2010, 179, 1143–1146.
- Li, Z.; Zhang, P.; Shao, T.; Li, X. In2O3 nanoporous nanosphere: A highly efficient photocatalyst for decomposition of perfluorooctanoic acid. Appl. Catal. B 2012, 125, 350–357.
- Liu, X.; Wei, W.; Xu, J.; Wang, D.; Song, L.; Ni, B.-J. Photochemical decomposition of perfluorochemicals in contaminated water. Water Res. 2020, 186, 116311.
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338.
- Bentel, M.J.; Yu, Y.; Xu, L.; Li, Z.; Wong, B.M.; Men, Y.; Liu, J. Defluorination of Per- and Polyfluoroalkyl Substances (PFASs) with Hydrated Electrons: Structural Dependence and Implications to PFAS Remediation and Management. Environ. Sci. Technol. 2019, 53, 3718–3728.
- Li, X.; Zhang, P.; Jin, L.; Shao, T.; Li, Z.; Cao, J. Efficient Photocatalytic Decomposition of Perfluorooctanoic Acid by Indium Oxide and Its Mechanism. Environ. Sci. Technol. 2012, 46, 5528–5534.
- Banayan Esfahani, E.; Asadi Zeidabadi, F.; Zhang, S.; Mohseni, M. Photo-chemical/catalytic oxidative/reductive decomposition of per- and poly-fluoroalkyl substances (PFAS), decomposition mechanisms and effects of key factors: A review. Environ. Sci. Water Res. Technol. 2022, 8, 698–728.
- Eberson, L. Electron-Transfer Reactions in Organic Chemistry. Adv. Phys. Org. Chem. 1982, 18, 79–185.
- Zhang, H.; Xie, C.; Chen, L.; Duan, J.; Li, F.; Liu, W. Different reaction mechanisms of SO4•− and •OH with organic compound interpreted at molecular orbital level in Co(II)/peroxymonosulfate catalytic activation system. Water Res. 2023, 229, 119392.
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517.
- Duan, L.; Wang, B.; Heck, K.N.; Clark, C.A.; Wei, J.; Wang, M.; Metz, J.; Wu, G.; Tsai, A.-L.; Guo, S.; et al. Titanium oxide improves boron nitride photocatalytic degradation of perfluorooctanoic acid. Chem. Eng. J. 2022, 448, 137735.
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B 2017, 202, 217–261.
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700.
- Wang, Y.; Zhang, P. Effects of pH on photochemical decomposition of perfluorooctanoic acid in different atmospheres by 185nm vacuum ultraviolet. J. Environ. Sci. 2014, 26, 2207–2214.
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem. Eng. J. 2012, 180, 197–203.
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506.
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chem. Eng. J. 2017, 328, 927–942.
- Phan Thi, L.-A.; Do, H.-T.; Lee, Y.-C.; Lo, S.-L. Photochemical decomposition of perfluorooctanoic acids in aqueous carbonate solution with UV irradiation. Chem. Eng. J. 2013, 221, 258–263.
- Qanbarzadeh, M.; Wang, D.; Ateia, M.; Sahu, S.P.; Cates, E.L. Impacts of Reactor Configuration, Degradation Mechanisms, and Water Matrices on Perfluorocarboxylic Acid Treatment Efficiency by the UV/Bi3O(OH)(PO4)2 Photocatalytic Process. ACS ES&T Eng. 2021, 1, 239–248.
- Hossain, M.S.; Mollah, M.Y.A.; Susan, M.A.B.H.; Islam, M.M. Role of in situ electrogenerated reactive oxygen species towards degradation of organic dye in aqueous solution. Electrochim. Acta 2020, 344, 136146.
- Liu, X.Y.; Zhang, Y.Q.; Xia, X.H.; Shi, S.J.; Lu, Y.; Wang, X.L.; Gu, C.D.; Tu, J.P. Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sour. 2013, 239, 157–163.
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569.
- Lin, H.; Niu, J.; Ding, S.; Zhang, L. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes. Water Res. 2012, 46, 2281–2289.
- Niu, J.; Lin, H.; Gong, C.; Sun, X. Theoretical and Experimental Insights into the Electrochemical Mineralization Mechanism of Perfluorooctanoic Acid. Environ. Sci. Technol. 2013, 47, 14341–14349.
