Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Blerida Banushi | -- | 3086 | 2023-11-06 16:50:25 | | | |
| 2 | Rita Xu | Meta information modification | 3086 | 2023-11-07 03:32:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Banushi, B.; Polito, V. Cellular Neurobiology of Psychedelics. Encyclopedia. Available online: https://encyclopedia.pub/entry/51201 (accessed on 02 March 2026).
Banushi B, Polito V. Cellular Neurobiology of Psychedelics. Encyclopedia. Available at: https://encyclopedia.pub/entry/51201. Accessed March 02, 2026.
Banushi, Blerida, Vince Polito. "Cellular Neurobiology of Psychedelics" Encyclopedia, https://encyclopedia.pub/entry/51201 (accessed March 02, 2026).
Banushi, B., & Polito, V. (2023, November 06). Cellular Neurobiology of Psychedelics. In Encyclopedia. https://encyclopedia.pub/entry/51201
Banushi, Blerida and Vince Polito. "Cellular Neurobiology of Psychedelics." Encyclopedia. Web. 06 November, 2023.
Copy Citation
Psychedelic substances have gained significant attention in recent years for their potential therapeutic effects on various psychiatric disorders.
psychedelics
5-HT2A
BDNF
TrkB
serotonergic
psilocybin
1. Introduction
Coined by Humphry Osmond in 1956, the term “psychedelic” originates from the Greek words meaning “mind manifesting” [1]. This term is used to describe the subjective effects of these substances, highlighting their ability to induce profound experiences and alter perception.
Psychedelics constitute a class of drugs obtained from specific plants, animals, and fungi, and they can be categorized into three primary classes based on their chemical structure: tryptamines, ergolines, and phenethylamines [2][3]. Tryptamines, such as psilocybin, N, N-dimethyltryptamine (DMT), and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) are characterized by an indole (aromatic group separated from a basic amine by a two-carbon linker) and share structural similarities with the neurotransmitter serotonin. Ergolines, such as lysergic acid diethylamide (LSD), are characterized by the presence of a tetracyclic ergoline ring, and were originally derived from the ergot fungus [4]. Phenethylamines, such as 2C-B, mescaline, amphetamine analogues—e.g., 2,5-Dimethoxy-4-iodoamphetamine (DOI) and 2,5-Dimethoxy-4-methylamphetamine (DOM), and derivatives such as 4-Iodo-2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamine (25I-NBOMe)—are characterized by a benzene ring with an amino group attached through a two-carbon chain [3]. In addition to classical psychedelics, there are atypical compounds like 3,4-Methylenedioxymethamphetamine (MDMA), muscimol, scopolamine, salvinorin A, ibogaine, nitrous oxide, phencyclidine (PCP), and ketamine that produce similar psychological effects but work through different mechanisms. These compounds are sometimes considered psychedelics under a broader definition [4].
Psychedelics have been used by humans for centuries. Historical records indicate that these substances have been consumed in ancient cultural rituals with the purpose of healing, attaining altered states of consciousness, and gaining spiritual insights, tracing back to prehistory [5][6]. The synthesis of mescaline in the early 1900s and the groundbreaking discovery of the classical hallucinogen LSD by Albert Hofmann in 1938 marked the beginning of Western psychedelic science [7][8][9]. During the 1950s and 1960s, these substances gained popularity in therapeutic and psychiatric settings due to their ability to facilitate psychotherapy [7]. However, in 1970, the U.S. Drug Enforcement Agency classified psychedelics as Schedule I drugs, which had a profound impact on research in the field [10]. Prior to their classification, over 1000 clinical studies were published, documenting promising therapeutic effects of psychedelics in more than 40,000 subjects. [7][11][12]. These studies indicated therapeutic benefits in various conditions such as anxiety and obsessive-compulsive disorders (OCD), depression, alcohol addiction, and sexual dysfunction, as well as pain and anxiety relief in patients with terminal cancer [10][13][14][15][16][17][18][19]. This regulatory decision effectively curtailed psychedelic research for approximately 30 years [20].
The advent of advanced neuroimaging techniques in the 1990s, including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), played a pivotal role in enhancing the comprehension of molecular and physiological mechanisms within the central nervous system [21]. These breakthroughs also sparked a renewed interest in exploring the effects of psychedelic substances [22]. In the last three decades, there has been a resurgence in the exploration of psychedelics, reigniting research interest in their therapeutic potential. This renewed focus has led to anticipated FDA approvals for the use of psychedelics in treating various conditions, marking a period of exponential scientific growth in this field [23][24][25].
In recent years a growing number of clinical trials and studies have been conducted or are currently underway, investigating the therapeutic potential of psychedelics such as LSD and psilocybin for various mental health conditions including depression, anxiety, cancer-related anxiety disorders, addiction, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder, terminal illness, stroke, traumatic brain injury (TBI), neurodegenerative disorders, and chronic pain [22][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41]. In response to this growing interest, the U.S. Food and Drug Administration recently released a new draft guidance aimed at emphasizing essential considerations for researchers exploring the use of psychedelic drugs as potential treatments for medical conditions [42].
Randomized Phase II trials have so far demonstrated significant reductions in symptoms and long-lasting benefits following psilocybin-assisted psychotherapy for major depressive disorder, anxiety, and treatment-resistant depression [43][44][45][46]. Remarkably, even one or two doses of psilocybin have led to rapid and sustained improvements in mood and perspective, with symptomatic relief lasting for at least 3–12 months [28][45][46][47]. Furthermore, enduring positive effects and improved well-being following psilocybin administration have also been observed in healthy individuals. Recent Phase 3 trials investigating MDMA for PTSD have shown promising results, and hint at a potential paradigm shift in psychiatry towards utilizing substances with acute psychoactive effects to generate long-term benefits for psychiatric patients [48].
2. Psychedelics Exert Their Effects on the Brain at Multiple Levels, Engaging in Intricate and Multifaceted Mechanisms
The impact of psychedelics on the brain can be examined from multiple perspectives, including molecular/cellular, circuit/network, and overall brain levels, all of which are inherently interconnected (Figure 1). At the molecular/cellular level, psychedelics stimulate the serotonin 2A receptor (5-HT2R) along with other serotonin sub-receptors, tropomyosin receptor kinase B (TrkB), and dopamine receptors [49][50]. Classic psychedelics have been shown to elevate levels of glutamate and oxytocin [22][51][52][53][54], promote the production of brain-derived neurotrophic factor (BDNF) [50][55][56], stimulate neurogenesis [22][51], and exhibit anti-inflammatory properties [57]. On a cellular level, psychedelics also induce an increase in the expression of various genes that encode for the synthesis of a range of proteins that facilitate neuroplasticity and learning, even following a single dose [58][59][60].
