Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rosa Paola Radice | -- | 1139 | 2023-11-09 10:10:49 | | | |
| 2 | Catherine Yang | Meta information modification | 1139 | 2023-11-10 02:11:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Innelli, P.; Lopizzo, T.; Paternò, G.; Bruno, N.; Radice, R.P.; Bertini, P.; Marabotti, A.; Luzi, G.; Stabile, E.; Di Fazio, A.; et al. Dipeptidyl Amino-Peptidase 3 (DPP3) in Cardiogenic Shock. Encyclopedia. Available online: https://encyclopedia.pub/entry/51342 (accessed on 06 February 2026).
Innelli P, Lopizzo T, Paternò G, Bruno N, Radice RP, Bertini P, et al. Dipeptidyl Amino-Peptidase 3 (DPP3) in Cardiogenic Shock. Encyclopedia. Available at: https://encyclopedia.pub/entry/51342. Accessed February 06, 2026.
Innelli, Pasquale, Teresa Lopizzo, Giovanni Paternò, Noemi Bruno, Rosa Paola Radice, Pietro Bertini, Alberto Marabotti, Giampaolo Luzi, Eugenio Stabile, Aldo Di Fazio, et al. "Dipeptidyl Amino-Peptidase 3 (DPP3) in Cardiogenic Shock" Encyclopedia, https://encyclopedia.pub/entry/51342 (accessed February 06, 2026).
Innelli, P., Lopizzo, T., Paternò, G., Bruno, N., Radice, R.P., Bertini, P., Marabotti, A., Luzi, G., Stabile, E., Di Fazio, A., Pittella, G., & Paternoster, G. (2023, November 09). Dipeptidyl Amino-Peptidase 3 (DPP3) in Cardiogenic Shock. In Encyclopedia. https://encyclopedia.pub/entry/51342
Innelli, Pasquale, et al. "Dipeptidyl Amino-Peptidase 3 (DPP3) in Cardiogenic Shock." Encyclopedia. Web. 09 November, 2023.
Copy Citation
Dipeptidyl amino-peptidase 3 (DPP3) is an aminopeptidase that is released into circulation upon cell death. DPP3 is involved in the degradation of angiotensins, enkephalines, and endomorphines. It has been shown that circulating DPP3 (cDPP3) plasma concentration increases in cardiogenic shock (CS) patients and correlates with high mortality risk. Cardiogenic shock is a life-threatening syndrome associated with organ hypoperfusion. One of the common causes of CS is acute myocardial infarction (AMI).
DPP3
cardiogenic shock
mechanical ventilation
1. Diseases of the Cardiovascular System
Cardiovascular disease accounts for over two-thirds of total mortality worldwide and is an emerging serious health issue in middle- to low-income countries as well as in high-income countries [1]. Atherosclerotic cardiovascular disease is a collective term comprising of a group of disorders of the heart and blood vessels. These disorders are the largest cause of morbidity and death worldwide. Coronary heart disease, cerebrovascular disease and peripheral arterial disease are the most frequently occurring cardiovascular diseases. Early diagnosis of these disorders by means of better diagnostic tools is fundamental.
2. Cardiogenic Shock
Cardiogenic shock (CS) is a clinical condition in which ineffective cardiac output, caused by a primary cardiac disorder, results in inadequate tissue perfusion. The clinical presentation is typically characterized by persistent hypotension unresponsive to volume replacement and is accompanied by clinical features of end-organ hypoperfusion requiring intervention with pharmacological or mechanical support [2][3].
In general, there is a profound myocardial contractility depression resulting in a deleterious spiral of reduced cardiac output, low blood pressure, and further coronary ischemia (Figure 1).