- Zhuo, Q.; Deng, S.; Yang, B.; Huang, J.; Yu, G. Efficient Electrochemical Oxidation of Perfluorooctanoate Using a Ti/SnO2-Sb-Bi Anode. Environ. Sci. Technol. 2011, 45, 2973–2979.
- Lin, H.; Niu, J.; Xu, J.; Huang, H.; Li, D.; Yue, Z.; Feng, C. Highly Efficient and Mild Electrochemical Mineralization of Long-Chain Perfluorocarboxylic Acids (C9–C10) by Ti/SnO2–Sb–Ce, Ti/SnO2–Sb/Ce–PbO2, and Ti/BDD Electrodes. Environ. Sci. Technol. 2013, 47, 13039–13046.
- Niu, J.; Lin, H.; Xu, J.; Wu, H.; Li, Y. Electrochemical Mineralization of Perfluorocarboxylic Acids (PFCAs) by Ce-Doped Modified Porous Nanocrystalline PbO2 Film Electrode. Environ. Sci. Technol. 2012, 46, 10191–10198.
- Ma, Q.; Liu, L.; Cui, W.; Li, R.; Song, T.; Cui, Z. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Yb-doped Ti/SnO2–Sb/PbO2 anodes and determination of the optimal conditions. RSC Adv. 2015, 5, 84856–84864.
- Wang, K.; Huang, D.; Wang, W.; Ji, Y.; Niu, J. Enhanced perfluorooctanoic acid degradation by electrochemical activation of peroxymonosulfate in aqueous solution. Environ. Int. 2020, 137, 105562.
- Fu, C.; Xu, X.; Zheng, C.; Liu, X.; Zhao, D.; Qiu, W. Photocatalysis of aqueous PFOA by common catalysts of In2O3, Ga2O3, TiO2, CeO2 and CdS: Influence factors and mechanistic insights. Environ. Geochem. Health 2022, 44, 2943–2953.
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Kuo, J.; Wu, C.-H. Decomposition of perfluorooctanoic acid by ultraviolet light irradiation with Pb-modified titanium dioxide. J. Hazard. Mater. 2016, 303, 111–118.
- Zhao, B.; Li, X.; Yang, L.; Wang, F.; Li, J.; Xia, W.; Li, W.; Zhou, L.; Zhao, C. ß-Ga2O3 Nanorod Synthesis with a One-step Microwave Irradiation Hydrothermal Method and its Efficient Photocatalytic Degradation for Perfluorooctanoic Acid. Photochem. Photobiol. 2015, 91, 42–47.
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38.
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Huang, C.-C. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide. J. Hazard. Mater. 2015, 288, 168–175.
- Chowdhury, N.; Choi, H. Photocatalytic degradation of perfluorooctanoic acid on Pb-doped TiO2 coated with reduced graphene oxide. Water Environ. Res. 2023, 95, e10871.
- Li, M.; Yu, Z.; Liu, Q.; Sun, L.; Huang, W. Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2. Chem. Eng. J. 2016, 286, 232–238.
- Liu, Q.; Yu, Z.-b.; Zhang, R.-h.; Li, M.-j.; Chen, Y.; Wang, L.; Kuang, Y.; Zhang, B.; Zhu, Y.-h. Photocatalytic Degradation of Perfluorooctanoic Acid by Pd-TiO2 Photocatalyst. Huanjing Kexue 2015, 36, 2138–2146.
- Tian, A.; Wu, Y.; Mao, K. Enhanced Performance of Surface Modified TiO2 Nanotubes for the Decomposition of Perfluorooctanoic Acid. In Proceedings of the International Conference on Materials Science, Resource and Environmental Engineering (MSREE), Xian, China, 10–11 December 2016.
- Estrellan, C.R.; Salim, C.; Hinode, H. Photocatalytic decomposition of perfluorooctanoic acid by iron and niobium co-doped titanium dioxide. J. Hazard. Mater. 2010, 179, 79–83.
- Li, Z.; Zhang, P.; Shao, T.; Wang, J.; Jin, L.; Li, X. Different nanostructured In2O3 for photocatalytic decomposition of perfluorooctanoic acid (PFOA). J. Hazard. Mater. 2013, 260, 40–46.
