Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marwyn Sowden and Version 2 by Jason Zhu.
Researchers have established that the preterm neonate is born with an immature gastrointestinal tract. The preterm neonate is thus susceptible to various complications often seen in the neonatal intensive care unit, e.g., feeding intolerances, necrotizing enterocolitis, and hospital-acquired bloodstream infections. These complications can be life-threatening, and if survived, can have an unfavorable effect on the neonate’s growth and development.
- microbiome
- preterm neonate
- dysbiosis
1. Microbiome Development in Utero
Colonization of the developing GIT starts in utero as early as 10 weeks post-conception when amniotic fluid is swallowed [1][2][3][4][5][22,23,24,25,26]. Commensal bacteria are present in the amniotic fluid [6][7][8][27,28,29]. Microbes are also found in the placenta [1][6][9][22,27,30], fetal membranes, and umbilical cord blood [10][31]. Microbes have been detected in the meconium of neonates shortly after birth, even before the initiation of any feeds [6][7][11][12][13][14][15][16][17][27,28,32,33,34,35,36,37,38].
An in vitro model has described amniotic fluid as promoting fetal intestinal growth since amniotic fluid contains multiple trophic factors, e.g., growth factors, epidermal growth factor, and insulin-like growth factors (IGF)-1 and IGF-2 [18][39]. By the end of the last trimester, the fetus swallows an average of 450 mL of amniotic fluid per day [19][2], a crucial period that preterm neonates miss out on.
It is hypothesized that maternal intestinal antigen-presenting cells such as dendritic cells can sample and phagocytize maternal intestinal flora, carrying live commensal bacteria to the bloodstream and maternal organs. These cells can then be found in the maternal organs such as the placenta and mammary gland. In this manner, maternal intestinal flora components may be able to cross over the placenta and be ingested by the fetus and, later, excreted in the breastmilk and ingested by the neonate [20][40].
The maternal microbiome affects the health of the neonate, since the maternal microbiome, linked with perinatal factors, determines the neonatal microbiome [21][41]. Various maternal factors may play a role in fetal microbiome development, e.g., stress, infection, illness, smoking, maternal diet, and obesity [22][23][42,43]. With respect to infection, the human immunodeficiency virus (HIV) leads to microbiome changes in the HIV-exposed but uninfected neonate, compared to the HIV-unexposed, uninfected neonate [24][44]. In terms of maternal diet, a high fat intake during pregnancy can lead to a reduced relative abundance of Bacteroides; a Western diet high in refined carbohydrates, fat, and animal protein leads to increased Clostridium innocuum, Eubacterium dolichum, Catenibacterium mitsuokai, and Enterococcus spp., and a reduced abundance of Bifidobacteria and Bacteroidetes [21][25][26][41,45,46]. Further, the presence of gestational diabetes can lead to a reduced relative abundance of Prevotella and Lactobacillus [25][26][45,46]. Some medications used during pregnancy, such as maternal biologics (e.g., tumor necrosis factor-α inhibitors), can cross the placental barrier, especially in the late second and third trimesters. Owing to the neonate’s immature reticuloendothelial system at birth, the drug can take up to 12 months to clear. The use of these immunosuppressants can thus lead to changes in the neonate’s immune system [27][28][47,48].
2. Microbiome Development from Birth Onwards
At birth, a neonate’s gestational age has the largest influence on their microbiome diversity [15][36]. The presence of Enterobacter, Enterococcus, Lactobacillus, Photorhabdus, and Tannerella in the meconium of neonates born < 33 weeks’ gestation is negatively correlated with the neonate’s gestational age and has been reported to provoke inflammatory responses, signifying a causative role in premature births [15][36].
Furthermore, lower gestational ages have been associated with a lower abundance of Bifidobacterium, Bacteroides, and Streptococcus [29][6]. One study found that the colonization of Bifidobacteria in very low birth weight neonates is delayed, and they only appear at a mean age of 10.6 days (versus as early as 4 days in full-term neonates). Bifidobacteria only became predominant at a mean age of 19.8 days in their group of preterm neonates [30][49].
Birth mode and associated complications also play a role. Neonates born following the premature rupture of membranes or intra-amniotic infection (chorioamnionitis) experienced microbe exposure in utero, since bacteria colonize the amniotic membrane, fetal skin, and mucosa [31][50]. Further, caesarean sections are associated with pathobionts, e.g., Staphylococcus, Corynebacterium and Propionibacterium species, compared to vaginal deliveries, which are characterized by Lactobacillus, Bacteroides, and Bifidobacteria [32][51].
