Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Iulia Gabriela David and Version 2 by Catherine Yang.
Curcumin (CU) is a polyphenolic compound extracted from turmeric, a well-known dietary spice. Since it has been shown that CU exerts beneficial effects on human health, interest has increased in its use but also in its analysis in different matrices. CU has an antioxidant character and is electroactive due to the presence of phenolic groups in its molecule.
- curcumin
- voltammetry
- electrochemistry
- antioxidant
1. Curcumin—History and Occurrence
Turmeric was used 4000 years ago in cuisine and traditional medicine in India and China [1], but curcumin (CU) as its main component was discovered in turmeric only in 1815 and obtained as a pure compound in 1842. Its chemical structure and its synthesis were reported in 1910 and 1913, respectively [2]. Nowadays, it is mainly cultivated in India and China, but also in other tropical regions from South Asia, Africa, South America [3], and the Pacific basin [4].
CU [(1E,6E)−1,7−bis(4−hydroxy−3-methoxyphenyl)hepta−1,6−diene−3,5−dione] or diferuloylmethane [5] (Figure 1) is a solid polyphenolic antioxidant with bitter taste [6], extracted from the rhizome of the perennial herb turmeric (Curcuma longa Linnaeus), but also from other plants of the ginger (Zingiberaceae) family, where it coexists with structurally related species, known under the collective name of curcuminoids [5]. The main curcuminoids are CU, demethoxycurcumin (DMCU), and bis-demethoxycurcumin (BDMCU) (Figure 1). They differ by the number of methoxy groups on the aromatic nuclei. All of them are bioactive, possessing antioxidant properties, but, among them, CU is the most effective, having higher antioxidant power than vitamins C and E [3].
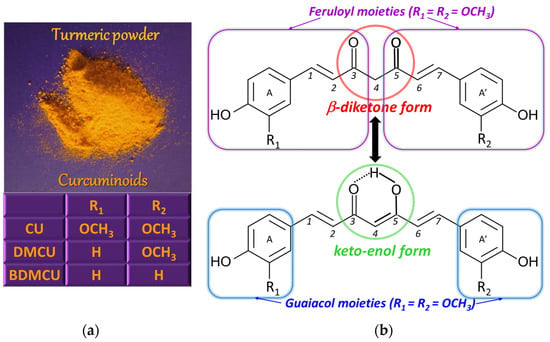
Figure 1.
Curcuminoids found in turmeric (
a
) and their tautomeric equilibrium (
b
).
The amount of curcuminoids (3–5%) [7]) in turmeric roots depends on the growing conditions, including soil type and climate [5], while the CU content of commercially available turmeric powder varies in the range 0.5–5.7%, also being influenced by the harvesting, extraction, and processing procedures [8]. Despite the fact that the literature reported somewhat different proportions of CU, DMCU, and BMCU, namely approximately 2:1:1 [8], 75%, 25%, and 5% [9] and 70%, 20%, and 10% [7], respectively, the major component of turmeric rhizome is CU.
2. Curcumin—Chemical Structure and Properties
From the chemical point of view, the CU molecule is composed of two ferulic acid molecules (feruloyl moieties) linked via a methylene group [10]. Going into more detail, the CU structure consists of two ortho-methoxy phenolic (guaiacol) groups bridged by a seven carbon atoms chain that contains an α,β-unsaturated β-diketone moiety, which determines the keto-enol tautomerism (Figure 1) [8], the enol proton being evenly distributed between the two oxygen atoms due to the symmetry of the CU molecule [11]. The ratio of the two tautomeric forms in solution depends on the solvent and its polarity, the solution pH, and temperature [7]. The keto form exists in acidic and neutral solutions (about 70%) [12] and in cell membranes, while the enol form is preponderant in alkaline media [9], in ethanol [8], in nonpolar organic solvents, and in solid phase, being stabilized by hydrogen bonds [10]. The CU biological properties are due to the guaiacol moieties as well as the keto-enol site [11].
CU undergoes three acid–base equilibria, the first one with pKa1 values reported in the range 7.43–8.55 and involving the transfer of the proton from the enol group. The next dissociation steps, attributed to the deprotonation of the two phenolic –OH moieties, have relatively close pKa values (pKa2 in the range 8.55–10.45 and pKa3 varying between 9.05 and 10.95) due to the symmetric position of the corresponding protogenic groups in the CU molecule [13].
