Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Iulia Gabriela David | -- | 1819 | 2023-11-10 05:53:55 | | | |
| 2 | Catherine Yang | Meta information modification | 1819 | 2023-11-10 06:47:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
David, I.G.; Iorgulescu, E.E.; Popa, D.E.; Buleandra, M.; Cheregi, M.C.; Noor, H. Curcumin Electrochemistry. Encyclopedia. Available online: https://encyclopedia.pub/entry/51397 (accessed on 07 March 2026).
David IG, Iorgulescu EE, Popa DE, Buleandra M, Cheregi MC, Noor H. Curcumin Electrochemistry. Encyclopedia. Available at: https://encyclopedia.pub/entry/51397. Accessed March 07, 2026.
David, Iulia Gabriela, Emilia Elena Iorgulescu, Dana Elena Popa, Mihaela Buleandra, Mihaela Carmen Cheregi, Hassan Noor. "Curcumin Electrochemistry" Encyclopedia, https://encyclopedia.pub/entry/51397 (accessed March 07, 2026).
David, I.G., Iorgulescu, E.E., Popa, D.E., Buleandra, M., Cheregi, M.C., & Noor, H. (2023, November 10). Curcumin Electrochemistry. In Encyclopedia. https://encyclopedia.pub/entry/51397
David, Iulia Gabriela, et al. "Curcumin Electrochemistry." Encyclopedia. Web. 10 November, 2023.
Copy Citation
Curcumin (CU) is a polyphenolic compound extracted from turmeric, a well-known dietary spice. Since it has been shown that CU exerts beneficial effects on human health, interest has increased in its use but also in its analysis in different matrices. CU has an antioxidant character and is electroactive due to the presence of phenolic groups in its molecule.
curcumin
voltammetry
electrochemistry
antioxidant
1. Curcumin—History and Occurrence
Turmeric was used 4000 years ago in cuisine and traditional medicine in India and China [1], but curcumin (CU) as its main component was discovered in turmeric only in 1815 and obtained as a pure compound in 1842. Its chemical structure and its synthesis were reported in 1910 and 1913, respectively [2]. Nowadays, it is mainly cultivated in India and China, but also in other tropical regions from South Asia, Africa, South America [3], and the Pacific basin [4].
CU [(1E,6E)−1,7−bis(4−hydroxy−3-methoxyphenyl)hepta−1,6−diene−3,5−dione] or diferuloylmethane [5] (Figure 1) is a solid polyphenolic antioxidant with bitter taste [6], extracted from the rhizome of the perennial herb turmeric (Curcuma longa Linnaeus), but also from other plants of the ginger (Zingiberaceae) family, where it coexists with structurally related species, known under the collective name of curcuminoids [5]. The main curcuminoids are CU, demethoxycurcumin (DMCU), and bis-demethoxycurcumin (BDMCU) (Figure 1). They differ by the number of methoxy groups on the aromatic nuclei. All of them are bioactive, possessing antioxidant properties, but, among them, CU is the most effective, having higher antioxidant power than vitamins C and E [3].
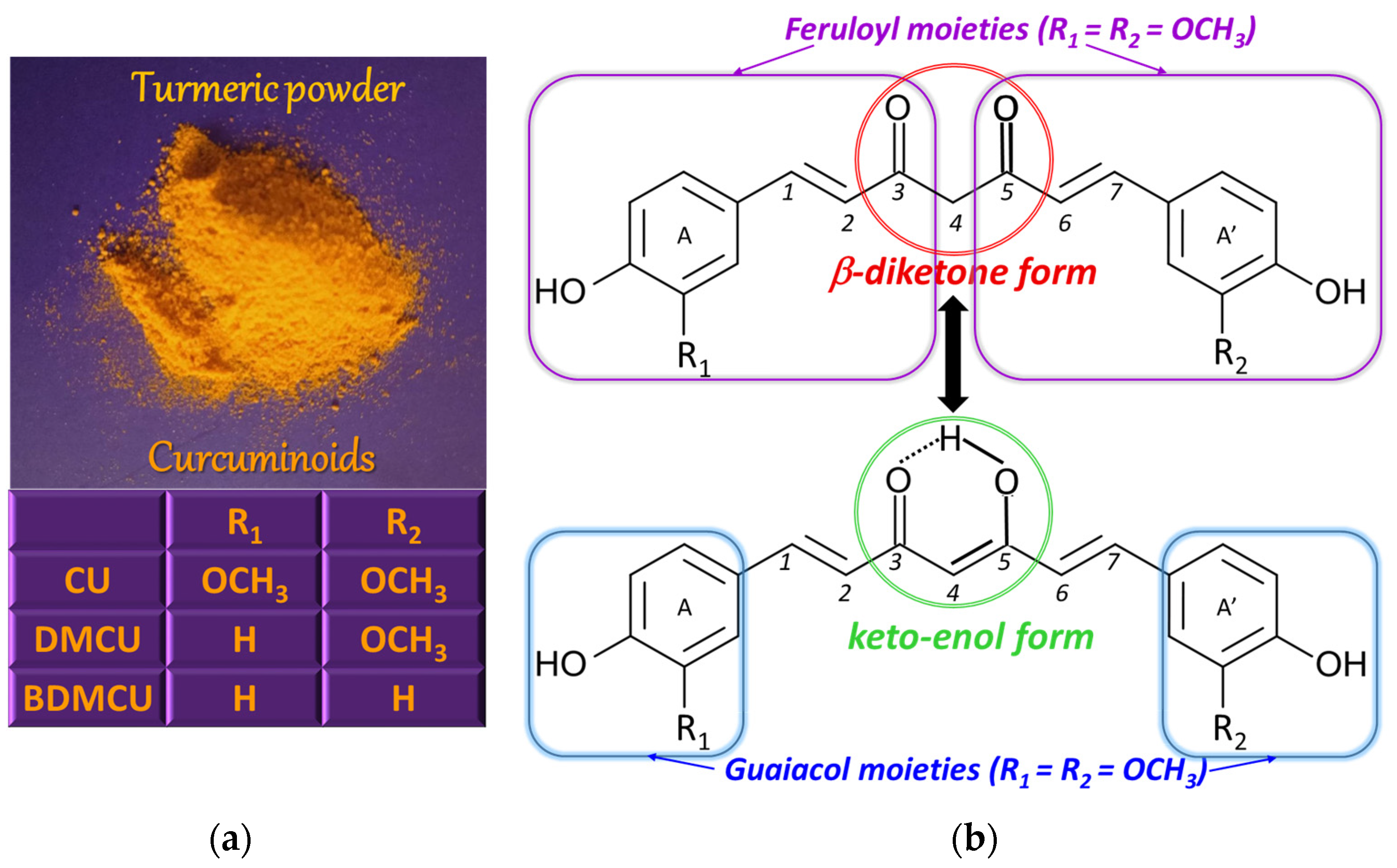
Figure 1. Curcuminoids found in turmeric (a) and their tautomeric equilibrium (b).