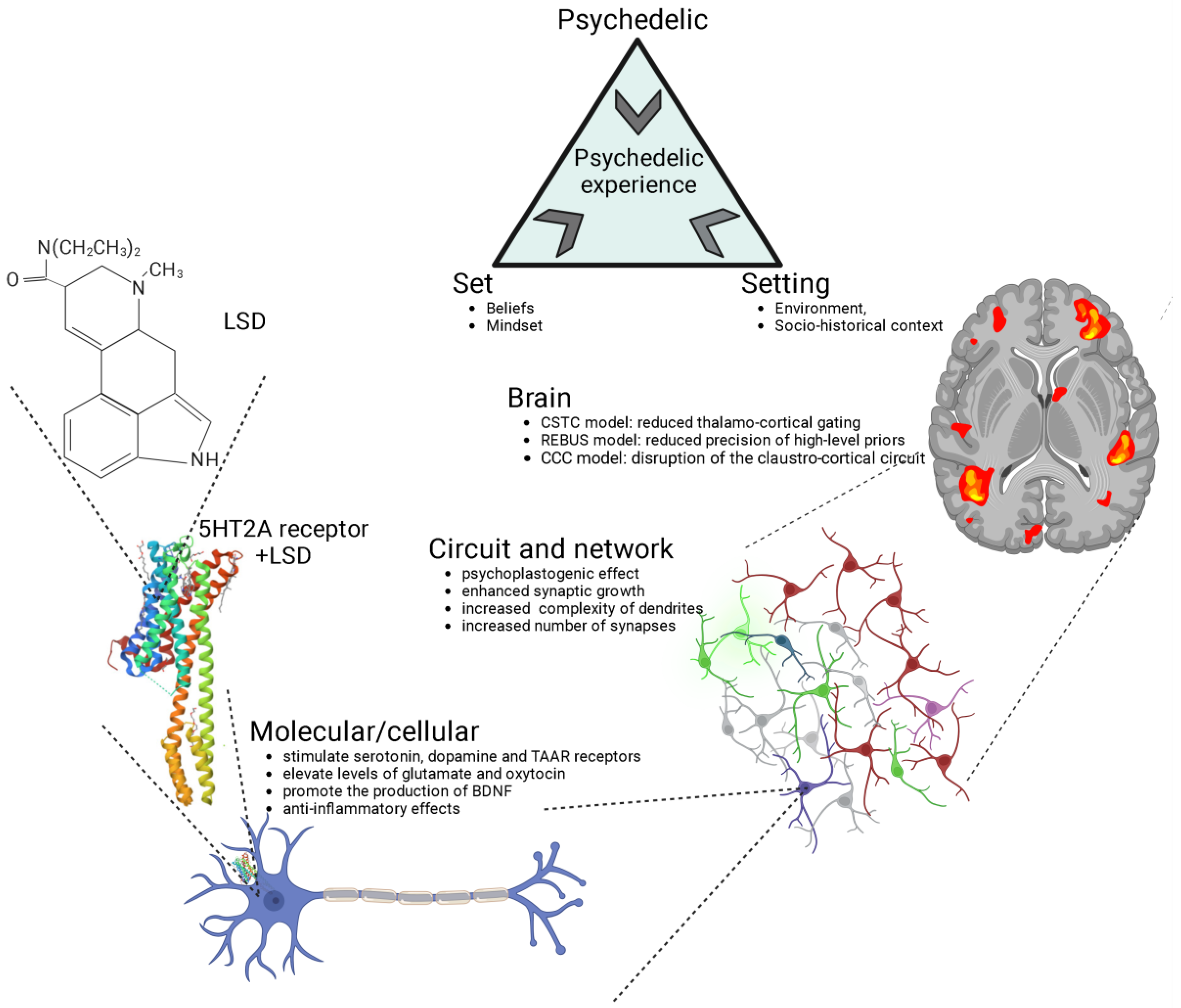
Figure 1. Psychedelics exert their effects through various levels of analysis, including the molecular/cellular, the circuit/network, and the overall brain. The crystal structure of serotonin 2A receptor in complex with LSD is sourced from the RCSB Protein Data Bank (RCSB PDB). LSD, lysergic acid diethylamide; 5-HT2A, serotonin 2A; CSTC, cortico-striato-thalamo-cortical; REBUS, relaxed beliefs under psychedelics model; CCC, claustro-cortical circuit.
At the circuit and network levels (Figure 1), both ketamine and serotonergic psychedelics have been observed to enhance synaptic growth and increase the complexity of dendrites, consequently leading to a greater number of synapses [61]. This, in turn, results in an increased connectivity among neurons [62][63][64][65][66][67][68].
The structural and neuroplastic changes induced by psychedelics originate from a series of processes discussed earlier at the molecular and cellular levels. This research will shed light on some of these mechanisms. This cascade of network changes has led to psychedelics being characterized as “psychoplastogens”, substances with the capability to facilitate rapid neural plasticity both in terms of structure and function [62][69].
At the level of the brain (Figure 1), several distinct and frequently complementary neuroscientific explanations have been put forth to elucidate the effects of psychedelics and the mechanisms underlying the psychedelic experience [70][71]. The three most prominent theories encompass the cortico-striato-thalamo-cortical (CSTC) model [72], the relaxed beliefs under psychedelics (REBUS) model [73], and the claustro-cortical circuit (CCC) model [74].
The CSTC model proposes that psychedelics, through the stimulation of 5-HT2ARs, disrupt information processing in the brain by altering the thalamo-cortical gating of external and internal information to the cortex [72]. This disruption leads to an increased flow of information or feedforward processing, resulting in various effects, including impaired sensorimotor gating, alterations in cognitive functioning, and changes in sensory and somatomotor cortical regions. This model is substantiated by both behavioral observations of impaired sensorimotor gating in humans following the administration of psilocybin [75][76], LSD [77] and ayahuasca [78], and neuroimaging findings demonstrating increased thalamic functional connectivity and synchronisation of cortical sensory regions in response to LSD [79][80].
The REBUS model suggests that psychedelics reduce the precision of high-level priors (or expectations and beliefs about the world) while simultaneously increasing the flow of bottom-up sensory information [73]. This model integrates the entropic brain hypothesis [81] and the free-energy principle, explaining how psychedelics influence brain function by altering the relative importance of prior beliefs and sensory information. This shift in signal weighting makes recurrent message transfer within the brain more responsive to modulation by incoming sensory signals, ultimately leading to increased complexity and entropy in neuronal dynamics. Preliminary empirical evidence supports this model, demonstrating that psychedelics like LSD, psilocybin, DMT, ketamine, and ayahuasca enhance signal diversity and measures of entropy in brain activity [82][83][84][85][86]. While the REBUS model has influenced the field of psychedelic research as a potential overarching framework, it has faced criticism both conceptually (such as regarding entropy definition, low- and high-level brain region demarcation) and methodologically (small sample sizes of the studies and analytical choices), necessitating further rigorous research for confirmation [70][71].