Initial studies reported that CU is stable in solutions with pH values below 7.00, its dissociation equilibrium being shifted towards the neutral form, with poor aqueous solubility when the environment becomes more acidic. CU instability in alkaline media was explained by its hydrolytic degradation [14]. After CV and spectrophotometric investigations of CU behavior in time at different pH values, Martínez-Guerra et al. [13] concluded that CU degradation is 20 times faster in acidic media than in neutral or basic solutions, but CU stability in aqueous environments can be improved by deaerating and protecting the solution from light.
CU is practically insoluble in acidic and neutral aqueous solutions and poorly soluble in hydrocarbon solvents [15], but it is soluble in alkali [14], in lipids [10], and in organic solvents like acetic acid, ethanol [7], methanol, DMSO [12], acetone, and dichloromethane, its extraction in acetone being more efficient than in ethanol [16].
3. Curcumin—Uses
CU popularity increased remarkably worldwide in recent years as a consequence of its nutritional, prophylactic, and therapeutic values. Due to its orange–yellow color, it found applications as a natural coloring agent for food (mustard, margarine, processed cheese [17], pastries, canned products, and beverages [18]), cosmetics, hair dyes [19], textiles, furniture, and lacquers [20]. CU can also legally be added to food [21] as a preservative, spice, flavoring agent [22], and antioxidant in dairy products, meat, and seafood (fish, shrimp) [7]. The CU content of foods varies between 5 mg/kg and 500 mg/kg [23]. Moreover, on the market, there is a large variety of nutraceuticals and dietary supplements containing turmeric or its most bioactive component, CU, which are consumed on a large scale by the population [10]. A daily dose of 12,000 mg CU, which corresponds to a concentration of 51.2 ng/mL in human serum [23], presented little to no side effects, and, therefore, the FDA classified it as GRAS [24], the daily consumption level approved by the WHO being 1–3 mg/kg body weight [4][6][4,6]. At higher concentrations and prolonged administration, CU can inhibit the activity of some enzymes and cause anemia in persons with reduced iron uptake or various gastrointestinal [25], liver, inflammatory, and anticoagulation [12] problems.
Photoactivated CU encapsulated in β-CD was used for the antibacterial treatment of berries, increasing their shelf life without changing their organoleptic properties [26], while recent studies emphasized that CU-NPs, even at low concentrations, improved soybean growth and could be employed as fertilizer [27]. CU loaded into a zein/shellac-based composite food packaging film conferred antioxidant properties, inhibited E. coli, and the color change with pH variation enabled the monitoring of the food freshness [28]. There are many such applications of CU in the development of bioactive thin-layer composite polymeric food packaging films, and they were recently reviewed by Roy et al. [7].
CU also has beneficial effects on the growth of chickens and their egg production, curcuma being used as a feed additive in the broiler poultry industry [29].
Due to its lipophilic character, CU can bind to the amyloid β-oligomers that generate brain dysfunctions [30], and, therefore, it presents a dose-dependent enhancement of learning ability and memory by leading to beneficial results in the treatment of Alzheimer’s disease, both alone or in combination with coenzyme Q10 [31] or in the combined treatment of Fabry disease [24]. It has cardioprotective effects [32], reduces inflammation in patients with chronic renal failure [33] or in patients recovered from COVID-19 [34], may prevent and treat liver injury caused by aflatoxin B1 [35] or its age-related senescence [36], has therapeutic effects on hyperglycemia, oxidative stress, kidney, and nonalcoholic fatty liver diseases induced by high fat diet [37][38][37,38], reduces muscle damage and inflammation and improves sport performances [39], can protect human or animal muscles from degeneration [40][41][40,41], the lungs against air-pollution-induced inflammation [42], and the skin against UV radiation, having antimelanogenic [43] and wound-healing properties [44], also being employed as an active ingredient in cosmetic products [45]. Due to its antioxidant and metal-chelating properties, CU could be a treatment for metal poisoning [10]. It was shown that CU at concentrations of 5.00 × 10−6–5.00 × 10−5 mol/L at the cancer cell level [46] has antitumoral effects [5][47][5,47]; for example, it inhibits the proliferation of breast cancer cells [48] and exerts a dose-dependent reduction in the growth and progression of adrenocortical carcinoma [49], prostate cancer [50], rhabdomyosarcoma [51], colorectal [52][53][52,53], and bladder tumoral cells [54]. Moreover, at high concentrations, it has pro-oxidant properties, generating intracellular ROS that induced apoptosis of human lung cancer cells resistant to docetaxel and vincristine [55]. Depending on its concentration, CU exerts an anti- or pro-oxidant effect on DNA [14][56][14,56]. Among the CU pro-health activities are also the antibacterial effect discovered in 1949 [12][15][12,15], antiviral (against HIV, HPV, hepatitis virus, etc.) [57], and immunomodulatory and potentially antiallergic ones [1].
