Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Blerida Banushi and Version 2 by Rita Xu.
Psychedelic substances have gained significant attention in recent years for their potential therapeutic effects on various psychiatric disorders.
- psychedelics
- 5-HT2A
- BDNF
- TrkB
- serotonergic
- psilocybin
1. Introduction
Coined by Humphry Osmond in 1956, the term “psychedelic” originates from the Greek words meaning “mind manifesting” [1]. This term is used to describe the subjective effects of these substances, highlighting their ability to induce profound experiences and alter perception.
Psychedelics constitute a class of drugs obtained from specific plants, animals, and fungi, and they can be categorized into three primary classes based on their chemical structure: tryptamines, ergolines, and phenethylamines [2][3][2,3]. Tryptamines, such as psilocybin, N, N-dimethyltryptamine (DMT), and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) are characterized by an indole (aromatic group separated from a basic amine by a two-carbon linker) and share structural similarities with the neurotransmitter serotonin. Ergolines, such as lysergic acid diethylamide (LSD), are characterized by the presence of a tetracyclic ergoline ring, and were originally derived from the ergot fungus [4]. Phenethylamines, such as 2C-B, mescaline, amphetamine analogues—e.g., 2,5-Dimethoxy-4-iodoamphetamine (DOI) and 2,5-Dimethoxy-4-methylamphetamine (DOM), and derivatives such as 4-Iodo-2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamine (25I-NBOMe)—are characterized by a benzene ring with an amino group attached through a two-carbon chain [3]. In addition to classical psychedelics, there are atypical compounds like 3,4-Methylenedioxymethamphetamine (MDMA), muscimol, scopolamine, salvinorin A, ibogaine, nitrous oxide, phencyclidine (PCP), and ketamine that produce similar psychological effects but work through different mechanisms. These compounds are sometimes considered psychedelics under a broader definition [4].
Psychedelics have been used by humans for centuries. Historical records indicate that these substances have been consumed in ancient cultural rituals with the purpose of healing, attaining altered states of consciousness, and gaining spiritual insights, tracing back to prehistory [5][6][5,6]. The synthesis of mescaline in the early 1900s and the groundbreaking discovery of the classical hallucinogen LSD by Albert Hofmann in 1938 marked the beginning of Western psychedelic science [7][8][9][7,8,9]. During the 1950s and 1960s, these substances gained popularity in therapeutic and psychiatric settings due to their ability to facilitate psychotherapy [7]. However, in 1970, the U.S. Drug Enforcement Agency classified psychedelics as Schedule I drugs, which had a profound impact on research in the field [10]. Prior to their classification, over 1000 clinical studies were published, documenting promising therapeutic effects of psychedelics in more than 40,000 subjects. [7][11][12][7,11,12]. These studies indicated therapeutic benefits in various conditions such as anxiety and obsessive-compulsive disorders (OCD), depression, alcohol addiction, and sexual dysfunction, as well as pain and anxiety relief in patients with terminal cancer [10][13][14][15][16][17][18][19][10,13,14,15,16,17,18,19]. This regulatory decision effectively curtailed psychedelic research for approximately 30 years [20].
The advent of advanced neuroimaging techniques in the 1990s, including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), played a pivotal role in enhancing theour comprehension of molecular and physiological mechanisms within the central nervous system [21]. These breakthroughs also sparked a renewed interest in exploring the effects of psychedelic substances [22]. In the last three decades, there has been a resurgence in the exploration of psychedelics, reigniting research interest in their therapeutic potential. This renewed focus has led to anticipated FDA approvals for the use of psychedelics in treating various conditions, marking a period of exponential scientific growth in this field [23][24][25][23,24,25].
In recent years a growing number of clinical trials and studies have been conducted or are currently underway, investigating the therapeutic potential of psychedelics such as LSD and psilocybin for various mental health conditions including depression, anxiety, cancer-related anxiety disorders, addiction, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder, terminal illness, stroke, traumatic brain injury (TBI), neurodegenerative disorders, and chronic pain [22][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][22,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. In response to this growing interest, the U.S. Food and Drug Administration recently released a new draft guidance aimed at emphasizing essential considerations for researchers exploring the use of psychedelic drugs as potential treatments for medical conditions [42].
Randomized Phase II trials have so far demonstrated significant reductions in symptoms and long-lasting benefits following psilocybin-assisted psychotherapy for major depressive disorder, anxiety, and treatment-resistant depression [43][44][45][46][43,44,45,46]. Remarkably, even one or two doses of psilocybin have led to rapid and sustained improvements in mood and perspective, with symptomatic relief lasting for at least 3–12 months [28][45][46][47][28,45,46,47]. Furthermore, enduring positive effects and improved well-being following psilocybin administration have also been observed in healthy individuals. Recent Phase 3 trials investigating MDMA for PTSD have shown promising results, and hint at a potential paradigm shift in psychiatry towards utilizing substances with acute psychoactive effects to generate long-term benefits for psychiatric patients [48].