The CCC model proposes that psychedelics interfere with coordination between cortical regions and the claustrum by directly activating 5-HT2ARs, which are abundant in the claustrum [74][87][88][89]. This coordination plays a crucial role in cognitive control, which is diminished by the effects of psychedelics [74][89]. This model has substantial support from neuroimaging studies that have demonstrated that psilocybin significantly disrupts networks associated with cognitive control and the proper functioning of the claustrum [63]. While the CCC model holds promise in explaining the widespread effects of psychedelics on various brain networks, it currently lacks specificity in detailing how these changes in the claustrum affect specific canonical circuits [70]. Advanced imaging techniques, such as higher field fMRI, will be needed to better understand the flow of information between the claustrum and other brain regions. Additionally, although reduced cognitive control may contribute to some psychedelic effects, it appears insufficient to explain all of the varied acute subjective experiences associated with these substances [70].
The models and levels of analysis presented in Figure 1 include extra-pharmacological factors, such as social, contextual, and cultural elements, commonly referred to as “set” (comprising individual beliefs, expectations, and mindset) and “setting” (involving the environment and socio-historical context). Of particular note, music has consistently played a pivotal role in guiding and enhancing the therapeutic experience in the history of psychedelic research, and recent findings emphasize its ability to support processes like meaning-making, emotional responses, and mental imagery following psychedelic administration, ultimately contributing to positive clinical outcomes in psychedelic therapy [90].
3. 5-HT2A Receptor Signaling
Psychedelics have a similar chemical structure to serotonin (5-HT) [91]. Recent studies using advanced techniques like X-ray diffraction and cryo-electron microscopy have revealed the crystal structures of serotonin receptors when they are bound to psychedelic substances like LSD, psilocin, and others [92][93]. These studies provide new insights into how psychedelics work at the molecular level and their potential use in developing psychedelic-based treatments.
Phenethylamines exhibit a greater level of specificity for 5-HT2A, 5-HT2B, and 5-HT2C receptors in comparison to tryptamines and ergolines [3][94]. In the past, it was widely believed that the therapeutic effects of psychedelics were mainly attributed to their activation of the 5-HT2AR [95][96][97][98], but recent research has revealed the involvement of other receptors in producing these effects [50]. This belief is substantiated by evidence showing that the particular neural circuits in the central nervous system impacted by serotonergic psychedelics align with the distribution of serotonin receptors (Figure 2) [99].
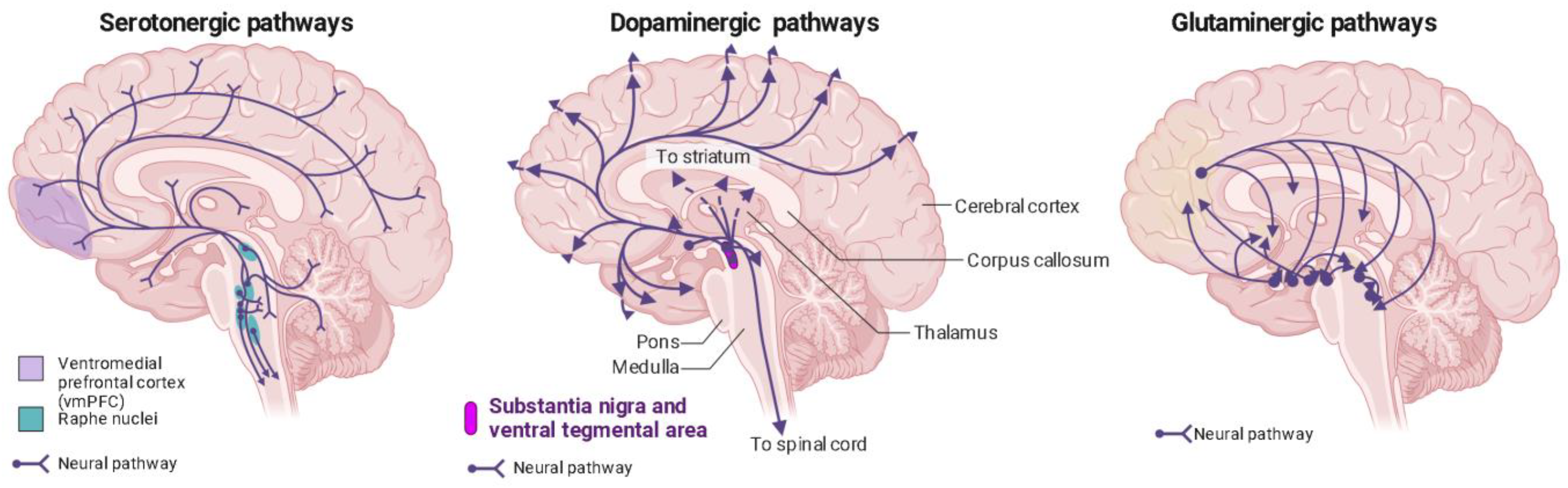
Figure 2. Distribution of serotonin, dopamine, and glutaminergic pathways in the human brain. Ventromedial prefrontal cortex (vmPFC) in purple; raphe nuclei in blue.
The occupancy of 5-HT2ARs also exhibits a correlation with the subjective effects induced by psychedelics and these effects are suppressed by serotonin-2A antagonists [65][99][100][101][102][103][104]. The 5-HT2ARs are prominently found in pyramidal neurons situated in layer 5 of the neocortex, the thalamus, and the reticular nucleus, with a significant concentration observed in the prefrontal cortex (PFC) [103][105][106][107][108][109][110][111][112]. These brain areas are associated with functions like visual perception and attention, which likely contribute to the broad and diverse effects of psychedelics on cognitive, perceptual, and emotional processes. However, it is worth noting that other studies have indicated that the greatest concentration of these receptors is found in the striate and extrastriate visual cortex [78][103]. This observation could explain the frequent occurrence of visual hallucinations, a distinctive hallmark of classic psychedelics. Moreover, a recent study used a whole-brain model of serotonergic neuromodulation to investigate the entropic effects of 5-HT2AR activation, confirming the earlier findings of increased entropy and emphasizing that the most significant changes in entropy occurred in the visuo-occipital regions. Interestingly, this study also revealed that the overall reorganization of brain activity was more strongly associated with the brain’s anatomical connectivity than to the density of 5-HT2AR, offering valuable insights into the mechanisms behind the psychedelic experience and the broader regulation of brain functions by pharmacological means [113].
The expression of HT2AR is highest in excitatory neurons within the cortex, but it is also found in inhibitory interneurons. Specifically, in the prefrontal cortex, 5-HT2ARs are predominantly located on the postsynaptic side [114].
The 5-HT2AR, classified as a Class A G-protein-coupled receptor, is activated by its natural ligand, 5-HT, acting as an agonist. However, in contrast to 5-HT, psychedelic agonists targeting this receptor induce profound changes in perception and cognition. These effects can be explained by the ternary complex model of receptor activity for agonists [115][116]. This model suggests that drug molecules can shift the equilibrium between different receptor conformations, leading to “bias” in activating either G-protein-dependent or β-arrestin-dependent signaling pathways (Figure 3) [117][118][119]. In a recent study, cryo-EM structures of LSD-bound HTR2B provided snapshots of LSD’s action. These revealed transitions from transducer-free, partially active states to transducer-coupled, fully active states, highlighting the potential for biased agonists with functional selectivity, which could be safer and more effective drugs for specific G-protein-coupled receptors (GPCRs) [120].