The amount of curcuminoids (3–5%) [7]) in turmeric roots depends on the growing conditions, including soil type and climate [5], while the CU content of commercially available turmeric powder varies in the range 0.5–5.7%, also being influenced by the harvesting, extraction, and processing procedures [8]. Despite the fact that the literature reported somewhat different proportions of CU, DMCU, and BMCU, namely approximately 2:1:1 [8], 75%, 25%, and 5% [9] and 70%, 20%, and 10% [7], respectively, the major component of turmeric rhizome is CU.
2. Curcumin—Chemical Structure and Properties
From the chemical point of view, the CU molecule is composed of two ferulic acid molecules (feruloyl moieties) linked via a methylene group [10]. Going into more detail, the CU structure consists of two ortho-methoxy phenolic (guaiacol) groups bridged by a seven carbon atoms chain that contains an α,β-unsaturated β-diketone moiety, which determines the keto-enol tautomerism (Figure 1) [8], the enol proton being evenly distributed between the two oxygen atoms due to the symmetry of the CU molecule [11]. The ratio of the two tautomeric forms in solution depends on the solvent and its polarity, the solution pH, and temperature [7]. The keto form exists in acidic and neutral solutions (about 70%) [12] and in cell membranes, while the enol form is preponderant in alkaline media [9], in ethanol [8], in nonpolar organic solvents, and in solid phase, being stabilized by hydrogen bonds [10]. The CU biological properties are due to the guaiacol moieties as well as the keto-enol site [11].
CU undergoes three acid–base equilibria, the first one with pKa1 values reported in the range 7.43–8.55 and involving the transfer of the proton from the enol group. The next dissociation steps, attributed to the deprotonation of the two phenolic –OH moieties, have relatively close pKa values (pKa2 in the range 8.55–10.45 and pKa3 varying between 9.05 and 10.95) due to the symmetric position of the corresponding protogenic groups in the CU molecule [13].
Initial studies reported that CU is stable in solutions with pH values below 7.00, its dissociation equilibrium being shifted towards the neutral form, with poor aqueous solubility when the environment becomes more acidic. CU instability in alkaline media was explained by its hydrolytic degradation [14]. After CV and spectrophotometric investigations of CU behavior in time at different pH values, Martínez-Guerra et al. [13] concluded that CU degradation is 20 times faster in acidic media than in neutral or basic solutions, but CU stability in aqueous environments can be improved by deaerating and protecting the solution from light.
CU is practically insoluble in acidic and neutral aqueous solutions and poorly soluble in hydrocarbon solvents [15], but it is soluble in alkali [14], in lipids [10], and in organic solvents like acetic acid, ethanol [7], methanol, DMSO [12], acetone, and dichloromethane, its extraction in acetone being more efficient than in ethanol [16].