- Li, Z.; Zhang, P.; Li, J.; Shao, T.; Wang, J.; Jin, L. Synthesis of In2O3 porous nanoplates for photocatalytic decomposition of perfluorooctanoic acid (PFOA). Catal. Commun. 2014, 43, 42–46.
- Xu, C.; Qiu, P.; Chen, H.; Jiang, F.; Wang, X. Fabrication of two-dimensional indium oxide nanosheets with graphitic carbon nitride nanosheets as sacrificial templates. Mater. Lett. 2019, 242, 24–27.
- Jiang, F.; Zhao, H.; Chen, H.; Xu, C.; Chen, J. Enhancement of photocatalytic decomposition of perfluorooctanoic acid on CeO2/In2O3. RSC Adv. 2016, 6, 72015–72021.
- Wu, Y.; Li, Y.; Fang, C.; Li, C. Highly Efficient Degradation of Perfluorooctanoic Acid over a MnOx-Modified Oxygen-Vacancy-Rich In2O3 Photocatalyst. ChemCatChem 2019, 11, 2297–2303.
- Xu, B.; Zhou, J.L.; Altaee, A.; Ahmed, M.B.; Johir, M.A.H.; Ren, J.; Li, X. Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate. Chemosphere 2020, 239, 124722.
- Wang, J.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Underneath mechanisms into the super effective degradation of PFOA by BiOF nanosheets with tunable oxygen vacancies on exposed (101) facets. Appl. Catal. B 2021, 286, 119911.
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A Chem. 2011, 334, 116–122.
- Li, T.; Wang, C.; Wang, T.; Zhu, L. Highly efficient photocatalytic degradation toward perfluorooctanoic acid by bromine doped BiOI with high exposure of (001) facet. Appl. Catal. B 2020, 268, 118442.
- Zhang, K.-L.; Liu, C.-M.; Huang, F.-Q.; Zheng, C.; Wang, W.-D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B 2006, 68, 125–129.
- Qian, L.; Georgi, A.; Gonzalez-Olmos, R.; Kopinke, F.-D. Degradation of perfluorooctanoic acid adsorbed on Fe-zeolites with molecular oxygen as oxidant under UV-A irradiation. Appl. Catal. B Environ. 2020, 278, 119283.
- Luo, D.; Yuan, J.; Zhou, J.; Zou, M.; Xi, R.; Qin, Y.; Shen, Q.; Hu, S.; Xu, J.; Nie, M.; et al. Synthesis of samarium doped ferrite and its enhanced photocatalytic degradation of perfluorooctanoic acid (PFOA). Opt. Mater. 2021, 122, 111636.
- Xu, J.; Wu, M.; Yang, J.; Wang, Z.; Chen, M.; Teng, F. Efficient photocatalytic degradation of perfluorooctanoic acid by a wide band gap p-block metal oxyhydroxide InOOH. Appl. Surf. Sci. 2017, 416, 587–592.
- Ochiai, T.; Iizuka, Y.; Nakata, K.; Murakami, T.; Tryk, D.A.; Fujishima, A.; Koide, Y.; Morito, Y. Efficient electrochemical decomposition of perfluorocarboxylic acids by the use of a boron-doped diamond electrode. Diamond Relat. Mater. 2011, 20, 64–67.
- Carter, K.E.; Farrell, J. Oxidative Destruction of Perfluorooctane Sulfonate Using Boron-Doped Diamond Film Electrodes. Environ. Sci. Technol. 2008, 42, 6111–6115.
- Zhi, J.-F.; Wang, H.-B.; Nakashima, T.; Rao, T.N.; Fujishima, A. Electrochemical Incineration of Organic Pollutants on Boron-Doped Diamond Electrode. Evidence for Direct Electrochemical Oxidation Pathway. J. Phys. Chem. B 2003, 107, 13389–13395.
- Sukeesan, S.; Boontanon, N.; Boontanon, S.K. Improved electrical driving current of electrochemical treatment of Per- and Polyfluoroalkyl Substances (PFAS) in water using Boron-Doped Diamond anode. Environ. Technol. Innov. 2021, 23, 101655.
- Barisci, S.; Suri, R. Electrooxidation of short and long chain perfluorocarboxylic acids using boron doped diamond electrodes. Chemosphere 2020, 243, 125349.