Figure 3. Schematic and simplified overview of the intracellular transduction cascades induced by 5-HT2AR TrkB and Sig-1R receptor activation by psychedelics. It is essential to emphasize that the understanding of the activation or inhibition of specific pathways and the precise molecular mechanisms responsible for triggering plasticity in specific neuron types remains incomplete. This figure illustrates the mechanisms associated with heightened plasticity within these pathways. Psychedelics (such as LSD, psilocin, and mescaline) bind to TrkB dimers, stabilizing their conformation. Furthermore, they enhance the localization of TrkB dimers within lipid rafts, thereby extending their signaling via PLCγ1. The BDNF/TrkB signaling pathway (black arrows) initiates with BDNF activating TrkB, prompting autophosphorylation of tyrosine residues within TrkB’s intracellular C-terminal domain (specifically Tyr490 and Tyr515), followed by the recruitment of SHC. This, in turn, leads to the binding of GRB2, which subsequently associates with SOS and GTPase RAS to form a complex, thereby initiating the ERK cascade. This cascade ultimately results in the activation of the CREB transcription factor. CREB, in turn, mediates the transcription of genes essential for neuronal survival, differentiation, BDNF production, neurogenesis, neuroprotection, neurite outgrowth, synaptic plasticity, and myelination. Activation of Tyr515 in TrkB also activates the PI3K signaling pathway through GAB1 and the SHC/GRB2/SOS complex, subsequently leading to the activation of protein kinase AKT and CREB. Both Akt and ERK activate mTOR, which is associated with downstream processes involving dendritic growth, AMPAR expression, and overall neuronal survival. Additionally, the phosphorylation of TrkB’s Tyr816 residue activates the phospholipase Cγ (PLCγ) pathway, generating IP3 and DAG. IP3 activates its receptor (IP3R) in the endoplasmic reticulum (ER), causing the release of calcium (Ca2+) from the ER and activating Ca2+/CaM/CaMKII which in turn activates CREB. DAG activates PKC, leading to ERK activation and synaptic plasticity. After being released into the extracellular space, glutamate binds to ionotropic glutamate receptors, including NMDA receptors (NMDARs) and AMPA receptors (AMPARs), as well as metabotropic glutamate receptors (mGluR1 to mGluR8), located on the membranes of both postsynaptic and presynaptic neurons. Upon binding, these receptors initiate various responses, such as membrane depolarization, activation of intracellular messenger cascades, modulation of local protein synthesis, and ultimately, gene expression. The surface expression and function of NMDARs and AMPARs are dynamically regulated through processes involving protein synthesis, degradation, and receptor trafficking between the postsynaptic membrane and endosomes. This insertion and removal of postsynaptic receptors provides a mechanism for the long-term modulation of synaptic strength. Psychedelic compounds exhibit a high affinity for 5-HT2R, leading to the activation of G-protein and β-arrestin signaling pathways (red arrows). Downstream for 5-HT2R activation, these pathways intersect with both PI3K/Akt and ERK kinases, similar to the BDNF/TrkB signaling pathway. This activation results in enhanced neural plasticity. A theoretical model illustrating the signaling pathway of DMT through Sig-1R at MAMs suggests that, at endogenous affinity concentrations (14 μM), DMT binds to Sig-1R, triggering the dissociation of Sig-1R from BiP. This enables Sig-1R to function as a molecular chaperone for IP3R, resulting in an increased flow of Ca2+ from the ER into the mitochondria. This, in turn, activates the TCA cycle and enhances the production of ATP. However, at higher concentrations (100 μM), DMT induces the translocation of Sig-1Rs from the MAM to the plasma membrane (dashed inhibitory lines), leading to the inhibition of ion channels. BDNF = brain-derived neurotrophic factor; TrkB = tropomyosin-related kinase B; LSD = lysergic acid diethylamide; SHC = src homology domain containing; SOS = son of sevenless; Ras = GTP binding protein; Raf = Ras associated factor; MEK = MAP/Erk kinase; mTOR = mammalian target of rapamycin; ERK = extracellular signal regulated kinase; GRB2 = growth factor receptor bound protein 2; GAB1 = GRB-associated binder 1; PLC = phospholipase C γ; IP3 = inositol-1, 4, 5-triphosphate; DAG = diacylglycerol; PI3K = phosphatidylinositol 3-kinase; CaMKII = calcium/calmodulin-dependent kinase; CREB = cAMP-calcium response element binding protein; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; Sig-1R = sigma-1 receptor; DMT = N,N-dimethyltryptamine; BiP = immunoglobulin protein; MAMs = mitochondria-associated ER membrane; ER = endoplasmic reticulum; TCA = tricarboxylic acid; AT P = adenosine triphosphate; ADP = adenosine diphosphate.
In the context of Gq-dependent signaling, both the psychedelic and non-psychedelic activators of 5-HT2AR stimulate Gq-like G proteins [121]. The process begins with the activation of PLCγ (Figure 3). This activation subsequently initiates the release of intracellular calcium through inositol trisphosphate (IP3) and triggers the activation of protein kinase C (PKC) via diacylglycerol (DAG). This, in turn, sets off various interconnected downstream pathways, including but not limited to the ERK, CREB, and mTOR pathways (Figure 3). Ultimately, these cascading events result in neuronal firing through multiple mechanisms, such as membrane depolarization, reduced afterhyperpolarization, and decreased spike frequency adaptation [122][123][124][125] (Figure 3). The entry of calcium, along with the activation of calmodulin-dependent protein kinase II (CaMKII), which interacts with NMDA-type glutamate receptors and phosphorylates AMPA-type glutamate receptor subunits, leads to the specific strengthening and structural fortification of synapses, contributing to the process of long-term potentiation (LTP) that enhances synaptic connections during learning (Figure 3) [126][127]. Beyond G-protein-coupled pathways, 5-HT2AR agonism can also engage in β-arrestin signaling via PI3K and AKT (Figure 3) [95][128][129]. Both of these signaling pathways can contribute to neural plasticity [130][131], and may underlie the persistent improvements observed in psychiatric disorders.
However, recent research conducted in mice suggests that the therapeutic effects of psychedelics may be most closely linked to the activation of the BNDF-TrkB signaling pathway, rather than the 5-HT2AR [50]. Specifically, several studies have shown that the “head twitch response” in rodents, which is used as a proxy for the hallucinogenic effects of psychedelics in humans [94], relies on 5-HT2AR activation [50][132][133], but that blocking the 5-HT2AR in mice did not prevent induced structural plasticity changes [50]. However, selectively restoring 5-HT2ARs in cortical pyramidal neurons was sufficient to rescue hallucinogen-induced head twitching in transgenic mice lacking 5-HT2Ars [134][135].