After delivery, rapid changes occur in the preterm intestinal microbiome in comparison with healthy, full-term neonates. As illustrated in Figure 1, environmental factors contribute to observed changes and include the NICU, with increased exposure to HA-BSI and reduced exposure to parental bacteria, owing to delayed skin-to-skin contact in comparison with healthy full-term neonates [33][19]. Hospitalized neonates have higher C. Difficile colonization rates. Brookes et al. showed that organisms present in the early phase of colonization of the GIT have reservoirs in the NICU [34][52].
The feeding journey of the preterm neonate is a challenge owing to factors such as a low gastric capacity, slow initiation of enteral feeds, and low maternal breastmilk availability at birth. For these reasons, many neonates receive TPN, leading to a loss of biodiversity and an altered gut microbial colonization [11][19][35][2,5,32].
The type of enteral feed also influences microbiome development. When comparing breastmilk, pasteurized donor breastmilk, and infant formula, the microbial content is distinctly different [36][53]. Breastmilk contains a high concentration of complex human milk oligosaccharides (HMOs). After lactose and lipids, HMOs are the third most abundant component of breastmilk. HMOs exert a powerful prebiotic effect: they are resistant to the gastric pH and can reach the neonate’s large intestine intact, where they modulate the composition of the gastrointestinal microbiome and act as a carbon source for the GIT microbiota [37][38][54,55]. HMOs have anti-inflammatory properties by regulating the production of interleukin, activating lymphocytes, and blocking the adhesion of microbial pathogens to the large intestine’s epithelial surface [37][39][54,56]. Breastmilk has its own core microbiome (Streptococci, Lactic acid bacteria, and Bifidobacteria) [40][18]. Apart from HMOs, breastmilk also contains lysosomes, lactoferrin, antibodies, and cytokines that stimulate the increase of Bacteroides, Bifidobacterium, and Lactobacillus spp. [5][36][37][38][40][18,26,53,54,55]. Moreover, breastmilk contains the peptide hormone insulin, leading to an increase in intestinal maturation [41][57]. Neonates who receive breastmilk have a lower microbiome diversity, but almost double the abundance of beneficial bacterial cells compared with neonates who receive infant formula [22][42]. Formula-fed neonates have a more diverse microbiome (Coliforms, Bacteroides, Clostridium difficile, and Lactobacilli) [42][43][44][58,59,60].
Almost all premature neonates receive antibiotics, either before, during, or after birth. Worldwide, an estimated 40% of pregnant mothers and neonates receive antibiotics for the prevention and control of infections [45][61]. Intrapartum antibiotic prophylaxis leads to decreased Bifidobacterial numbers directly after birth [40][18]. Empirical antibiotic therapy is often initiated in preterm neonates [45][46][47][61,62,63]. Antibiotic use early in life is detrimental to microbiome progression. It affects the timing of the progression of the microbiome, as well as the organisms present [24][29][48][6,44,64]. Administering antibiotics at an early age, when the immune system is still maturing, increases the risk of developing HA-BSI and NEC [49][50][51][65,66,67].
While it was previously believed that genetics influence the microbiome, new studies in twins refute this, indicating the importance (or influence) of environmental factors [40][18]. Figure 1 provides a visual overview of various factors that play a role in the development of the microbiome in the preterm neonate. The various factors will be described in the section below, from birth and onwards.
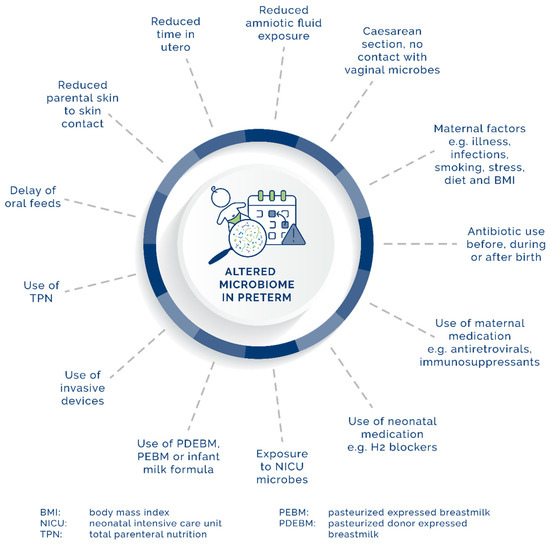

Figure 1.
Maternal, neonatal, and environmental factors affecting the development of the GIT microbiome in preterm neonates.