- Zhao, G.; Zhang, Y.; Lei, Y.; Lv, B.; Gao, J.; Zhang, Y.; Li, D. Fabrication and Electrochemical Treatment Application of a Novel Lead Dioxide Anode with Superhydrophobic Surfaces, High Oxygen Evolution Potential, and Oxidation Capability. Environ. Sci. Technol. 2010, 44, 1754–1759.
- Lou, Z.; Wang, J.; Wang, S.; Xu, Y.; Wang, J.; Liu, B.; Yu, C.; Yu, J. Strong hydrophobic affinity and enhanced •OH generation boost energy-efficient electrochemical destruction of perfluorooctanoic acid on robust ceramic/PbO2-PTFE anode. Sep. Purif. Technol. 2022, 280, 119919.
- Yang, B.; Jiang, C.; Yu, G.; Zhuo, Q.; Deng, S.; Wu, J.; Zhang, H. Highly efficient electrochemical degradation of perfluorooctanoic acid (PFOA) by F-doped Ti/SnO2 electrode. J. Hazard. Mater. 2015, 299, 417–424.
- Zhuo, Q.; Xiang, Q.; Yi, H.; Zhang, Z.; Yang, B.; Cui, K.; Bing, X.; Xu, Z.; Liang, X.; Guo, Q.; et al. Electrochemical oxidation of PFOA in aqueous solution using highly hydrophobic modified PbO2 electrodes. J. Electroanal. Chem. 2017, 801, 235–243.
- Duan, T.; Wen, Q.; Chen, Y.; Zhou, Y.; Duan, Y. Enhancing electrocatalytic performance of Sb-doped SnO2 electrode by compositing nitrogen-doped graphene nanosheets. J. Hazard. Mater. 2014, 280, 304–314.
- Zhao, H.; Gao, J.; Zhao, G.; Fan, J.; Wang, Y.; Wang, Y. Fabrication of novel SnO2-Sb/carbon aerogel electrode for ultrasonic electrochemical oxidation of perfluorooctanoate with high catalytic efficiency. Appl. Catal. B 2013, 136–137, 278–286.
- Aquino, J.M.; Rocha-Filho, R.C.; Ruotolo, L.A.M.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of a real textile wastewater using β-PbO2 and DSA® anodes. Chem. Eng. J. 2014, 251, 138–145.
- Mukimin, A.; Vistanty, H.; Zen, N. Oxidation of textile wastewater using cylinder Ti/β-PbO2 electrode in electrocatalytic tube reactor. Chem. Eng. J. 2015, 259, 430–437.
- Song, S.; Zhan, L.; He, Z.; Lin, L.; Tu, J.; Zhang, Z.; Chen, J.; Xu, L. Mechanism of the anodic oxidation of 4-chloro-3-methyl phenol in aqueous solution using Ti/SnO2–Sb/PbO2 electrodes. J. Hazard. Mater. 2010, 175, 614–621.
- Zhong, C.; Wei, K.; Han, W.; Wang, L.; Sun, X.; Li, J. Electrochemical degradation of tricyclazole in aqueous solution using Ti/SnO2–Sb/PbO2 anode. J. Electroanal. Chem. 2013, 705, 68–74.
- Song, S.; Fan, J.; He, Z.; Zhan, L.; Liu, Z.; Chen, J.; Xu, X. Electrochemical degradation of azo dye C.I. Reactive Red 195 by anodic oxidation on Ti/SnO2–Sb/PbO2 electrodes. Electrochim. Acta 2010, 55, 3606–3613.
- Yang, B.; Wang, J.; Jiang, C.; Li, J.; Yu, G.; Deng, S.; Lu, S.; Zhang, P.; Zhu, C.; Zhuo, Q. Electrochemical mineralization of perfluorooctane sulfonate by novel F and Sb co-doped Ti/SnO2 electrode containing Sn-Sb interlayer. Chem. Eng. J. 2017, 316, 296–304.
- Xu, Z.; Yu, Y.; Liu, H.; Niu, J. Highly efficient and stable Zr-doped nanocrystalline PbO2 electrode for mineralization of perfluorooctanoic acid in a sequential treatment system. Sci. Total Environ. 2017, 579, 1600–1607.