2. Psychedelics Exert Their Effects on the Brain at Multiple Levels, Engaging in Intricate and Multifaceted Mechanisms
The impact of psychedelics on the brain can be examined from multiple perspectives, including molecular/cellular, circuit/network, and overall brain levels, all of which are inherently interconnected (Figure 1). At the molecular/cellular level, psychedelics stimulate the serotonin 2A receptor (5-HT2R) along with other serotonin sub-receptors, tropomyosin receptor kinase B (TrkB), and dopamine receptors [49][50][50,51]. Classic psychedelics have been shown to elevate levels of glutamate and oxytocin [22][51][52][53][54][22,52,53,54,55], promote the production of brain-derived neurotrophic factor (BDNF) [50][55][56][51,56,57], stimulate neurogenesis [22][51][22,52], and exhibit anti-inflammatory properties [57][58]. On a cellular level, psychedelics also induce an increase in the expression of various genes that encode for the synthesis of a range of proteins that facilitate neuroplasticity and learning, even following a single dose [58][59][60][59,60,61].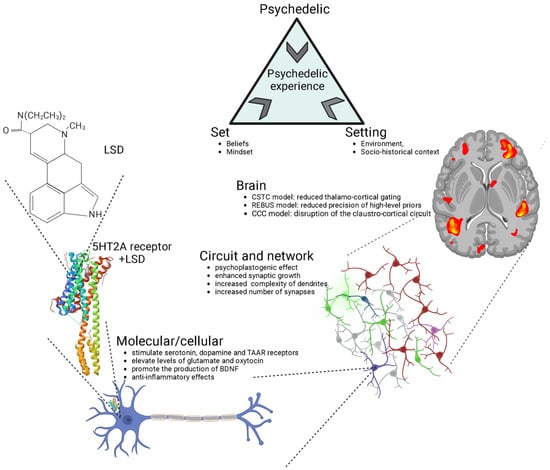
Figure 1. Psychedelics exert their effects through various levels of analysis, including the molecular/cellular, the circuit/network, and the overall brain. The crystal structure of serotonin 2A receptor in complex with LSD is sourced from the RCSB Protein Data Bank (RCSB PDB). LSD, lysergic acid diethylamide; 5-HT2A, serotonin 2A; CSTC, cortico-striato-thalamo-cortical; REBUS, relaxed beliefs under psychedelics model; CCC, claustro-cortical circuit.
3. 5-HT2A Receptor Signaling
Psychedelics have a similar chemical structure to serotonin (5-HT) [91][93]. Recent studies using advanced techniques like X-ray diffraction and cryo-electron microscopy have revealed the crystal structures of serotonin receptors when they are bound to psychedelic substances like LSD, psilocin, and others [92][93][62,94]. These studies provide new insights into how psychedelics work at the molecular level and their potential use in developing psychedelic-based treatments. Phenethylamines exhibit a greater level of specificity for 5-HT2A, 5-HT2B, and 5-HT2C receptors in comparison to tryptamines and ergolines [3][94][3,95]. In the past, it was widely believed that the therapeutic effects of psychedelics were mainly attributed to their activation of the 5-HT2AR [95][96][97][98][96,97,98,99], but recent research has revealed the involvement of other receptors in producing these effects [50][51]. This belief is substantiated by evidence showing that the particular neural circuits in the central nervous system impacted by serotonergic psychedelics align with the distribution of serotonin receptors (Figure 2) [99][100].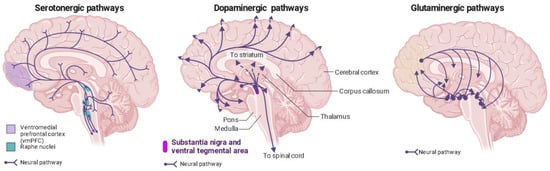
Figure 2. Distribution of serotonin, dopamine, and glutaminergic pathways in the human brain. Ventromedial prefrontal cortex (vmPFC) in purple; raphe nuclei in blue.
