Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marwyn Sowden | -- | 1159 | 2023-11-10 09:57:01 | | | |
| 2 | Jason Zhu | Meta information modification | 1159 | 2023-11-13 07:27:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sowden, M.; Van Niekerk, E.; Bulabula, A.N.H.; Van Weissenbruch, M.M. Development of the Microbiome in Preterm Neonates. Encyclopedia. Available online: https://encyclopedia.pub/entry/51413 (accessed on 25 January 2026).
Sowden M, Van Niekerk E, Bulabula ANH, Van Weissenbruch MM. Development of the Microbiome in Preterm Neonates. Encyclopedia. Available at: https://encyclopedia.pub/entry/51413. Accessed January 25, 2026.
Sowden, Marwyn, Evette Van Niekerk, Andre Nyandwe Hamama Bulabula, Mirjam Maria Van Weissenbruch. "Development of the Microbiome in Preterm Neonates" Encyclopedia, https://encyclopedia.pub/entry/51413 (accessed January 25, 2026).
Sowden, M., Van Niekerk, E., Bulabula, A.N.H., & Van Weissenbruch, M.M. (2023, November 10). Development of the Microbiome in Preterm Neonates. In Encyclopedia. https://encyclopedia.pub/entry/51413
Sowden, Marwyn, et al. "Development of the Microbiome in Preterm Neonates." Encyclopedia. Web. 10 November, 2023.
Copy Citation
Researchers have established that the preterm neonate is born with an immature gastrointestinal tract. The preterm neonate is thus susceptible to various complications often seen in the neonatal intensive care unit, e.g., feeding intolerances, necrotizing enterocolitis, and hospital-acquired bloodstream infections. These complications can be life-threatening, and if survived, can have an unfavorable effect on the neonate’s growth and development.
microbiome
preterm neonate
dysbiosis
1. Microbiome Development in Utero
Colonization of the developing GIT starts in utero as early as 10 weeks post-conception when amniotic fluid is swallowed [1][2][3][4][5]. Commensal bacteria are present in the amniotic fluid [6][7][8]. Microbes are also found in the placenta [1][6][9], fetal membranes, and umbilical cord blood [10]. Microbes have been detected in the meconium of neonates shortly after birth, even before the initiation of any feeds [6][7][11][12][13][14][15][16][17].
An in vitro model has described amniotic fluid as promoting fetal intestinal growth since amniotic fluid contains multiple trophic factors, e.g., growth factors, epidermal growth factor, and insulin-like growth factors (IGF)-1 and IGF-2 [18]. By the end of the last trimester, the fetus swallows an average of 450 mL of amniotic fluid per day [19], a crucial period that preterm neonates miss out on.
It is hypothesized that maternal intestinal antigen-presenting cells such as dendritic cells can sample and phagocytize maternal intestinal flora, carrying live commensal bacteria to the bloodstream and maternal organs. These cells can then be found in the maternal organs such as the placenta and mammary gland. In this manner, maternal intestinal flora components may be able to cross over the placenta and be ingested by the fetus and, later, excreted in the breastmilk and ingested by the neonate [20].
The maternal microbiome affects the health of the neonate, since the maternal microbiome, linked with perinatal factors, determines the neonatal microbiome [21]. Various maternal factors may play a role in fetal microbiome development, e.g., stress, infection, illness, smoking, maternal diet, and obesity [22][23]. With respect to infection, the human immunodeficiency virus (HIV) leads to microbiome changes in the HIV-exposed but uninfected neonate, compared to the HIV-unexposed, uninfected neonate [24]. In terms of maternal diet, a high fat intake during pregnancy can lead to a reduced relative abundance of Bacteroides; a Western diet high in refined carbohydrates, fat, and animal protein leads to increased Clostridium innocuum, Eubacterium dolichum, Catenibacterium mitsuokai, and Enterococcus spp., and a reduced abundance of Bifidobacteria and Bacteroidetes [21][25][26]. Further, the presence of gestational diabetes can lead to a reduced relative abundance of Prevotella and Lactobacillus [25][26]. Some medications used during pregnancy, such as maternal biologics (e.g., tumor necrosis factor-α inhibitors), can cross the placental barrier, especially in the late second and third trimesters. Owing to the neonate’s immature reticuloendothelial system at birth, the drug can take up to 12 months to clear. The use of these immunosuppressants can thus lead to changes in the neonate’s immune system [27][28].