3. Curcumin—Uses
CU popularity increased remarkably worldwide in recent years as a consequence of its nutritional, prophylactic, and therapeutic values. Due to its orange–yellow color, it found applications as a natural coloring agent for food (mustard, margarine, processed cheese [17], pastries, canned products, and beverages [18]), cosmetics, hair dyes [19], textiles, furniture, and lacquers [20]. CU can also legally be added to food [21] as a preservative, spice, flavoring agent [22], and antioxidant in dairy products, meat, and seafood (fish, shrimp) [7]. The CU content of foods varies between 5 mg/kg and 500 mg/kg [23]. Moreover, on the market, there is a large variety of nutraceuticals and dietary supplements containing turmeric or its most bioactive component, CU, which are consumed on a large scale by the population [10]. A daily dose of 12,000 mg CU, which corresponds to a concentration of 51.2 ng/mL in human serum [23], presented little to no side effects, and, therefore, the FDA classified it as GRAS [24], the daily consumption level approved by the WHO being 1–3 mg/kg body weight [4][6]. At higher concentrations and prolonged administration, CU can inhibit the activity of some enzymes and cause anemia in persons with reduced iron uptake or various gastrointestinal [25], liver, inflammatory, and anticoagulation [12] problems.
Photoactivated CU encapsulated in β-CD was used for the antibacterial treatment of berries, increasing their shelf life without changing their organoleptic properties [26], while recent studies emphasized that CU-NPs, even at low concentrations, improved soybean growth and could be employed as fertilizer [27]. CU loaded into a zein/shellac-based composite food packaging film conferred antioxidant properties, inhibited E. coli, and the color change with pH variation enabled the monitoring of the food freshness [28]. There are many such applications of CU in the development of bioactive thin-layer composite polymeric food packaging films, and they were recently reviewed by Roy et al. [7].
CU also has beneficial effects on the growth of chickens and their egg production, curcuma being used as a feed additive in the broiler poultry industry [29].
Due to its lipophilic character, CU can bind to the amyloid β-oligomers that generate brain dysfunctions [30], and, therefore, it presents a dose-dependent enhancement of learning ability and memory by leading to beneficial results in the treatment of Alzheimer’s disease, both alone or in combination with coenzyme Q10 [31] or in the combined treatment of Fabry disease [24]. It has cardioprotective effects [32], reduces inflammation in patients with chronic renal failure [33] or in patients recovered from COVID-19 [34], may prevent and treat liver injury caused by aflatoxin B1 [35] or its age-related senescence [36], has therapeutic effects on hyperglycemia, oxidative stress, kidney, and nonalcoholic fatty liver diseases induced by high fat diet [37][38], reduces muscle damage and inflammation and improves sport performances [39], can protect human or animal muscles from degeneration [40][41], the lungs against air-pollution-induced inflammation [42], and the skin against UV radiation, having antimelanogenic [43] and wound-healing properties [44], also being employed as an active ingredient in cosmetic products [45]. Due to its antioxidant and metal-chelating properties, CU could be a treatment for metal poisoning [10]. It was shown that CU at concentrations of 5.00 × 10−6–5.00 × 10−5 mol/L at the cancer cell level [46] has antitumoral effects [5][47]; for example, it inhibits the proliferation of breast cancer cells [48] and exerts a dose-dependent reduction in the growth and progression of adrenocortical carcinoma [49], prostate cancer [50], rhabdomyosarcoma [51], colorectal [52][53], and bladder tumoral cells [54]. Moreover, at high concentrations, it has pro-oxidant properties, generating intracellular ROS that induced apoptosis of human lung cancer cells resistant to docetaxel and vincristine [55]. Depending on its concentration, CU exerts an anti- or pro-oxidant effect on DNA [14][56]. Among the CU pro-health activities are also the antibacterial effect discovered in 1949 [12][15], antiviral (against HIV, HPV, hepatitis virus, etc.) [57], and immunomodulatory and potentially antiallergic ones [1].
Despite its many health benefits, which were also discussed in different sections of some reviews [10][12][58][59], CU therapeutic use is limited by its reduced bioavailability generated by its low aqueous solubility, poor intestinal absorption, rapid metabolism and excretion from the body, 75% of the administrated CU dose being found in animal feces [10]. The highest CU concentration is in the intestine, while, in plasma or other tissues, it can be smaller than the quantification limits of the commonly applied analytical methods. In plasma, CU could be detected only after oral administration of high doses (at g levels), the maximum plasmatic level being reached 1–2 h after ingestion [58]. That is why researchers are continuously concerned to enhance the stability and bioavailability of CU in various ways (Figure 2), some of them being summarized in different review papers [9][10][12][15][58][60][61][62].