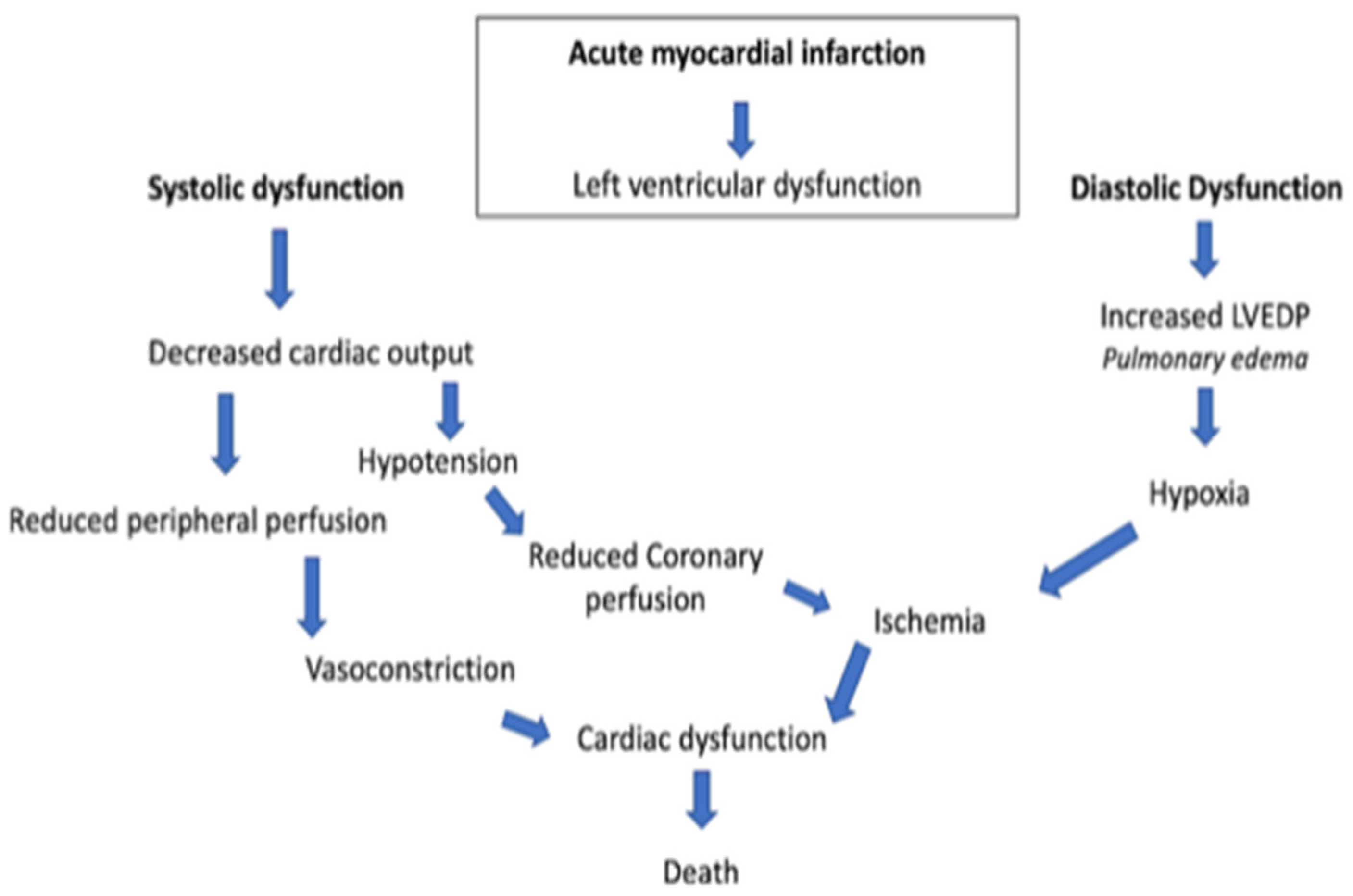
Figure 1. Acute myocardial infarction spiral. LVEDP, left ventricular end-diastolic pressure.