2. Microbiome Development from Birth Onwards
At birth, a neonate’s gestational age has the largest influence on their microbiome diversity [15]. The presence of Enterobacter, Enterococcus, Lactobacillus, Photorhabdus, and Tannerella in the meconium of neonates born < 33 weeks’ gestation is negatively correlated with the neonate’s gestational age and has been reported to provoke inflammatory responses, signifying a causative role in premature births [15].
Furthermore, lower gestational ages have been associated with a lower abundance of Bifidobacterium, Bacteroides, and Streptococcus [29]. One study found that the colonization of Bifidobacteria in very low birth weight neonates is delayed, and they only appear at a mean age of 10.6 days (versus as early as 4 days in full-term neonates). Bifidobacteria only became predominant at a mean age of 19.8 days in their group of preterm neonates [30].
Birth mode and associated complications also play a role. Neonates born following the premature rupture of membranes or intra-amniotic infection (chorioamnionitis) experienced microbe exposure in utero, since bacteria colonize the amniotic membrane, fetal skin, and mucosa [31]. Further, caesarean sections are associated with pathobionts, e.g., Staphylococcus, Corynebacterium and Propionibacterium species, compared to vaginal deliveries, which are characterized by Lactobacillus, Bacteroides, and Bifidobacteria [32].
After delivery, rapid changes occur in the preterm intestinal microbiome in comparison with healthy, full-term neonates. As illustrated in Figure 1, environmental factors contribute to observed changes and include the NICU, with increased exposure to HA-BSI and reduced exposure to parental bacteria, owing to delayed skin-to-skin contact in comparison with healthy full-term neonates [33]. Hospitalized neonates have higher C. Difficile colonization rates. Brookes et al. showed that organisms present in the early phase of colonization of the GIT have reservoirs in the NICU [34].
The feeding journey of the preterm neonate is a challenge owing to factors such as a low gastric capacity, slow initiation of enteral feeds, and low maternal breastmilk availability at birth. For these reasons, many neonates receive TPN, leading to a loss of biodiversity and an altered gut microbial colonization [11][19][35].
The type of enteral feed also influences microbiome development. When comparing breastmilk, pasteurized donor breastmilk, and infant formula, the microbial content is distinctly different [36]. Breastmilk contains a high concentration of complex human milk oligosaccharides (HMOs). After lactose and lipids, HMOs are the third most abundant component of breastmilk. HMOs exert a powerful prebiotic effect: they are resistant to the gastric pH and can reach the neonate’s large intestine intact, where they modulate the composition of the gastrointestinal microbiome and act as a carbon source for the GIT microbiota [37][38]. HMOs have anti-inflammatory properties by regulating the production of interleukin, activating lymphocytes, and blocking the adhesion of microbial pathogens to the large intestine’s epithelial surface [37][39]. Breastmilk has its own core microbiome (Streptococci, Lactic acid bacteria, and Bifidobacteria) [40]. Apart from HMOs, breastmilk also contains lysosomes, lactoferrin, antibodies, and cytokines that stimulate the increase of Bacteroides, Bifidobacterium, and Lactobacillus spp. [5][36][37][38][40]. Moreover, breastmilk contains the peptide hormone insulin, leading to an increase in intestinal maturation [41]. Neonates who receive breastmilk have a lower microbiome diversity, but almost double the abundance of beneficial bacterial cells compared with neonates who receive infant formula [22]. Formula-fed neonates have a more diverse microbiome (Coliforms, Bacteroides, Clostridium difficile, and Lactobacilli) [42][43][44].
Almost all premature neonates receive antibiotics, either before, during, or after birth. Worldwide, an estimated 40% of pregnant mothers and neonates receive antibiotics for the prevention and control of infections [45]. Intrapartum antibiotic prophylaxis leads to decreased Bifidobacterial numbers directly after birth [40]. Empirical antibiotic therapy is often initiated in preterm neonates [45][46][47]. Antibiotic use early in life is detrimental to microbiome progression. It affects the timing of the progression of the microbiome, as well as the organisms present [24][29][48]. Administering antibiotics at an early age, when the immune system is still maturing, increases the risk of developing HA-BSI and NEC [49][50][51].
While it was previously believed that genetics influence the microbiome, new studies in twins refute this, indicating the importance (or influence) of environmental factors [40]. Figure 1 provides a visual overview of various factors that play a role in the development of the microbiome in the preterm neonate. The various factors will be described in the section below, from birth and onwards.