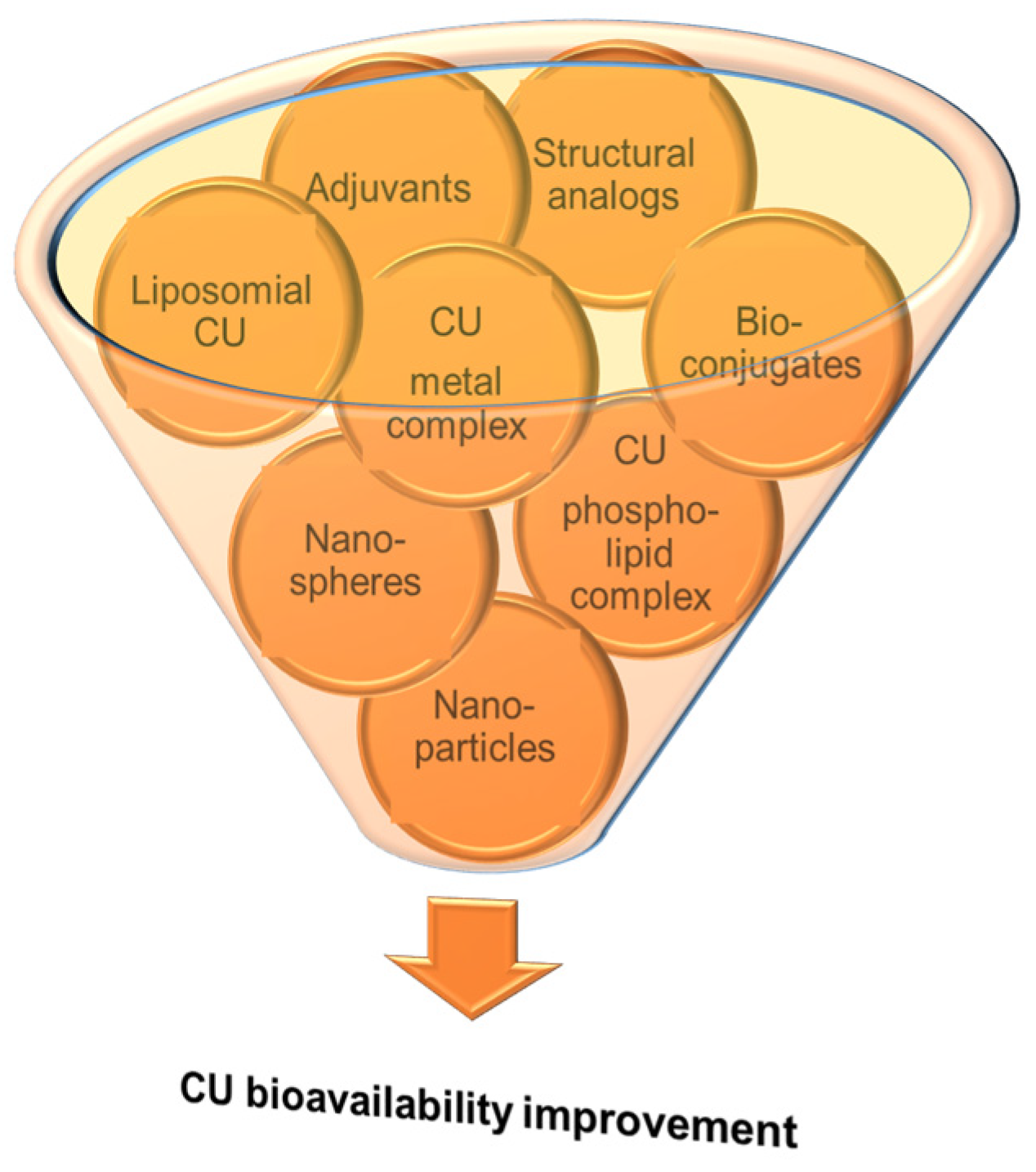
Figure 2. Different methodologies used to enhance the bioavailability of curcumin (adapted after [10]).
There are also reports presenting an interesting application of CU and its derivatives as a corrosion inhibitor [63][64].
The multiple different applications of CU, generated by its various beneficial functional properties, have increased its demand worldwide in recent years, and this trend continues such that the global CU market was estimated to be USD 104.19 million in 2025 [7]. However, CU’s main use still remains in the food and dietary supplements industry.
Despite the fact that CU ingestion presents a high degree of safety for animals, considering its concentration-dependent anti- and pro-oxidant activity towards DNA and also other possible side effects that could appear after the administration of high CU doses, it is important to have simple and rapid methods for its sensitive and selective quantification in foods and dietary supplements, as well as in biological samples. The recent literature includes reports related to the spectrometric [65][66][67][68], fluorimetric [57][69][70][71], chromatographic [72][73][74][75], and electrochemical [19][23][76][77][78] analysis of CU. Analytical methods applied to curcuminoids assessment in turmeric, including CU, were reviewed in 2019 by Kotra et al. [79], with special emphasis on the chromatographic and spectrometric ones, electrochemical methods being very briefly mentioned. A few examples of voltammetric determination of CU in spices were discussed in 2018 by Ziyatidinova and Budnikov [80] in a review paper related to the analytical chemistry of spice antioxidants. The examples of CU electrochemical detection presented in 2019 by Mohajeri et al. [81] in a synthesizing article that addressed the interaction between CU and carbon-based nanomaterials were limited only to the biosensors using this type of sensing material. In a recent review on the CU extraction and analysis procedures, electrochemical methods and sensors are mentioned [12]. Each of the analytical methods have certain advantages and drawbacks, but the electrochemical ones are simpler, user-friendly, more rapid, involve fewer reagents (thus being eco-friendly), and, most importantly, voltammetry allows the explanation of certain reaction mechanisms that are the basis of some biological activities, such as antioxidant activity.
Therefore, based on the data published in the specialized literature in the last 20 years, this review discusses in detail the role of the electrochemical sensors and methods in investigating diverse aspects of CU analysis, including not only its quantification in various matrices but also its redox behavior and some of its biological activities, especially the antioxidant and antitumoral ones. In addition, CU interaction with various chemical species and its application in the development of electrochemical sensors for the assessment of different analytes were addressed.