References
- Osmond, H. A Review of the Clinical Effects of Psychotomimetic Agents. Ann. N. Y. Acad. Sci. 1957, 66, 418–434.
- Kelmendi, B.; Kaye, A.P.; Pittenger, C.; Kwan, A.C. Psychedelics. Curr. Biol. 2022, 32, R63–R67.
- Nichols, D.E. Chemistry and Structure-Activity Relationships of Psychedelics. In Behavioral Neurobiology of Psychedelic Drugs; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2018; Volume 36, pp. 1–43.
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The Pharmacology of Lysergic Acid Diethylamide: A Review. CNS Neurosci. Ther. 2008, 14, 295–314.
- Carod-Artal, F.J. Hallucinogenic Drugs in Pre-Columbian Mesoamerican Cultures. Neurologia 2015, 30, 42–49.
- Guerra-Doce, E. Psychoactive Substances in Prehistoric Times: Examining the Archaeological Evidence. Time Mind 2015, 8, 91–112.
- Nichols, D.E.; Walter, H. The History of Psychedelics in Psychiatry. Pharmacopsychiatry 2021, 54, 151–166.
- Swanson, L.R. Unifying Theories of Psychedelic Drug Effects. Front. Pharmacol. 2018, 9, 172.
- Hofmann, A. Chemical Constitution and Pharmacodynamic Actions; Dekker: New York, NY, USA, 1968.
- Pletscher, A.; Ladewig, D.; Symposium Schweizerische Akademie der Medizinischen Wissenschaften (Eds.) 50 Years of LSD: Current Status and Perspectives of Hallucinogens: A Symposium of the Swiss Academy of Medical Sciences, Lugano-Agno, Switzerland, 21–22 October 1993; Parthenon Pub. Group: New York, NY, USA, 1994.
- Vattano, A.J. Psychedelic Drugs Reconsidered. By Lester Grinspoon and James B. Bakalar. New York: Basic Books, 1979. 343 pp. $15.95 cloth. Child. Sch. 1981, 4, 71–72.
- Malleson, N. Acute Adverse Reactions to LSD in Clinical and Experimental Use in the United Kingdom. Br. J. Psychiatry 1971, 118, 229–230.
- Hoffer, A. The Uses and Implications of Hallucinogenic Drugs; Nature Publishing Group, a Division of Macmillan Publishers Limited: London, UK, 1970.
- Abramson, H.A. The Use of LSD in Psychotherapy and Alcoholism; Bobbs-Merrill: Indianapolis, IN, USA, 1967.
- Solomon, D. LSD: The Consciousness-Expanding Drug; Putnam: New York, NY, USA, 1964.
- Pahnke, W.N.; Kurland, A.A.; Goodman, L.E.; Richards, W.A. LSD-Assisted Psychotherapy with Terminal Cancer Patients. Curr. Psychiatr. Ther. 1969, 9, 144–152.
- Ladewig, D.; Pletscher, A. Fifty Years of LSD: Current Status and Perspectives of Hallucinogens; CRC Press: Boca Raton, FL, USA, 1994.
- Rucker, J.J.; Jelen, L.A.; Flynn, S.; Frowde, K.D.; Young, A.H. Psychedelics in the Treatment of Unipolar Mood Disorders: A Systematic Review. J. Psychopharmacol. 2016, 30, 1220–1229.
- Pare, C.M.B. Neuro-Psychopharmacology. Proceedings of the Fifth International Congress of the Collegium Internationale Neuro-Psychopharmacologicum. Washington, D.C. March, 1966. Edited by H. Brill. Excerpta Medica Foundation. 1967. Pp. 1278. Price £21 15s. Br. J. Psychiatry 1968, 114, 1043.
- Dyck, E. Flashback: Psychiatric Experimentation with LSD in Historical Perspective. Can. J. Psychiatry 2005, 50, 381–388.
- Cowan, W.M.; Harter, D.H.; Kandel, E.R. The Emergence of Modern Neuroscience: Some Implications for Neurology and Psychiatry. Annu. Rev. Neurosci. 2000, 23, 343–391.
- Vollenweider, F.X.; Kometer, M. The Neurobiology of Psychedelic Drugs: Implications for the Treatment of Mood Disorders. Nat. Rev. Neurosci. 2010, 11, 642–651.
- Hadar, A.; David, J.; Shalit, N.; Roseman, L.; Gross, R.; Sessa, B.; Lev-Ran, S. The Psychedelic Renaissance in Clinical Research: A Bibliometric Analysis of Three Decades of Human Studies with Psychedelics. J. Psychoact. Drugs 2023, 55, 1–10.
- Sessa, B. The 21st Century Psychedelic Renaissance: Heroic Steps Forward on the Back of an Elephant. Psychopharmacology 2018, 235, 551–560.
- Rivera-García, M.T.; Cruz, S.L. The Resurgence of Hallucinogen Drugs in Clinical Research. Rev. Investig. Clin. 2023, 75, 169–178.
- Mithoefer, M.C.; Grob, C.S.; Brewerton, T.D. Novel Psychopharmacological Therapies for Psychiatric Disorders: Psilocybin and MDMA. Lancet Psychiatry 2016, 3, 481–488.
- Nutt, D.; Erritzoe, D.; Carhart-Harris, R. Psychedelic Psychiatry’s Brave New World. Cell 2020, 181, 24–28.
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-Term Follow-up of Psilocybin-Facilitated Smoking Cessation. Am. J. Drug Alcohol Abus. 2017, 43, 55–60.
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin-Assisted Treatment for Alcohol Dependence: A Proof-of-Concept Study. J. Psychopharmacol. 2015, 29, 289–299.
- Andersen, K.A.A.; Carhart-Harris, R.; Nutt, D.J.; Erritzoe, D. Therapeutic Effects of Classic Serotonergic Psychedelics: A Systematic Review of Modern-Era Clinical Studies. Acta Psychiatr. Scand. 2021, 143, 101–118.
- Bogenschutz, M.P.; Johnson, M.W. Classic Hallucinogens in the Treatment of Addictions. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 250–258.
- Krebs, T.S.; Johansen, P.-Ø. Lysergic Acid Diethylamide (LSD) for Alcoholism: Meta-Analysis of Randomized Controlled Trials. J. Psychopharmacol. 2012, 26, 994–1002.
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, Tolerability, and Efficacy of Psilocybin in 9 Patients with Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2006, 67, 1735–1740.
- Ehrmann, K.; Allen, J.J.B.; Moreno, F.A. Psilocybin for the Treatment of Obsessive-Compulsive Disorders. Curr. Top. Behav. Neurosci. 2022, 56, 247–259.
- Rucker, J.J.; Iliff, J.; Nutt, D.J. Psychiatry & the Psychedelic Drugs. Past, Present & Future. Neuropharmacology 2018, 142, 200–218.