This classic paradigm also includes a lot of compensatory mechanisms, such as systemic vasoconstriction, that result in an ineffective stroke volume [4][5]. Consequently, CS and the downstream mechanisms induced thereby are strongly related to reduced end-organ perfusion with high risk of mortality and morbidity [6][7]. The management of CS frequently includes vasoactive drugs, circulatory and ventilatory support, and coronary revascularization in case of an acute myocardial infarction [8][9]. The management of CS is mostly based on clinicians’ experience rather than evidence-based recommendations since, for many treatments, either no adequately designed randomized clinical trials (RCT) exist or their results failed to show relevant beneficial effects. Hence, scarce advances have been achieved in the treatment of CS and patients’ outcomes remain poor [10]. In this context, particular attention is given to delineation of inclusion and exclusion criteria in RCTs in order to better understand the pathophysiology of patients that will and will not respond to medical treatment. Refractory cardiogenic shock refers to an ill-defined severity of CS not responding to standard therapies. In the pre-mechanical circulatory support (MCS) era, refractoriness to standard medical treatment led inevitably to death. The implementation of MCS opened new doors for the treatment of CS and modified the interpretation of “refractory” shock and its prognosis. However, the lack of a standard definition and nomenclature undermines research and clinical decision making in refractory CS.
3. Dipeptidyl Amino-Peptidase 3 in the Context of Cardiovascular Diseases
Dipeptidyl amino-peptidase 3 (DPP3) is an intracellular zinc-dependent enzyme [11] and is ubiquitously expressed in many cell types and tissues, including neutrophils, lungs, liver, kidney, and heart [12][13]. It has been reported that during cell death, intracellular DPP3 is released into circulation and, consequently, has been named circulating DPP3 (cDPP3) [14][15]. cDPP3 degrades angiotensins [16][17], enkephalines [17][18], and endorphines [19]. Therefore, it has been implicated in the modulation of many physiological processes, such as blood pressure regulation [16], inflammatory processes [13], and pain modulation [19]. The most notable DPP3 substrate is angiotensin II (Ang II), which serves as the primary effector molecule of the renin–angiotensin–aldosterone system (RAAS). The RAAS plays a vital role in regulating the cardiovascular system homeostasis, modulating blood pressure through the sympathetic system, glomerular filtration, releasing endogenous catecholamine and vasopressin, and stimulating vascular smooth muscle cells [20][21].
Increased blood levels of cDPP3 have been observed in critically ill patients suffering from septic, cardiogenic and haemorrhagic shock [22][23]. Furthermore, an association between high cDPP3 levels in the blood and high mortality in shocked patients in the ICU [22][23][24] has been shown. CS is a heterogenous syndrome associated with low cardiac output, organ hypoperfusion, and hypoxia, which leads to multi-organ failure and death [25][26]. The most common cause of cardiogenic shock remains acute myocardial infarction (AMI), representing 5–8% of patients with AMI [27]. Deniau et al. have shown that non-survivor CS patients had higher cDPP3 levels at all time points compared to survivors [24]. Furthermore, high cDPP3 levels in CS patients were associated with severe organ dysfunction. Consequently, cDPP3 levels at admission were predictive for 90-day mortality. Interestingly, a decrease in cDPP3 levels within 24 h of admission was associated with reduced 90-day mortality, decreased cardiovascular support, and renal function improvement [25].
Takagi et al. attempted to delineate a clinical role for cDPP3 and confirm the relationship between cDPP3 and clinical outcomes measured in patients with ST-elevation myocardial infarction (STEMI) and CS from the OptimaCC trial. The authors established the relationship between cDPP3 and the development of refractory CS, defined as shock non-responsive to inotropes and vasopressors with optimal cardiac filling pressures [22]. The authors have shown that cDPP3 levels are higher in patients that develop refractory CS within 72 h from admission to the ICU [22]. In addition, cDPP3 levels at admission could discriminate patients who did develop refractory shock from those who did not. Patients with high cDPP3 levels also exhibited lower cardiac index, low glomerular filtration rate, and higher Simplified Acute Physiology Score II (SAPS II) [22]. More importantly, CS patients with high cDPP3 levels at inclusion but decreased levels at 24 h showed a striking reduction in refractory shock and death [22].
Similar results have been observed in severe sepsis and septic shock patients. In this patient population, there is also a significant association between high cDPP3 blood levels upon ICU admission and 28-day mortality, high need for organ support, including prolonged need for vasopressor(s), mechanical ventilation and renal replacement therapy [28][29]. Interestingly, intravenous injection of DPP3 induced myocardial depression in healthy mice while inhibition of cDPP3 with Procizumab (a specific monoclonal antibody against cDPP3) normalized myocardial function in an acute heart failure mouse model, suggesting cDPP3 as a potential therapeutic target in patients with acute heart failure and shock. Pharmacological inhibition of cDPP3 by Procizumab also improved cardiac dysfunction and reduced mortality in septic rats [30].