References
- Lis, K.; Bartuzi, Z. Plant Food Dyes with Antioxidant Properties and Allergies—Friend or Enemy? Antioxidants 2023, 12, 1357.
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299.
- Rahimnejad, M.; Zokhtareh, R.; Moghadamnia, A.A.; Asghary, M. An Electrochemical Sensor Based on Reduced Graphene Oxide Modified Carbon Paste Electrode for Curcumin Determination in Human Blood Serum. Port. Electrochim. Acta 2020, 38, 29–42.
- Sun, X.; Follett, P.A.; Wall, M.M.; Duff, K.S.; Wu, X.; Shu, C.; Plotto, A.; Liang, P.; Stockton, D.G. Physical, Chemical, and Sensory Properties of a Turmeric-Fortified Pineapple Juice Beverage. Foods 2023, 12, 2323.
- Mishra, A.P.; Swetanshu; Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the Dietary Phytochemical Curcumin in Targeting Cancer Cell Signalling Pathways. Plants 2023, 12, 1782.
- Burç, M.; Gungor, O.; Duran, S.T. Voltammetric Determination of Curcumin in Spices using Platinum Electrode Electrochemically Modified with Poly (Vanillin-co-Caffeic Acid). Anal. Bioanal. Chem. 2020, 12, 625–643.
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its Uses in Active and Smart Food Packaging Applications—A Comprehensive Review. Food Chem. 2022, 375, 131885.
- Chaisiwamongkhol, K.; Ngamchuea, K.; Batchelor-McAuley, C.; Compton, R.G. Multiwalled Carbon Nanotube Modified Electrodes for the Adsorptive Stripping Voltammetric Determination and Quantification of Curcumin in Turmeric. Electroanalysis 2017, 29, 1049–1055.
- Stanić, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12.
- Smirnova, E.; Moniruzzaman, M.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Do, K.; Min, T. A Review of the Role of Curcumin in Metal Induced Toxicity. Antioxidants 2023, 12, 243.
- Serpi, C.; Stanić, Z.; Girousi, S. Electroanalytical Study of the Interaction Between dsDNA and Curcumin in the Presence of Copper(II). Talanta 2010, 81, 1731–1734.
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874.
- Martínez-Guerra, J.; Palomar-Pardavé, M.; Romero-Romo, M.; Corona-Avendaño, S.; Rojas-Hernández, A.; Ramírez-Silva, M.T. New Insights on the Chemical Stability of Curcumin in Aqueous Media at Different pH: Influence of the Experimental Conditions. Int. J. Electrochem. Sci. 2019, 14, 5373–5385.
- Jain, R.; Haque, A.; Verma, A. Voltammetric Quantification of Surfactant Stabilized Curcumin at MWCNT/GCE Sensor. J. Mol. Liq. 2017, 230, 600–607.
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336.
- Basmaz, G.; Öztürk, N. Determination of Curcumin in Turmeric Sample Using Edge Plane Pyrolytic Graphite Electrode. Celal Bayar Univ. J. Sci. 2017, 13, 689–694.
- Daneshgar, P.; Norouzi, P.; Moosavi-Movahedi, A.A.; Ganjali, M.R.; Haghshenas, E.; Dousty, F.; Farhadi, M. Fabrication of Carbon Nanotube and Dysprosium Nanowire Modified Electrodes as a Sensor for Determination of Curcumin. J. Appl. Electrochem. 2009, 39, 1983–1992.
- Raril, C.; Manjunatha, J.G.; Tigari, G. Low-Cost Voltammetric Sensor Based on an Anionic Surfactant Modified Carbon Nanocomposite Material for the Rapid Determination of Curcumin in Natural Food Supplement. Instrumen. Sci. Technol. 2020, 48, 561–582.
- Deng, P.; Wei, Y.; Li, W.; Shi, S.; Zhou, C.; Li, J.; Yao, L.; Ding, J.; He, Q. A Novel Platform Based on MnO2 Nanoparticles and Carboxylated Multi-walled Carbon Nanotubes Composite for Accurate and Rapid Determination of Curcumin in Commercial Food Products. J. Food Compos. Anal. 2023, 115, 104940.
- Stanić, Z.; Voulgaropoulos, A.; Girousi, S. Electroanalytical Study of the Antioxidant and Antitumor Agent Curcumin. Electroanalysis 2008, 20, 1263–1266.
- Zhou, Q.; Zhai, H.Y.; Pan, Y.F.; Li, K. A Simple and Sensitive Sensor Based on a Molecularly Imprinted Polymer-Modified Carbon Paste Electrode for the Determination of Curcumin in Foods. RSC Adv. 2017, 7, 22913–22918.
- Suhito, I.R.; Lee, W.; Baek, S.; Lee, D.; Min, J.; Kim, T.-H. Rapid and Sensitive Electrochemical Detection of Anticancer Effects of Curcumin on Human Glioblastoma Cells. Sens. Actuators B Chem. 2019, 288, 527–534.
