Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jin-Xian Gao | -- | 2991 | 2023-11-02 08:36:42 | | | |
| 2 | Lindsay Dong | Meta information modification | 2991 | 2023-11-06 01:32:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gao, J.; Yan, G.; Li, X.; Xie, J.; Spruyt, K.; Shao, Y.; Hou, Y. The Ponto-Geniculo-Occipital Waves in Dreaming. Encyclopedia. Available online: https://encyclopedia.pub/entry/51072 (accessed on 07 January 2026).
Gao J, Yan G, Li X, Xie J, Spruyt K, Shao Y, et al. The Ponto-Geniculo-Occipital Waves in Dreaming. Encyclopedia. Available at: https://encyclopedia.pub/entry/51072. Accessed January 07, 2026.
Gao, Jin-Xian, Guizhong Yan, Xin-Xuan Li, Jun-Fan Xie, Karen Spruyt, Yu-Feng Shao, Yi-Ping Hou. "The Ponto-Geniculo-Occipital Waves in Dreaming" Encyclopedia, https://encyclopedia.pub/entry/51072 (accessed January 07, 2026).
Gao, J., Yan, G., Li, X., Xie, J., Spruyt, K., Shao, Y., & Hou, Y. (2023, November 02). The Ponto-Geniculo-Occipital Waves in Dreaming. In Encyclopedia. https://encyclopedia.pub/entry/51072
Gao, Jin-Xian, et al. "The Ponto-Geniculo-Occipital Waves in Dreaming." Encyclopedia. Web. 02 November, 2023.
Copy Citation
Rapid eye movement (REM) sleep is the main sleep correlate of dreaming. Ponto-geniculo-occipital (PGO) waves are a signature of REM sleep. They represent the physiological mechanism of REM sleep that specifically limits the processing of external information. PGO waves look just like a message sent from the pons to the lateral geniculate nucleus of the visual thalamus, the occipital cortex, and other areas of the brain. The dedicated visual pathway of PGO waves can be interpreted by the brain as visual information, leading to the visual hallucinosis of dreams. PGO waves are considered to be both a reflection of REM sleep brain activity and causal to dreams due to their stimulation of the cortex.
rapid eye movement sleep
ponto-geniculo-occipital waves
non-rapid eye movement sleep
1. Introduction
Dreams are images and experiences that people have while they sleep. In ancient cultures, dreams were believed to contain messages from the gods or omens of the future. In the late 19th century, dreams became a subject of study for psychologists and psychoanalysts. Sigmund Freud’s The Interpretation of Dreams [1] initially developed the most prominent psychoanalytic theory of dreams. This model has been quite influential in sleep research and continues to have strong adherents to this day. For Freud, the dream is a highly meaningful mental product that is the result of specific mental processes under the conditions of sleep. Dreams that have “hidden meanings” and “repressed desires” are well established in popular folk psychology. Although Freud theorized that the purpose of dreaming is wish fulfillment, there is little experimental evidence to support this concept.
Modern sleep science has evolved significantly since the late 1950s, largely due to the seminal discoveries of rapid eye movement (REM) sleep [2][3][4][5]. Aserinsky and Kleitman [2] found that 74% of awakenings from REM sleep resulted in the recall of a dream, as compared to only 9% of awakenings from non-REM (NREM) sleep. The linking of REM sleep to dreaming ushered in a new era in the study of dreams [6]. During REM sleep, in addition to rapid eye movements (REMs), cortical electroencephalogram (EEG) desynchronization (or activation), loss of muscle tone, and autonomic fluctuations [2][3][4][5], many other physiological and behavioral features have also been found in humans and other mammals. These include high-amplitude spiky potentials of ponto-geniculo-occipital (PGO) waves, high-amplitude hippocampal EEG theta waves, penile erections, sporadic limb twitching, increases in brain/body temperature, and an elevated arousal threshold [7][8][9][10][11][12][13][14][15][16][17]. In these characteristic components of REM sleep, PGO waves are undoubtedly powerful internal sensory signals that convey a large amount of information to the visual cortex and seem to “compose the song sheet of dreams” [18]. Accordingly, a “dream state generator”, located mainly in the pontine reticular formation and producing PGO waves, has been postulated to be the cause of both REMs and the periodic intrusion of new content into hallucinatory dreams [19][20][21][22].
2. The Neural Mechanisms Underlying Dreaming
2.1. PGO Waves
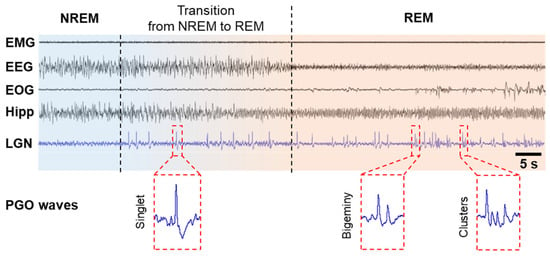
PGO wave activity was first discovered in cats in the 1950s [5][23][24]. Because these local field potentials originate in the pons (P) and propagate to the lateral geniculate nucleus (G) of the visual thalamus and the occipital cortex (O), they are called PGO waves [18][25][26]. PGO waves occur just before the onset of REM sleep and continue throughout its duration. They are characterized as biphasic, sharp field potentials lasting 60–120 ms, with an amplitude of 200–300 μV, occurring as singlets and clusters (Figure 1). These spikes during REM sleep period are in parallel to eye saccades and are observed not only in cats [5][25][27][28][29] but also in rats [30][31], mice [32], in non-human primates such as macaques [33][34], baboons [35], and in humans [7][36][37][38]. Cholinergic/glutamatergic neurons in the pontine brainstem have been shown to generate PGO waves by burst firing. PGO waves look just like a message sent from the pons to the lateral geniculate nucleus (LGN), occipital cortex, and other brain regions, including the temporal and prefrontal cortices and the amygdala. The dedicated visual pathway of PGO waves could be interpreted by the brain as visual information, thereby leading to the visual hallucinosis of dreams. These waves are not only associated with dream production but also limit the cortex’s ability to process external inputs [39][40].