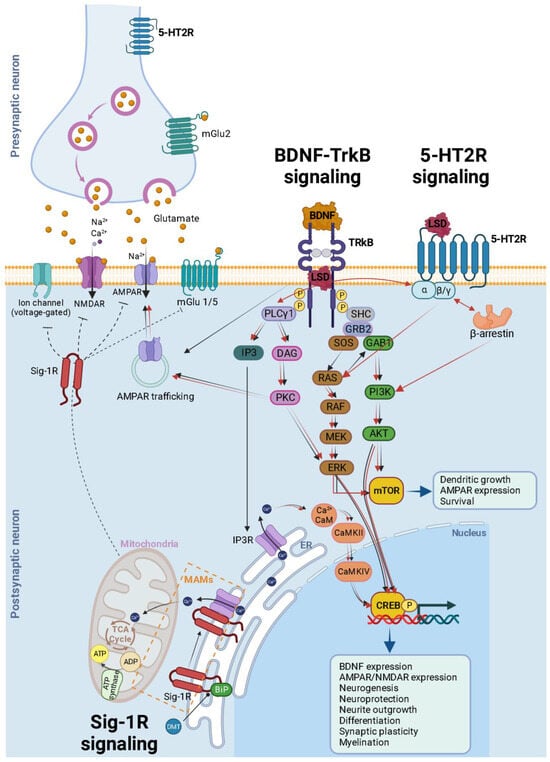
Figure 3. Schematic and simplified overview of the intracellular transduction cascades induced by 5-HT2AR TrkB and Sig-1R receptor activation by psychedelics. It is essential to emphasize that theour understanding of the activation or inhibition of specific pathways and the precise molecular mechanisms responsible for triggering plasticity in specific neuron types remains incomplete. This figure illustrates the mechanisms associated with heightened plasticity within these pathways. Psychedelics (such as LSD, psilocin, and mescaline) bind to TrkB dimers, stabilizing their conformation. Furthermore, they enhance the localization of TrkB dimers within lipid rafts, thereby extending their signaling via PLCγ1. The BDNF/TrkB signaling pathway (black arrows) initiates with BDNF activating TrkB, prompting autophosphorylation of tyrosine residues within TrkB’s intracellular C-terminal domain (specifically Tyr490 and Tyr515), followed by the recruitment of SHC. This, in turn, leads to the binding of GRB2, which subsequently associates with SOS and GTPase RAS to form a complex, thereby initiating the ERK cascade. This cascade ultimately results in the activation of the CREB transcription factor. CREB, in turn, mediates the transcription of genes essential for neuronal survival, differentiation, BDNF production, neurogenesis, neuroprotection, neurite outgrowth, synaptic plasticity, and myelination. Activation of Tyr515 in TrkB also activates the PI3K signaling pathway through GAB1 and the SHC/GRB2/SOS complex, subsequently leading to the activation of protein kinase AKT and CREB. Both Akt and ERK activate mTOR, which is associated with downstream processes involving dendritic growth, AMPAR expression, and overall neuronal survival. Additionally, the phosphorylation of TrkB’s Tyr816 residue activates the phospholipase Cγ (PLCγ) pathway, generating IP3 and DAG. IP3 activates its receptor (IP3R) in the endoplasmic reticulum (ER), causing the release of calcium (Ca2+) from the ER and activating Ca2+/CaM/CaMKII which in turn activates CREB. DAG activates PKC, leading to ERK activation and synaptic plasticity. After being released into the extracellular space, glutamate binds to ionotropic glutamate receptors, including NMDA receptors (NMDARs) and AMPA receptors (AMPARs), as well as metabotropic glutamate receptors (mGluR1 to mGluR8), located on the membranes of both postsynaptic and presynaptic neurons. Upon binding, these receptors initiate various responses, such as membrane depolarization, activation of intracellular messenger cascades, modulation of local protein synthesis, and ultimately, gene expression. The surface expression and function of NMDARs and AMPARs are dynamically regulated through processes involving protein synthesis, degradation, and receptor trafficking between the postsynaptic membrane and endosomes. This insertion and removal of postsynaptic receptors provides a mechanism for the long-term modulation of synaptic strength. Psychedelic compounds exhibit a high affinity for 5-HT2R, leading to the activation of G-protein and β-arrestin signaling pathways (red arrows). Downstream for 5-HT2R activation, these pathways intersect with both PI3K/Akt and ERK kinases, similar to the BDNF/TrkB signaling pathway. This activation results in enhanced neural plasticity. A theoretical model illustrating the signaling pathway of DMT through Sig-1R at MAMs suggests that, at endogenous affinity concentrations (14 μM), DMT binds to Sig-1R, triggering the dissociation of Sig-1R from BiP. This enables Sig-1R to function as a molecular chaperone for IP3R, resulting in an increased flow of Ca2+ from the ER into the mitochondria. This, in turn, activates the TCA cycle and enhances the production of ATP. However, at higher concentrations (100 μM), DMT induces the translocation of Sig-1Rs from the MAM to the plasma membrane (dashed inhibitory lines), leading to the inhibition of ion channels. BDNF = brain-derived neurotrophic factor; TrkB = tropomyosin-related kinase B; LSD = lysergic acid diethylamide; SHC = src homology domain containing; SOS = son of sevenless; Ras = GTP binding protein; Raf = Ras associated factor; MEK = MAP/Erk kinase; mTOR = mammalian target of rapamycin; ERK = extracellular signal regulated kinase; GRB2 = growth factor receptor bound protein 2; GAB1 = GRB-associated binder 1; PLC = phospholipase C γ; IP3 = inositol-1, 4, 5-triphosphate; DAG = diacylglycerol; PI3K = phosphatidylinositol 3-kinase; CaMKII = calcium/calmodulin-dependent kinase; CREB = cAMP-calcium response element binding protein; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; Sig-1R = sigma-1 receptor; DMT = N,N-dimethyltryptamine; BiP = immunoglobulin protein; MAMs = mitochondria-associated ER membrane; ER = endoplasmic reticulum; TCA = tricarboxylic acid; AT P = adenosine triphosphate; ADP = adenosine diphosphate.