The use of cDPP3 could be beneficial in helping clinicians make decisions on how to approach cardiogenic shock, such as recommendation of rapid transfer to a tertiary care center or the use of an intra-aortic balloon pump for high cDPP3 patients. Additionally, cDPP3 could be used to monitor a patient’s progress and help guide therapeutic decisions. Decreasing cDPP3 levels could lead to a recommendion for a less aggressive approach. Furthermore, cDPP3 together with other clinical and biological parameters could be used as a population-enrichment strategy in CS trials [31]. Finally, randomized clinical trials should clarify whether pharmacological inhibition of cDPP3 could be a novel therapeutic approach to improve hemodynamics and cardiovascular prognosis in CS patients.
References
- Palacios Ordonez, C.; Garan, A.R. The landscape of cardiogenic shock: Epidemiology and current definitions. Curr. Opin. Cardiol. 2022, 37, 236–240.
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434.
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E876–E894.
- Thiele, H.; Ohman, E.M.; Desch, S.; Eitel, I.; De Waha, S. Management of cardiogenic shock. Eur. Heart J. 2015, 36, 1223–1230.
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, 1–12.
- Krittanawong, C.; Rivera, M.R.; Shaikh, P.; Kumar, A.; May, A.; Mahtta, D.; Jentzer, J.; Civitello, A.; Katz, J.; Naidu, S.S.; et al. Key Concepts Surrounding Cardiogenic Shock. Curr. Probl. Cardiol. 2022, 47, 101303.
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672.
- Biswas, S.; Malik, A.H.; Bandyopadhyay, D.; Gupta, R.; Goel, A.; Briasoulis, A.; Fonarow, G.C.; Lanier, G.M.; Naidu, S.S. Matanalysis comparing the efficacy of dobutamine versus Milrinone in acute decompensated heart failure. Curr. Probl. Cardiol. 2022, 8, 101245.
- Paternoster, G.; Bertini, P.; Innelli, P.; Trambaiolo, P.; Landoni, G.; Franchi, F.; Scolletta, S.; Guarracino, F. Right Ventricular Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3319–3324.
- Ashish, A.H.; Puri, R.; Kalra, A. Management of cardiogenixc shock complicating acute myocardila infarction: A review. Clin. Cardiol. 2019, 42, 484–493.
- Prajapati, S.C.; Chauhan, S.S. Dipeptidyl peptidase III: A multifaceted oligopeptide N-end cutter. FEBS J. 2011, 278, 3256–3276.
- Ohkubo, I.; Li, Y.H.; Maeda, T.; Yamamoto, Y.; Yamane, T.; Du, P.G.; Nishi, K. Molecular cloning and immunohistochemical localization of rat dipeptidyl peptidase III. Forensic Sci. Int. 2000, 113, 147–151.
- Hashimoto, J.I.; Yamamoto, Y.; Kurosawa, H.; Nishimura, K.; Hazato, T. Identification of dipeptidyl peptidase III in human neutrophils. Biochem. Biophys. Res. Commun. 2000, 273, 393–397.
- Wattiaux, R.; Wattiaux-de coninck, S.; Thirion, J.; Gasingirwa, M.-C.; Jadot, M. Lysosomes and Fas-mediated liver cell death. Biochem. J. 2007, 403, 89–95.
- Rehfeld, L.; Funk, E.; Jha, S.; Macheroux, P.; Melander, O.; Bergmann, A. Novel Methods for the Quantification of Dipeptidyl Peptidase 3 (DPP3) Concentration and Activity in Human Blood Samples. J. Appl. Lab. Med. 2019, 3, 943–953.
- Jha, S.; Taschler, U.; Domenig, O.; Poglitsch, M.; Bourgeois, B.; Pollheimer, M.; Pusch, L.M.; Malovan, G.; Frank, S.; Madl, T.; et al. Dipeptidyl peptidase 3 modulates the renin–Angiotensin system in mice. J. Biol. Chem. 2020, 295, 13711–13723.