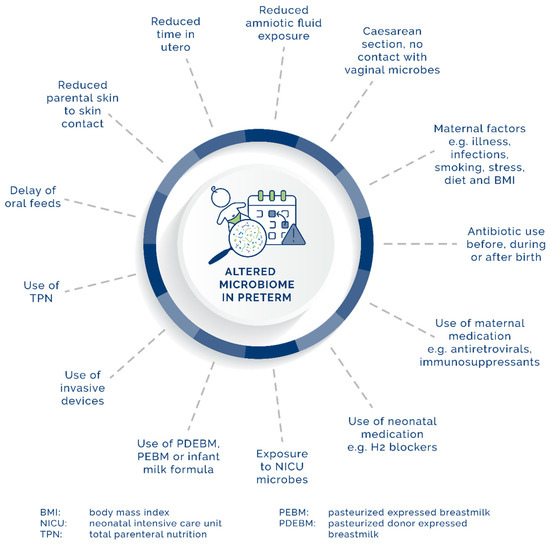

Figure 1. Maternal, neonatal, and environmental factors affecting the development of the GIT microbiome in preterm neonates.
References
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci Transl. Med. 2014, 6, 237ra65.
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 2018, 10, 274.
- Hitti, J.; Riley, D.E.; Krohn, M.A.; Hillier, S.L.; Agnew, K.J.; Krieger, J.N.; Eschenbach, D.A. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin. Infect. Dis. 1997, 24, 1228–1232.
- Pettker, C.M.; Buhimschi, I.A.; Magloire, L.K.; Sfakianaki, A.K.; Hamar, B.D.; Cuhimschi, C.S. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet. Gynecol. 2007, 109, 739–749.
- Thum, C.; Cookson, A.L.; Otter, D.E.; McNabb, W.C.; Hodgkinson, A.J.; Dyer, J.; Roy, N.C. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J. Nutr. 2012, 142, 1921–1928.
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129.
- Liu, C.J.; Liang, X.; Niu, Z.Y.; Jin, Q.; Zeng, X.Q.; Wang, W.X.; Li, M.Y.; Chen, X.R.; Meng, H.Y.; Shen, R.; et al. Is the delivery mode a critical factor for the microbial communities in the meconium? EBioMedicine 2019, 49, 354–363.
- He, Q.; Kwok, L.Y.; Xi, X.; Zhong, Z.; Ma, T.; Xu, H.; Meng, H.; Zhao, F.; Zhang, H. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 2020, 12, 1794266.
- Satokari, R.; Grönroos, T.; Laitinen, K.; Salminen, S.; Isolauri, E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett. Appl. Microbiol. 2009, 48, 8–12.
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martin, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguex, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274.
- Dahlgren, A.F.; Pan, A.; Lam, V.; Gouthro, K.C.; Simpson, P.M.; Salzman, N.H.; Nghiem-Rao, T.H. Longitudinal changes in the gut microbiome of infants on total parenteral nutrition. Pediatr. Res. 2019, 86, 107–114.
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, D.; Li, N.; Mai, V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J. Pediatr. 2010, 156, 20–25.
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975.
- Hansen, R.; Scott, K.P.; Khan, S.; Martin, J.C.; Berry, S.H.; Stevenson, M.; Okpapi, A.; Munro, M.J.; Hold, G.L. First-Pass Meconium samples from healthy term vaginally-delivered neonates: An analysis of the microbiota. PLoS ONE 2015, 10, e0133320.
- Ardissone, A.N.; de la Cruz, D.M.; Davis-Richardson, A.G.; Rechcigl, K.T.; Li, N.; Drew, J.C.; Murgas-Torrazza, R.; Sharma, R.; Hudak, M.L.; Triplett, E.W.; et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE 2014, 9, e90784.
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The not-so-sterile womb: Evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 2019, 10, 1124.
- Gosalbes, M.J.; Llop, S.; Vallès, Y.; Moya, A.; Ballester, F.; Francino, M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 2013, 43, 198–211.
- Hirai, C.; Ichiba, H.; Saito, M.; Shintaku, H.; Yamano, T.; Kusuda, S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 524–528.
- Neu, J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am. J. Clin. Nutr. 2007, 85, 629S–634S.
- Elgin, T.G.; Kern, S.L.; McElroy, S.J. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin. Ther. 2016, 38, 706–715.
- Mack, D.R. Probiotics-mixed messages. Can. Fam. Physician 2005, 51, 1455–1457, 1462–1464.
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38.