- Ahmed, A.H.M.T.; Naskar, H.; Banerjee, S.; Ghatak, B.; Das, N.; Tudu, B.; Bandyopadhyay, R. Electrochemical Sensor Based on Molecularly Imprinted Polymer Embedded Graphite Electrode for Detecting Curcumin. Sens. Actuator A Phys. 2022, 344, 113748.
- Monticelli, M.; Hay Mele, B.; Allocca, M.; Liguori, L.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Andreotti, G. Curcumin Has Beneficial Effects on Lysosomal Alpha-Galactosidase: Potential Implications for the Cure of Fabry Disease. Int. J. Mol. Sci. 2023, 24, 1095.
- Dey, N.; Devasena, T.; Verma, R.S. Validation of Copper Decorated Graphene Oxide Material for Assaying Curcumin. Front. Nanosci. Nanotech. 2021, 7, 1–11.
- Stura, I.; Munir, Z.; Cavallo, L.; Torri, L.; Mandras, N.; Banche, G.; Spagnolo, R.; Pertusio, R.; Cavalli, R.; Guiot, C. Combining Blue Light and Yellow Curcumin to Obtain a “Green” Tool for Berry Preservation against Bacterial Contamination: A Preliminary Investigation. Foods 2023, 12, 2038.
- Salama, A.M.; Ramadan, A.M.; Alakhdar, H.H.; Khan, T.K.; El-Garhy, H.A.S.; Shoala, T. Influence of Spraying Nano-Curcumin and Nano-Glycyrrhizic Acid on Resistance Enhancement and Some Growth Parameters of Soybean (Glycine max) in Response to Tetranychus urticae Infestation and Drought Stress. Plants 2023, 12, 114.
- Han, T.; Chen, W.; Zhong, Q.; Chen, W.; Xu, Y.; Wu, J.; Chen, H. Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin. Foods 2023, 12, 1577.
- Bondar, A.; Horodincu, L.; Solcan, G.; Solcan, C. Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry. Agriculture 2023, 13, 1553.
- Qin, J.; Park, J.S.; Jo, D.G.; Cho, M.; Lee, Y. Curcumin-Based Electrochemical Sensor of Amyloid-β Oligomer for the Early Detection of Alzheimer’s Disease. Sens. Actuators B Chem. 2018, 273, 1593–1599.
- Kumar, P.; Singh, A.; Kumar, A.; Kumar, R.; Pal, R.; Sachan, A.K.; Dixit, R.K.; Nath, R. Effect of Curcumin and Coenzyme Q10 Alone and in Combination on Learning and Memory in an Animal Model of Alzheimer’s Disease. Biomedicines 2023, 11, 1422.
- Li, H.; Sureda, A.; Devkota, H.P.; Pittala, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343.
- D’andurain, J.; López, V.; Arazo-Rusindo, M.; Tiscornia, C.; Aicardi, V.; Simón, L.; Mariotti-Celis, M.S. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients 2023, 15, 2239.
- Fessler, S.N.; Chang, Y.; Liu, L.; Johnston, C.S. Curcumin Confers Anti-Inflammatory Effects in Adults Who Recovered from COVID-19 and Were Subsequently Vaccinated: A Randomized Controlled Trial. Nutrients 2023, 15, 1548.
- Cui, Y.; Wang, Q.; Zhang, X.; Yang, X.; Shi, Y.; Li, Y.; Song, M. Curcumin Alleviates Aflatoxin B1-Induced Liver Pyroptosis and Fibrosis by Regulating the JAK2/NLRP3 Signaling Pathway in Ducks. Foods 2023, 12, 1006.
- Lee, D.-Y.; Lee, S.-J.; Chandrasekaran, P.; Lamichhane, G.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Dietary Curcumin Attenuates Hepatic Cellular Senescence by Suppressing the MAPK/NF-κB Signaling Pathway in Aged Mice. Antioxidants 2023, 12, 1165.
- Chang, G.-R.; Hsieh, W.-T.; Chou, L.-S.; Lin, C.-S.; Wu, C.-F.; Lin, J.-W.; Lin, W.-L.; Lin, T.-C.; Liao, H.-J.; Kao, C.-Y.; et al. Curcumin Improved Glucose Intolerance, Renal Injury, and Nonalcoholic Fatty Liver Disease and Decreased Chromium Loss through Urine in Obese Mice. Processes 2021, 9, 1132.
- Zeng, Y.; Luo, Y.; Wang, L.; Zhang, K.; Peng, J.; Fan, G. Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. Int. J. Mol. Sci. 2023, 24, 3323.
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371.
- Gorza, L.; Germinario, E.; Vitadello, M.; Guerra, I.; De Majo, F.; Gasparella, F.; Caliceti, P.; Vitiello, L.; Danieli-Betto, D. Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants 2023, 12, 1181.
- Saud Gany, S.L.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a Therapeutic Agent for Sarcopenia. Nutrients 2023, 15, 2526.
- Lee, M.K.; Kim, H.D.; Lee, S.H.; Lee, J.H. Curcumin Ameliorates Particulate Matter-Induced Pulmonary Injury through Bimodal Regulation of Macrophage Inflammation via NF-κB and Nrf2. Int. J. Mol. Sci. 2023, 24, 1858.