- Kyzar, E.J.; Nichols, C.D.; Gainetdinov, R.R.; Nichols, D.E.; Kalueff, A.V. Psychedelic Drugs in Biomedicine. Trends Pharmacol. Sci. 2017, 38, 992–1005.
- De Vos, C.M.; Mason, N.L.; Kuypers, K.P. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 724606.
- Elman, I.; Pustilnik, A.; Borsook, D. Beating Pain with Psychedelics: Matter over Mind? Neurosci. Biobehav. Rev. 2022, 134, 104482.
- Lukasiewicz, K.; Baker, J.J.; Zuo, Y.; Lu, J. Serotonergic Psychedelics in Neural Plasticity. Front. Mol. Neurosci. 2021, 14, 748359.
- Saeger, H.N.; Olson, D.E. Psychedelic-Inspired Approaches for Treating Neurodegenerative Disorders. J. Neurochem. 2022, 162, 109–127.
- Leger, R.F.; Unterwald, E.M. Assessing the Effects of Methodological Differences on Outcomes in the Use of Psychedelics in the Treatment of Anxiety and Depressive Disorders: A Systematic Review and Meta-Analysis. J. Psychopharmacol. 2022, 36, 20–30.
- Center for Drug Evaluation and Research. Psychedelic Drugs: Considerations for Clinical Investigations Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2023.
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489.
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin Versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411.
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197.
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180.
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A. Psilocybin with Psychological Support for Treatment-Resistant Depression: An Open-Label Feasibility Study. Lancet Psychiatry 2016, 3, 619–627.
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033.
- Halberstadt, A.L.; Geyer, M.A. Multiple Receptors Contribute to the Behavioral Effects of Indoleamine Hallucinogens. Neuropharmacology 2011, 61, 364–381.
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics Promote Plasticity by Directly Binding to BDNF Receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041.
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a Convergent Mechanism of Ketamine and Classical Psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942.
- Mason, N.; Kuypers, K.; Müller, F.; Reckweg, J.; Tse, D.; Toennes, S.; Hutten, N.; Jansen, J.; Stiers, P.; Feilding, A. Me, myself, bye: Regional Alterations in Glutamate and the Experience of Ego Dissolution with Psilocybin. Neuropsychopharmacology 2020, 45, 2003–2011.
- Nardou, R.; Lewis, E.M.; Rothhaas, R.; Xu, R.; Yang, A.; Boyden, E.; Dölen, G. Oxytocin-Dependent Reopening of a Social Reward Learning Critical Period with MDMA. Nature 2019, 569, 116–120.
- Holze, F.; Avedisian, I.; Varghese, N.; Eckert, A.; Liechti, M.E. Role of the 5-HT(2A) Receptor in Acute Effects of LSD on Empathy and Circulating Oxytocin. Front. Pharmacol. 2021, 12, 711255.
- Hutten, N.R.; Mason, N.L.; Dolder, P.C.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Varghese, N.; Eckert, A.; Feilding, A.; Ramaekers, J.G. Low Doses of LSD Acutely Increase BDNF Blood Plasma Levels in Healthy Volunteers. ACS Pharmacol. Transl. Sci. 2020, 4, 461–466.
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S. Acute Dose-Dependent Effects of Lysergic acid Diethylamide in a Double-Blind Placebo-Controlled Study in Healthy Subjects. Neuropsychopharmacology 2021, 46, 537–544.
- Flanagan, T.W.; Nichols, C.D. Psychedelics as Anti-Inflammatory Agents. Int. Rev. Psychiatry 2018, 30, 363–375.
- Nichols, C.D.; Sanders-Bush, E. A Single Dose of Lysergic Acid Diethylamide Influences Gene Expression Patterns within the Mammalian Brain. Neuropsychopharmacology 2002, 26, 634–642.
- Kanen, J.W.; Luo, Q.; Rostami Kandroodi, M.; Cardinal, R.N.; Robbins, T.W.; Nutt, D.J.; Carhart-Harris, R.L.; den Ouden, H.E.M. Effect of Lysergic acid Diethylamide (LSD) on Reinforcement Learning in Humans. Psychol. Med. 2022, 53, 6434–6445.
- Knudsen, G.M. Sustained Effects of Single Doses of Classical Psychedelics in Humans. Neuropsychopharmacology 2023, 48, 145–150.
- Dai, R.; Larkin, T.E.; Huang, Z.; Tarnal, V.; Picton, P.; Vlisides, P.E.; Janke, E.; McKinney, A.; Hudetz, A.G.; Harris, R.E. Classical and Non-Classical Psychedelic Drugs Induce Common Network Changes in Human Cortex. NeuroImage 2023, 273, 120097.
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182.
- Barrett, F.S.; Krimmel, S.R.; Griffiths, R.R.; Seminowicz, D.A.; Mathur, B.N. Psilocybin Acutely Alters the Functional Connectivity of the Claustrum with Brain Networks that Support Perception, Memory, and Attention. Neuroimage 2020, 218, 116980.
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C. Neural Correlates of the LSD Experience Revealed by Multimodal Neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858.
- Preller, K.H.; Duerler, P.; Burt, J.B.; Ji, J.L.; Adkinson, B.; Stńmpfli, P.; Seifritz, E.; Repovš, G.; Krystal, J.H.; Murray, J.D. Psilocybin Induces Time-Dependent Changes in Global Functional Connectivity. Biol. Psychiatry 2020, 88, 197–207.
- Kaelen, M.; Roseman, L.; Kahan, J.; Santos-Ribeiro, A.; Orban, C.; Lorenz, R.; Barrett, F.S.; Bolstridge, M.; Williams, T.; Williams, L. LSD Modulates Music-Induced Imagery via Changes in Parahippocampal Connectivity. Eur. Neuropsychopharmacol. 2016, 26, 1099–1109.
- Petri, G.; Expert, P.; Turkheimer, F.; Carhart-Harris, R.; Nutt, D.; Hellyer, P.J.; Vaccarino, F. Homological Scaffolds of Brain Functional Networks. J. R. Soc. Interface 2014, 11, 20140873.
- Smigielski, L.; Scheidegger, M.; Kometer, M.; Vollenweider, F.X. Psilocybin-Assisted Mindfulness Training Modulates Self-Consciousness and Brain Default Mode Network Connectivity with Lasting Effects. NeuroImage 2019, 196, 207–215.
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508.
- van Elk, M.; Yaden, D.B. Pharmacological, Neural, and Psychological Mechanisms Underlying Psychedelics: A Critical Review. Neurosci. Biobehav. Rev. 2022, 140, 104793.
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The Neural Basis of Psychedelic Action. Nat. Neurosci. 2022, 25, 1407–1419.
- Vollenweider, F.X.; Geyer, M.A. A Systems Model of Altered Consciousness: Integrating Natural and Drug-Induced Psychoses. Brain Res. Bull. 2001, 56, 495–507.