- Ellis, D.A.; Denkenberger, K.A.; Burrow, T.E.; Mabury, S.A. The Use of 19F NMR to Interpret the Structural Properties of Perfluorocarboxylate Acids: A Possible Correlation with Their Environmental Disposition. J. Phys. Chem. A 2004, 108, 10099–10106.
- Duan, X.; Wang, W.; Wang, Q.; Sui, X.; Li, N.; Chang, L. Electrocatalytic degradation of perfluoroocatane sulfonate (PFOS) on a 3D graphene-lead dioxide (3DG-PbO2) composite anode: Electrode characterization, degradation mechanism and toxicity. Chemosphere 2020, 260, 127587.
- Ma, Q.; Gao, J.; Moussa, B.; Young, J.; Zhao, M.; Zhang, W. Electrosorption, Desorption, and Oxidation of Perfluoroalkyl Carboxylic Acids (PFCAs) via MXene-Based Electrocatalytic Membranes. ACS Appl. Mater. Interfaces 2023, 15, 29149–29159.
- Han, J.; Zhao, M.; Wu, K.; Hong, Y.; Huang, T.; Gu, X.; Wang, Z.; Liu, S.; Zhu, G. A bifunctional single-atom catalyst assisted by Fe2O3 for efficiently electrocatalytic perfluorooctanoic acid degradation by integrating reductive and oxidative processes. Chem. Eng. J. 2023, 470, 144270.
- Wang, Q.; Liu, M.; Zhao, H.; Chen, Y.; Xiao, F.; Chu, W.; Zhao, G. Efficiently degradation of perfluorooctanoic acid in synergic electrochemical process combining cathodic electro-Fenton and anodic oxidation. Chem. Eng. J. 2019, 378, 122071.
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144.
- Wang, C.; Zhang, T.; Yin, L.; Ni, C.; Ni, J.; Hou, L.-A. Enhanced perfluorooctane acid mineralization by electrochemical oxidation using Ti3+ self-doping TiO2 nanotube arrays anode. Chemosphere 2022, 286, 131804.
- Liu, X.; Duan, X.; Bao, T.; Hao, D.; Chen, Z.; Wei, W.; Wang, D.; Wang, S.; Ni, B.-J. High-performance photocatalytic decomposition of PFOA by BiOX/TiO2 heterojunctions: Self-induced inner electric fields and band alignment. J. Hazard. Mater. 2022, 430, 128195.
- Yao, X.; Zuo, J.; Wang, Y.-J.; Song, N.-N.; Li, H.-H.; Qiu, K. Enhanced Photocatalytic Degradation of Perfluorooctanoic Acid by Mesoporous Sb2O3/TiO2 Heterojunctions. Front. Chem. 2021, 9, 690520.
- Yang, Y.; Zheng, Z.; Yang, M.; Chen, J.; Li, C.; Zhang, C.; Zhang, X. In-situ fabrication of a spherical-shaped Zn-Al hydrotalcite with BiOCl and study on its enhanced photocatalytic mechanism for perfluorooctanoic acid removal performed with a response surface methodology. J. Hazard. Mater. 2020, 399, 123070.
- McQueen, A.D.; Tedrow, O.N.; Ballentine, M.L.; Kennedy, A.J. Demonstration of Photocatalytic Degradation of Per- and Polyfluoroalkyl Substances (PFAS) in Landfill Leachate Using 3D Printed TiO2 Composite Tiles. Water Air Soil Pollut. 2022, 233, 444.
- Huang, G.; Zhang, G.; Gao, Z.; Cao, J.; Li, D.; Yun, H.; Zeng, T. Enhanced visible-light-driven photocatalytic activity of BiFeO3 via electric-field control of spontaneous polarization. J. Alloys Compd. 2019, 783, 943–951.
- Wang, Y.; Wang, C.; Luo, P.; Hu, Q. Removal of perfluorooctanoic acid by MWCNT-modified carbon-doped titanium dioxide in a peroxymonosulfate/simulated sunlight system. Appl. Surf. Sci. 2023, 614, 156251.
- Zhuo, Q.; Li, X.; Yan, F.; Yang, B.; Deng, S.; Huang, J.; Yu, G. Electrochemical oxidation of 1H,1H,2H,2H-perfluorooctane sulfonic acid (6:2 FTS) on DSA electrode: Operating parameters and mechanism. J. Environ. Sci. 2014, 26, 1733–1739.