- Jeon, H.-J.; Kim, K.; Kim, C.; Lee, S.-E. Antimelanogenic Effects of Curcumin and Its Dimethoxy Derivatives: Mechanistic Investigation Using B16F10 Melanoma Cells and Zebrafish (Danio rerio) Embryos. Foods 2023, 12, 926.
- Farhat, F.; Sohail, S.S.; Siddiqui, F.; Irshad, R.R.; Madsen, D.Ø. Curcumin in Wound Healing—A Bibliometric Analysis. Life 2023, 13, 143.
- Scomoroscenco, C.; Teodorescu, M.; Nistor, C.L.; Gifu, I.C.; Petcu, C.; Banciu, D.D.; Banciu, A.; Cinteza, L.O. Preparation and In Vitro Characterization of Alkyl Polyglucoside-Based Microemulsion for Topical Administration of Curcumin. Pharmaceutics 2023, 15, 1420.
- Mirzaei, B.; Zarrabi, A.; Noorbakhsh, A.; Amini, A.; Makvandi, P. A reduced graphene oxide-b-cyclodextrin nanocomposite-based electrode for electrochemical detection of curcumin. RSC Adv. 2021, 11, 7862–7872.
- Nasery, M.M.; Varzandeh, M.; Pahlavanneshan, S.; Mohamadi, N.; Sarhadi, S.; Fekri, H.S.; Mohammadinejad, R.; Ahn, K.S. Curcumin: A Potential Therapeutic Natural Product for Adenocarcinomas. Phytochem. Lett. 2022, 49, 45–55.
- Barcelos, K.A.; Mendonça, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165.
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050.
- Boccellino, M.; Ambrosio, P.; Ballini, A.; De Vito, D.; Scacco, S.; Cantore, S.; Feola, A.; Di Donato, M.; Quagliuolo, L.; Sciarra, A.; et al. The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers 2022, 14, 3348.
- Salucci, S.; Bavelloni, A.; Stella, A.B.; Fabbri, F.; Vannini, I.; Piazzi, M.; Volkava, K.; Scotlandi, K.; Martinelli, G.; Faenza, I.; et al. The Cytotoxic Effect of Curcumin in Rhabdomyosarcoma is Associated with the Modulation of AMPK, AKT/mTOR, STAT, and p53 Signaling. Nutrients 2023, 15, 740.
- Güllü, N.; Smith, J.; Herrmann, P.; Stein, U. MACC1-Dependent Antitumor Effect of Curcumin in Colorectal Cancer. Nutrients 2022, 14, 4792.
- Shih, K.-C.; Chan, H.-W.; Wu, C.-Y.; Chuang, H.-Y. Curcumin Enhances the Abscopal Effect in Mice with Colorectal Cancer by Acting as an Immunomodulator. Pharmaceutics 2023, 15, 1519.
- Piwowarczyk, L.; Stawny, M.; Mlynarczyk, D.T.; Muszalska-Kolos, I.; Goslinski, T.; Jelińska, A. Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers 2020, 12, 1801.
- Wu, M.-F.; Huang, Y.-H.; Chiu, L.-Y.; Cherng, S.-H.; Sheu, G.-T.; Yang, T.-Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248.
- Gao, Q.; Zang, Y.; Zhang, Y.; Xie, J.; Li, J.; Gao, J.; Xue, H. Composite Polymerized Molecular Imprinting Membrane-Based Electrochemical Sensor for Sensitive Determination of Curcumin by Using 4-Pentenoyl-Aminoacyl-Chitosan Oligosaccharide as Functional Monomer Oligomer. J. Electroanal. Chem. 2020, 879, 114793.
- Sravani, A.B.; Mathew, E.M.; Ghate, V.; Levis, S.A. A Sensitive Spectrofluorimetric Method for Curcumin Analysis. J. Fluoresc. 2022, 32, 1517–1527.
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332.
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476.
- Deljoo, S.; Rabiee, N.; Rabiee, M. Curcumin-hybrid Nanoparticles in Drug Delivery System. Asian J. Nano. Mat. 2019, 2, 66–91.
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513.
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in Advanced Oral Drug Delivery System for Curcumin: A Systematic Review. J. Control. Release 2022, 348, 335–345.
- Flores-Frias, E.A.; Barba, V.; Lucio-Garcia, M.A.; Lopez-Cecenes, R.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G. Use of Curcuma and Curcumin as a Green Corrosion Inhibitors for Carbon Steel in Sulfuric Acid. Int. J. Electrochem. Sci. 2019, 14, 5026–5041.
- Ashwini, N.; Dileep, R.; Ranganatha, S. Curcumin and Curcumin Derivatives as Green Corrosion Inhibitor-A Review. Phys. Chem. Res. 2023, 11, 825–835.
- Rapalli, V.K.; Kaul, V.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Pandey, M.M.; Singhvi, G. UV Spectrophotometric Method for Characterization of Curcumin Loaded Nanostructured Lipid Nanocarriers in Simulated Conditions: Method Development, in-vitro and ex-vivo Applications in Topical Delivery. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117392.