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344.
- Doss, M.K.; Madden, M.B.; Gaddis, A.; Nebel, M.B.; Griffiths, R.R.; Mathur, B.N.; Barrett, F.S. Models of Psychedelic Drug Action: Modulation of Cortical-Subcortical Circuits. Brain 2022, 145, 441–456.
- Vollenweider, F.X.; Csomor, P.A.; Knappe, B.; Geyer, M.A.; Quednow, B.B. The Effects of the Preferential 5-HT2A Agonist Psilocybin on Prepulse Inhibition of Startle in Healthy Human Volunteers Depend on Interstimulus Interval. Neuropsychopharmacology 2007, 32, 1876–1887.
- Quednow, B.B.; Kometer, M.; Geyer, M.A.; Vollenweider, F.X. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition Are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology 2012, 37, 630–640.
- Schmid, Y.; Enzler, F.; Gasser, P.; Grouzmann, E.; Preller, K.H.; Vollenweider, F.X.; Brenneisen, R.; Müller, F.; Borgwardt, S.; Liechti, M.E. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol. Psychiatry 2015, 78, 544–553.
- Riba, J.; Rodríguez-Fornells, A.; Barbanoj, M.J. Effects of Ayahuasca on Sensory and Sensorimotor Gating in Humans as Measured by P50 Suppression and Prepulse Inhibition of the Startle Reflex, Respectively. Psychopharmacology 2002, 165, 18–28.
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D. Changes in Global and Thalamic Brain Connectivity in LSD-Induced Altered States of Consciousness Are Attributable to the 5-HT2A Receptor. Elife 2018, 7, e35082.
- Müller, F.; Lenz, C.; Dolder, P.; Lang, U.; Schmidt, A.; Liechti, M.; Borgwardt, S. Increased Thalamic Resting-State Connectivity as a Core Driver of LSD-Induced Hallucinations. Acta Psychiatr. Scand. 2017, 136, 648–657.
- Carhart-Harris, R.L. The Entropic Brain-Revisited. Neuropharmacology 2018, 142, 167–178.
- Schartner, M.M.; Carhart-Harris, R.L.; Barrett, A.B.; Seth, A.K.; Muthukumaraswamy, S.D. Increased Spontaneous MEG Signal Diversity for Psychoactive Doses of Ketamine, LSD and Psilocybin. Sci. Rep. 2017, 7, 46421.
- Timmermann, C.; Roseman, L.; Schartner, M.; Milliere, R.; Williams, L.T.; Erritzoe, D.; Muthukumaraswamy, S.; Ashton, M.; Bendrioua, A.; Kaur, O. Neural Correlates of the DMT Experience Assessed with Multivariate EEG. Sci. Rep. 2019, 9, 16324.
- Lebedev, A.V.; Kaelen, M.; Lövdén, M.; Nilsson, J.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. LSD-Induced Entropic Brain Activity Predicts Subsequent Personality Change. Human Brain Mapp. 2016, 37, 3203–3213.
- Atasoy, S.; Roseman, L.; Kaelen, M.; Kringelbach, M.L.; Deco, G.; Carhart-Harris, R.L. Connectome-Harmonic Decomposition of Human Brain Activity Reveals Dynamical Repertoire Re-Organization under LSD. Sci. Rep. 2017, 7, 17661.
- Viol, A.; Palhano-Fontes, F.; Onias, H.; de Araujo, D.B.; Viswanathan, G.M. Shannon Entropy of Brain Functional Complex Networks under the Influence of the Psychedelic Ayahuasca. Sci. Rep. 2017, 7, 7388.
- Mathur, B.N. The Claustrum in Review. Front. Syst. Neurosci. 2014, 8, 48.
- Krimmel, S.R.; White, M.G.; Panicker, M.H.; Barrett, F.S.; Mathur, B.N.; Seminowicz, D.A. Resting State Functional Connectivity and Cognitive Task-Related Activation of the Human Claustrum. Neuroimage 2019, 196, 59–67.
- White, M.G.; Mathur, B.N. Claustrum Circuit Components for Top–Down Input Processing and Cortical Broadcast. Brain Struct. Funct. 2018, 223, 3945–3958.
- Barrett, F.S.; Preller, K.H.; Kaelen, M. Psychedelics and Music: Neuroscience and Therapeutic Implications. Int. Rev. Psychiatry 2018, 30, 350–362.
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355.
- Cao, D.; Yu, J.; Wang, H.; Luo, Z.; Liu, X.; He, L.; Qi, J.; Fan, L.; Tang, L.; Chen, Z.; et al. Structure-Based Discovery of Nonhallucinogenic Psychedelic Analogs. Science 2022, 375, 403–411.
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017, 168, 377–389.e12.
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; Wallach, J.; Brandt, S.D. Correlation between the Potency of Hallucinogens in the Mouse Head-Twitch Response Assay and Their Behavioral and Subjective Effects in Other Species. Neuropharmacology 2020, 167, 107933.
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 2020, 182, 1574–1588.e19.
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457.
- Kraehenmann, R.; Pokorny, D.; Aicher, H.; Preller, K.H.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. LSD Increases Primary Process Thinking via Serotonin 2A Receptor Activation. Front. Pharmacol. 2017, 8, 814.
- Kraehenmann, R.; Pokorny, D.; Vollenweider, L.; Preller, K.H.; Pokorny, T.; Seifritz, E.; Vollenweider, F.X. Dreamlike Effects of LSD on Waking Imagery in Humans Depend on Serotonin 2A Receptor Activation. Psychopharmacology 2017, 234, 2031–2046.
- Delli Pizzi, S.; Chiacchiaretta, P.; Sestieri, C.; Ferretti, A.; Onofrj, M.; Della Penna, S.; Roseman, L.; Timmermann, C.; Nutt, D.J.; Carhart-Harris, R.L.; et al. Spatial Correspondence of LSD-Induced Variations on Brain Functioning at Rest With Serotonin Receptor Expression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 768–776.
- Becker, A.M.; Klaiber, A.; Holze, F.; Istampoulouoglou, I.; Duthaler, U.; Varghese, N.; Eckert, A.; Liechti, M.E. Ketanserin Reverses the Acute Response to LSD in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Participants. Int. J. Neuropsychopharmacol. 2023, 26, 97–106.
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin Induces Schizophrenia-Like Psychosis in Humans via a Serotonin-2 Agonist Action. Neuroreport 1998, 9, 3897–3902.
- Singleton, S.P.; Timmermann, C.; Luppi, A.I.; Eckernas, E.; Roseman, L.; Carhart-Harris, R.L.; Kuceyeski, A. Time-Resolved Network Control Analysis Links Reduced Control Energy under DMT with the Serotonin 2a Receptor, Signal Diversity, and Subjective Experience. bioRxiv 2023.
- Quednow, B.B.; Geyer, M.A.; Halberstadt, A.L. Serotonin and Schizophrenia; Elsevier: Amsterdam, The Netherlands, 2010; pp. 585–620.