- Pravia, C.I.; Benny, M. Long-term consequences of prematurity. Cleve Clin. J. Med. 2020, 87, 759–767.
- Bender, J.M.; Li, F.; Martelly, S.; Byrt, E.; Rouzier, V.; Leo, M.; Tobin, N.; Pannaraj, P.S.; Adisetiyo, H.; Rollie, A.; et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci. Transl. Med. 2016, 8, 349ra100.
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77.
- Su, M.; Nie, Y.; Shao, R.; Duan, S.; Jiang, Y.; Wang, M.; Xing, Z.; Sun, Q.; Liu, X.; Xu, W. Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS ONE 2018, 13, e0205695.
- Bortlik, M.; Machkova, N.; Duricova, D.; Malickova, K.; Hrdlicka, L.; Lukas, M.; Kohout, P.; Shonova, O.; Lukas, M. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-α therapy during pregnancy: Three-center study. Scand. J. Gastroenterol. 2013, 48, 951–958.
- Dai, F.F.; Hu, M.; Zhang, Y.W.; Zhu, R.H.; Chen, L.P.; Li, Z.D.; Haung, Y.J.; Hu, W.; Cheng, Y.X. TNF-α/anti-TNF-α drugs and its effect on pregnancy outcomes. Expert Rev. Mol. Med. 2022, 24, e26.
- Chernikova, D.A.; Madan, J.C.; Housman, M.L.; Zain-Ul-Abideen, M.; Lundgren, S.N.; Morrison, H.G.; Sogin, M.L.; Williams, S.M.; Moore, J.H.; Karagas, M.R.; et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr. Res. 2018, 84, 71–79.
- Sakata, H.; Yoshioka, H.; Fujita, K. Development of the intestinal flora in very low birth weight infants compared to normal full-term newborns. Eur. J. Pediatr. 1985, 144, 186–190.
- Tchirikov, M.; Schlabritz-Loutsevitch, N.; Maher, J.; Buchmann, J.; Naberezhnev, Y.; Winarno, A.S.; Seliger, G. Mid-trimester preterm premature rupture of membranes (PPROM): Etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 2018, 46, 465–488.
- Clarke, G.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Priming for health: Gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014, 103, 812–819.
- Ahearn-Ford, S.; Berrington, J.E.; Stewart, C.J. Development of the gut microbiome in early life. Exp. Physiol. 2022, 107, 415–421.
- Brooks, B.; Firek, B.A.; Miller, C.S.; Sharon, I.; Thomas, B.C.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2014, 2, 1.
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123.
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039.
- Singh, R.P.; Niharika, J.; Kondepudi, K.K.; Bishnoi, M.; Tingirikari, J.M.R. Recent understanding of human milk oligosaccharides in establishing infant gut microbiome and roles in immune system. Food Res. Int. 2022, 151, 110884.
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99.
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4653–4658.
- Bertelsen, R.J.; Jensen, E.T.; Ringel-Kulka, T. Use of probiotics and prebiotics in infant feeding. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 39–48.
- Mank, E.; Sáenz de Pipaón, M.; Lapillonne, A.; Carnielli, V.P.; Senterre, T.; Shamir, R.; van Toledo, L.; van Goudoever, J.B.; FIT-04 Study Group. Efficacy and safety of enteral recombinant human insulin in preterm infants: A randomized clinical trial. JAMA Pediatr. 2022, 176, 452–460.
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—Intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438.
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521.
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494.
- Shekhar, S.; Petersen, F.C. The dark side of antibiotics: Adverse effects on the infant immune defense against infection. Front. Pediatr. 2020, 8, 544460.
- Mukhopadhyay, S.; Sengupta, S.; Puopolo, K.M. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F327–F332.
- Cotten, C.M. Adverse consequences of neonatal antibiotic exposure. Curr. Opin. Pediatr. 2016, 28, 141–149.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Mai, V.; Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Li, N.; Shuster, J.; Sharma, R.; Hudak, M.L.; Neu, J. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 2013, 8, e52876.
- Raba, A.A.; O’Sullivan, A.; Semberova, J.; Martin, A. Are antibiotics a risk factor for the development of necrotizing enterocolitis-case-control retrospective study. Eur. J. Pediatr. 2019, 178, 923–928.
- Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sharma, R.; Hudak, M.L.; Neu, J.; Mai, V. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS ONE 2013, 8, e83304.
More
Information
Subjects:
Pediatrics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
442
Revisions:
2 times
(View History)
Update Date:
13 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