- Shokrollahi, A.; Hesampour, S. Spectrophotometric Determination of Curcumin after Preconcentration by Ultrasonic Assisted Supramolecular Dispersive Liquid-liquid Microextraction based on Solidification of Floating Organic Drops using Taguchi Design Method. Adv. Mater. Lett. 2021, 12, 21111680.
- Suryawanshi, B.; Nehete, J.Y. Qualitative Analysis of Curcumin in Marketed Dosage Form by Using UV Spectroscopy. Int. J. Pharm. Res. Appl. 2021, 6, 845–850.
- Liu, Y.; Jiang, H. Qualitative and Quantitative Analysis of Curcumin in Dried Ginger by the Resonance Rayleigh Scattering Technique and Absorption Spectroscopy. J. Food Comp. Anal. 2023, 115, 104923.
- Wang, Y.-Q.; Li, L.; Yin, J.; Yu, X.; Wu, X.; Xu, L. Turn on Fluorescence Detection of Curcumin in Food Matrices by the Novel Fluorescence Sensitizer. Anal. Chim. Acta 2023, 1254, 341094.
- Fang, F.; Zhang, S.; Chen, C.; Wang, X.; Luo, C.; Wei, Q. A Fluorescent Nanoprobe Based on N/S co-Doped Carbon Dots Coupled with Molecularly Imprinted Polymers for the Detection of Curcumin. Opt. Mater. 2023, 139, 113800.
- Tang, S.; Wang, Y.; Guo, G.; Li, T.; Xing, H.; Hu, H.; Leng, X.; Gu, C.; Chen, D. Activated Cascade Effect for Dual-Mode Ratiometric and Smartphone-Assisted Visual Detection of Curcumin and F− Based on Nitrogen-Doped Carbon Dots. Sci. Total Environ. 2023, 872, 162277.
- Kushwaha, P.; Shukla, B.; Dwivedi, J.; Saxena, S. Validated high-performance thin-layer chromatographic analysis of curcumin in the methanolic fraction of Curcuma longa L. rhizomes. Futur. J. Pharm. Sci. 2021, 7, 178.
- Alvarado, H.-L.; Limón, D.; Calpena-Campmany, A.-C.; Mallandrich, M.; Rodríguez-Cid, L.; Aliaga-Alcalde, N.; González-Campo, A.; Pérez-García, L. Intrinsic Permeation and Anti-Inflammatory Evaluation of Curcumin, Bisdemethoxycurcumin and Bisdemethylcurcumin by a Validated HPLC-UV Method. Int. J. Mol. Sci. 2023, 24, 6640.
- Mohammed, H.A.; Alsahabi, D.S.; Hegazy, A.M.; Khan, R.A.; Ahmed, A.M. Analytical Purity Determinations of Universal Food-Spice Curcuma longa through a QbD Validated HPLC Approach with Critical Parametric Predictors and Operable-Design’s Monte Carlo Simulations: Analysis of Extracts, Forced-Degradants, and Capsules and Tablets-Based Pharmaceutical Dosage Forms. Foods 2023, 12, 1010.
- Kroon, M.A.G.M.; van Laarhoven, H.W.M.; Swart, E.L.; Kemper, E.M.; van Tellingen, O. A Validated HPLC-MS/MS Method for Simultaneously Analyzing Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin, Tetra-hydrocurcumin and Piperine in Human Plasma, Urine or Feces. Heliyon 2023, 9, e15540.
- Mohammadinejad, A.; Abouzari-Lotf, E.; Aleyaghoob, G.; Rezayi, M.; Oskuee, R.K. Application of a Transition Metal Oxide/Carbon-Based Nanocomposite for Designing a Molecularly Imprinted Poly (l-Cysteine) Electrochemical Sensor for Curcumin. Food Chem. 2022, 386, 132845.
- Martínez-Guerra, J.; Palomar-Pardavé, M.; Romero-Romo, M.; Corona-Avendaño, S.; Guzmán-Hernández, D.-S.; Rojas-Hernández, A.; Ramírez-Silva, M.T. On the Curcumin and β-Cyclodextrin Interaction in Aqueous Media. Spectrophotometric and Electrochemical Study. ChemElectroChem 2022, 9, 202101534.
- Modarresi, M.; Harfbol, M.R.; Eshraghi, A.; Ahmadi, F. Development of Ternary H-Point Standard Addition Method for Simultaneous Analysis of Curcuminoids by Differential Pulse Voltammetry. Food Res. Int. 2022, 161, 111802.
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A Critical Review of Analytical Methods for Determination of Curcuminoids in Turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166.
- Ziyatdinova, G.K.; Budnikov, H.C. Spice Antioxidants as Objects of Analytical Chemistry. J. Anal. Chem. 2018, 73, 946–965.
- Mohajeri, M.; Behnam, B.; Tasbandi, A.; Jamialahmadi, T.; Sahebkar, A. Carbon-based Nanomaterials and Curcumin: A Review of Biosensing Applications. In Studies on Biomarkers and New Targets in Aging Research in Iran; Advances in Experimental Medicine and Biology; Guest, P.C., Ed.; Springer: Cham, Switzerland, 2021; Volume 1291.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
834
Revisions:
2 times
(View History)
Update Date:
10 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