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic Effects of Psilocybin Correlate with Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels. Neuropsychopharmacology 2019, 44, 1328–1334.
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624.
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. 2017, 37, 120–128.
- Weber, E.T.; Andrade, R. Htr2a Gene and 5-HT2A Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front. Neurosci. 2010, 4, 36.
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin Modulation of Cortical Neurons and Networks. Front. Integr. Neurosci. 2013, 7, 25.
- Jakab, R.L.; Goldman-Rakic, P.S. 5-Hydroxytryptamine2A Serotonin Receptors in the Primate Cerebral Cortex: Possible Site of Action of Hallucinogenic and Antipsychotic Drugs in Pyramidal Cell Apical Dendrites. Proc. Natl. Acad. Sci. USA 1998, 95, 735–740.
- Hall, H.; Farde, L.; Halldin, C.; Lundkvist, C.; Sedvall, G. Autoradiographic Localization of 5-HT2A Receptors in the Human Brain Using M100907 and M100907. Synapse 2000, 38, 421–431.
- Saulin, A.; Savli, M.; Lanzenberger, R. Serotonin and Molecular Neuroimaging in Humans Using PET. Amino Acids 2012, 42, 2039–2057.
- Willins, D.L.; Deutch, A.Y.; Roth, B.L. Serotonin 5-HT2A Receptors Are Expressed on Pyramidal Cells and Interneurons in the Rat Cortex. Synapse 1997, 27, 79–82.
- Herzog, R.; Mediano, P.A.M.; Rosas, F.E.; Lodder, P.; Carhart-Harris, R.; Perl, Y.S.; Tagliazucchi, E.; Cofre, R. A Whole-Brain Model of the Neural Entropy Increase Elicited by Psychedelic Drugs. Sci. Rep. 2023, 13, 6244.
- Wood, J.; Kim, Y.; Moghaddam, B. Disruption of Prefrontal Cortex Large Scale Neuronal Activity by Different Classes of Psychotomimetic Drugs. J. Neurosci. 2012, 32, 3022–3031.
- Miner, L.; Backstrom, J.; Sanders-Bush, E.; Sesack, S. Ultrastructural Localization of Serotonin2A Receptors in the Middle Layers of the Rat Prelimbic Prefrontal Cortex. Neuroscience 2003, 116, 107–117.
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and Serotonin 5-HT 2A Receptor-Mediated Signaling Pathways. Behav. Neurobiol. Psychedelic Drugs 2018, 36, 45–73.
- Sleight, A.J.; Stam, N.J.; Mutel, V.; Vanderheyden, P.M. Radiolabelling of the Human 5-HT2A Receptor with an Agonist, a Partial Agonist and an Antagonist: Effects on Apparent Agonist Affinities. Biochem. Pharmacol. 1996, 51, 71–76.
- Kenakin, T.P. Biased Signalling and Allosteric Machines: New Vistas and Challenges for Drug Discovery. Br. J. Pharmacol. 2012, 165, 1659–1669.
- Urban, J.D.; Clarke, W.P.; Zastrow, M.v.; Nichols, D.E.; Kobilka, B.; Weinstein, H.; Javitch, J.A.; Roth, B.L.; Christopoulos, A.; Sexton, P.M.; et al. Functional Selectivity and Classical Concepts of Quantitative Pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 1–13.
- Kenakin, T. Functional Selectivity and Biased Receptor Signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302.
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The Stressed Synapse: The Impact of Stress and Glucocorticoids on Glutamate Transmission. Nat. Rev. Neurosci. 2012, 13, 22–37.
- Egan, C.; Grinde, E.; Dupre, A.; Roth, B.L.; Hake, M.; Teitler, M.; Herrick-Davis, K. Agonist High and Low Affinity State Ratios Predict Drug Intrinsic Activity and a Revised Ternary Complex Mechanism at Serotonin 5-HT(2A) and 5-HT(2C) Receptors. Synapse 2000, 35, 144–150.
- Roth, B.L.; Nakaki, T.; Chuang, D.M.; Costa, E. Aortic Recognition Sites for Serotonin (5HT) Are Coupled to Phospholipase C and Modulate Phosphatidylinositol Turnover. Neuropharmacology 1984, 23, 1223–1225.
- Roth, B.L.; Nakaki, T.; Chuang, D.M.; Costa, E. 5-Hydroxytryptamine2 Receptors Coupled to Phospholipase C in Rat Aorta: Modulation of Phosphoinositide Turnover by Phorbol Ester. J. Pharmacol. Exp. Ther. 1986, 238, 480–485.
- Banerjee, A.A.; Vaidya, V.A. Differential Signaling Signatures Evoked by DOI Versus Lisuride Stimulation of the 5-HT2A Receptor. Biochem. Biophys. Res. Commun. 2020, 531, 609–614.
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-Hydroxytryptamine1a Receptors Mediate Opposing Responses on Membrane Excitability in Rat Association Cortex. Neuroscience 1991, 40, 399–412.
- Pilc, A.; Machaczka, A.; Kawalec, P.; Smith, J.L.; Witkin, J.M. Where Do We Go Next in Antidepressant Drug Discovery? A New Generation of Antidepressants: A Pivotal Role of AMPA Receptor Potentiation and mGlu2/3 Receptor Antagonism. Expert. Opin. Drug Discov. 2022, 17, 1131–1146.
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII Action in Long-Term Potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182.
- Pottie, E.; Dedecker, P.; Stove, C.P. Identification of Psychedelic New Psychoactive Substances (NPS) Showing Biased Agonism at the 5-HT(2A)R through Simultaneous Use of β-Arrestin 2 and miniGα(q) Bioassays. Biochem. Pharmacol. 2020, 182, 114251.
- Gray, J.A.; Bhatnagar, A.; Gurevich, V.V.; Roth, B.L. The Interaction of a Constitutively Active Arrestin with the Arrestin-Insensitive 5-HT(2A) Receptor Induces Agonist-Independent Internalization. Mol. Pharmacol. 2003, 63, 961–972.
- Tedford, H.W.; Zamponi, G.W. Direct G Protein Modulation of Cav2 Calcium Channels. Pharmacol. Rev. 2006, 58, 837–862.
- Betke, K.M.; Wells, C.A.; Hamm, H.E. GPCR Mediated Regulation of Synaptic Transmission. Progress. Neurobiol. 2012, 96, 304–321.
- Benneyworth, M.A.; Smith, R.L.; Barrett, R.J.; Sanders-Bush, E. Complex Discriminative Stimulus Properties of (+) Lysergic Acid Diethylamide (LSD) in C57Bl/6J Mice. Psychopharmacology 2005, 179, 854–862.
- Winter, J.; Rice, K.; Amorosi, D.; Rabin, R. Psilocybin-Induced Stimulus Control in the Rat. Pharmacol. Biochem. Behav. 2007, 87, 472–480.
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
07 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