Despite its many health benefits, which were also discussed in different sections of some reviews [10][12][58][59][10,12,58,59], CU therapeutic use is limited by its reduced bioavailability generated by its low aqueous solubility, poor intestinal absorption, rapid metabolism and excretion from the body, 75% of the administrated CU dose being found in animal feces [10]. The highest CU concentration is in the intestine, while, in plasma or other tissues, it can be smaller than the quantification limits of the commonly applied analytical methods. In plasma, CU could be detected only after oral administration of high doses (at g levels), the maximum plasmatic level being reached 1–2 h after ingestion [58]. That is why researchers are continuously concerned to enhance the stability and bioavailability of CU in various ways (Figure 2), some of them being summarized in different review papers [9][10][12][15][58][60][61][62][9,10,12,15,58,60,61,62].
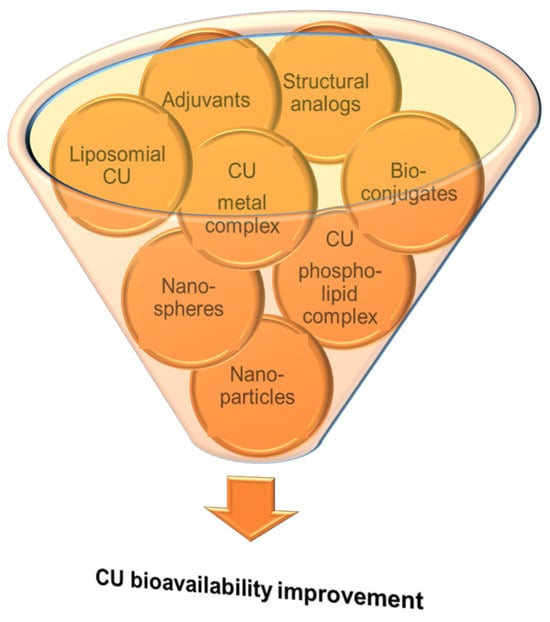
Figure 2.
There are also reports presenting an interesting application of CU and its derivatives as a corrosion inhibitor [63][64][63,64].
The multiple different applications of CU, generated by its various beneficial functional properties, have increased its demand worldwide in recent years, and this trend continues such that the global CU market was estimated to be USD 104.19 million in 2025 [7]. However, CU’s main use still remains in the food and dietary supplements industry.
Despite the fact that CU ingestion presents a high degree of safety for animals, considering its concentration-dependent anti- and pro-oxidant activity towards DNA and also other possible side effects that could appear after the administration of high CU doses, it is important to have simple and rapid methods for its sensitive and selective quantification in foods and dietary supplements, as well as in biological samples. The recent literature includes reports related to the spectrometric [65][66][67][68][65,66,67,68], fluorimetric [57][69][70][71][57,69,70,71], chromatographic [72][73][74][75][72,73,74,75], and electrochemical [19][23][76][77][78][19,23,76,77,78] analysis of CU. Analytical methods applied to curcuminoids assessment in turmeric, including CU, were reviewed in 2019 by Kotra et al. [79], with special emphasis on the chromatographic and spectrometric ones, electrochemical methods being very briefly mentioned. A few examples of voltammetric determination of CU in spices were discussed in 2018 by Ziyatidinova and Budnikov [80] in a review paper related to the analytical chemistry of spice antioxidants. The examples of CU electrochemical detection presented in 2019 by Mohajeri et al. [81] in a synthesizing article that addressed the interaction between CU and carbon-based nanomaterials were limited only to the biosensors using this type of sensing material. In a recent review on the CU extraction and analysis procedures, electrochemical methods and sensors are mentioned [12]. Each of the analytical methods have certain advantages and drawbacks, but the electrochemical ones are simpler, user-friendly, more rapid, involve fewer reagents (thus being eco-friendly), and, most importantly, voltammetry allows the explanation of certain reaction mechanisms that are the basis of some biological activities, such as antioxidant activity.
Therefore, based on the data published in the specialized literature in the last 20 years, this review discusses in detail the role of the electrochemical sensors and methods in investigating diverse aspects of CU analysis, including not only its quantification in various matrices but also its redox behavior and some of its biological activities, especially the antioxidant and antitumoral ones. In addition, CU interaction with various chemical species and its application in the development of electrochemical sensors for the assessment of different analytes were addressed.