- Lee, C.; Snyder, S.H. Dipeptidyl-aminopeptidase III of Rat Brain. J. Biol. Chem. 1982, 257, 12043–12050.
- Bezerra, G.A.; Dobrovetsky, E.; Viertlmayr, R.; Dong, A.; Binter, A.; Abramić, M.; Macheroux, P.; Dhe-Paganon, S.; Gruber, K. Entropy-driven binding of opioid peptides induces a large domain motion in human dipeptidyl peptidase III. Proc. Natl. Acad. Sci. USA 2012, 109, 6525–6530.
- Baršun, M.; Jajčanin, N.; Vukelić, B.; Špoljarić, J.; Abramić, M. Human dipeptidyl peptidase III acts as a post-proline-cleaving enzyme on endomorphins. Biol. Chem. 2007, 388, 343–348.
- Pang, X.; Shimizu, A.; Kurita, S.; Zankov, D.P.; Takeuchi, K.; Yasuda-Yamahara, M.; Kume, S.; Ishida, T.; Ogita, H. Novel therapeutic role for dipeptidyl peptidase III in the treatment of hypertension. Hypertension 2016, 68, 630–641.
- Malovan, G.; Hierzberger, B.; Suraci, S.; Schaefer, M.; Santos, K.; Jha, S.; Macheroux, P. The emerging role of dipeptidyl peptidase 3 in pathophysiology. FEBS J. 2022, 1–17.
- Takagi, K.; Blet, A.; Levy, B.; Deniau, B.; Azibani, F.; Feliot, E.; Bergmann, A.; Santos, K.; Hartmann, O.; Gayat, E.; et al. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: Results from the OptimaCC trial. Eur. J. Heart Fail. 2020, 22, 279–286.
- Frigyesi, A.; Lengquist, M.; Spångfors, M.; Annborn, M.; Cronberg, T.; Nielsen, N.; Levin, H.; Friberg, H. Circulating dipeptidyl peptidase 3 on intensive care unit admission is a predictor of organ dysfunction and mortality. J. Intensive Care 2021, 9, 52.
- Deniau, B.; Rehfeld, L.; Santos, K.; Dienelt, A.; Azibani, F.; Sadoune, M.; Kounde, P.R.; Samuel, J.L.; Tolpannen, H.; Lassus, J.; et al. Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: Dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur. J. Heart Fail. 2020, 22, 290–299.
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.P.; Antohi, E.L.; Arrigo, M.; Gal, T.B.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Reynolds, H.R.; Hochman, J.S. Cardiogenic shock current concepts and improving outcomes. Circulation 2008, 117, 686–697.
- Blet, A.; Deniau, B.; Santos, K.; Van Lier, D.P.T.; Azibani, F.; Wittebole, X.; Chousterman, B.G.; Gayat, E.; Hartmann, O.; Struck, J.; et al. Monitoring Circulating dipeptidyl peptidase 3 (DPP3) predicts improvement of organ failure and survival in sepsis: A prospective observational multinational study. Crit. Care 2021, 3, 1–10.
- Deniau, B.; Picod, A.; Van Lier, D.; Vaittinada Ayar, P.; Santos, K.; Hartmann, O.; Gayat, E.; Mebazaa, A.; Blet, A.; Azibani, F. High plasma dipeptidyl peptidase 3 levels are associated with mortality and organ failure in shock: Results from the international, prospective and observational FROG-ICU cohort. Br. J. Anaesth. 2022, 128, e54–e57.
- Deniau, B.; Blet, A.; Santos, K.; Ayar, P.V.; Genest, M.; Kästorf, M.; Sadoune, M.; de Sousa Jorge, A.; Samuel, J.L.; Vodovar, N.; et al. Inhibition of circulating dipeptidyl-peptidase 3 restores cardiac function in a sepsis-induced model in rats: A proof of concept study. PLoS ONE 2020, 15, e0238039.
- Magliocca, A.; Omland, T.; Latini, R. Dipeptidyl peptidase 3, a biomarker in cardiogenic shock and hopefully much more. Eur. J. Heart Fail. 2020, 22, 300–302.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
737
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
10 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