Figure 1. PGO waves in cats. PGO waves occur just before the onset of REM sleep, i.e., during the transition from NREM to REM, and during REM sleep period. PGO wave singlets occur predominantly during the transition from NREM to REM sleep and are not time-locked to rapid eye movements (REMs), but PGO wave clusters (≥3 waves) occur predominantly during REM sleep and correlate strongly with REMs and typical hippocampal theta oscillations. Abbreviations: EEG, electroencephalogram of the neocortex; EMG, electromyogram; EOG, electro-oculogram; Hipp, EEG of the hippocampus; LGN, EEG of the lateral geniculate nucleus.
In humans, many invasive and non-invasive studies provide insight into how PGO waves occur during REM sleep and how they contribute to dreaming. For example, in two invasive studies in Parkinson’s disease patients, deep brain stimulation electrodes were surgically implanted in the pedunculopontine nucleus of the pontine tegmentum [36] and the subthalamic nucleus (STN) [7]. The results showed that PGO-like waves were observed during REM sleep. Much like feline models, PGO wave singlets and clusters were recorded within STN during pre-REM and REM sleep [7].
Overall, much of the detailed understanding of PGO waves and their mechanisms still depends on animal studies. However, direct translation to humans may require further investigation and validation. Using noninvasive methods such as fMRI and magnetoencephalogram, the future of PGO wave research undoubtedly lies in broad studies that combine behavioral, pharmacological, physiological, and cognitive experiments in human subjects [38].
2.2. Activation–Synthesis Hypothesis
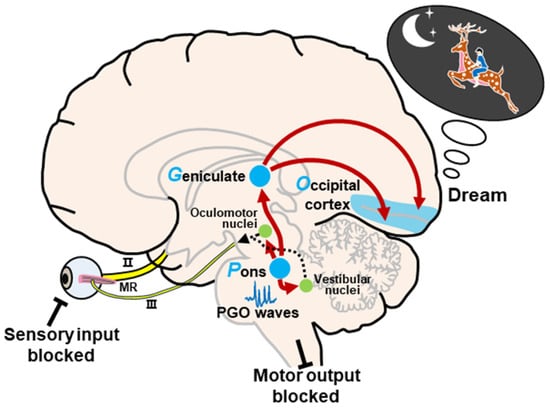
Hobson and McCarley, following the pioneering work of Jouvet [41], proposed the activation–synthesis hypothesis in 1977 [19]. This hypothesis is based primarily on microelectrode recordings of PGO waves in cats, which were found to occur primarily during REM sleep. The dreaming process consists of activation and synthesis. In short, the neural activity in the pons activates the brain, especially the LGN and visual cortex, to generate information during REM sleep. Under these conditions, in the absence of external stimuli, internally generated inputs randomly activate sensorimotor information, and the passive synthesis of this information (perceptual, conceptual, and emotional) creates dreams (Figure 2). During REM sleep, these internal inputs generated by PGO waves continuously activate the forebrain via the

Figure 2. Schematic representation of the activation–synthesis model. Neural activity of PGO waves in the pons activates the lateral geniculate nucleus of the visual thalamus and the visual cortex. The passive synthesis of information (perceptual, conceptual, and emotional) generates dreams in the cortex. Meanwhile, the neural activity in the pons also activates the vestibular nucleus and oculomotor-related nuclei, such as the oculomotor nucleus, the trochlear nucleus, and the abducens nucleus, to induce REMs during REM sleep. In this state, external input and motor output are blocked. Abbreviations: II, optic nerve; III, oculomotor nerve; MR, medial rectus.
2.3. Activation, Input, and Modulation (AIM) Model
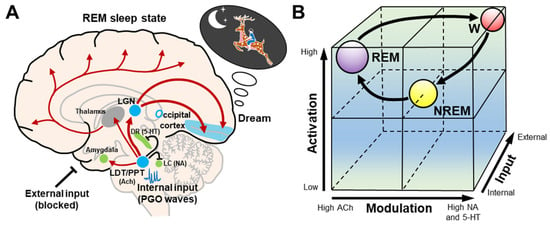
The activation–synthesis hypothesis of dreaming was updated and extended by Hobson et al. [21][22], and transformed into the AIM state-space model of the brain–mind isomorphism, where a conscious state can be understood as a point in a three-dimensional state space. In the AIM model, the variables consist of activation (A), input (I), and modulation (M) (Figure 3). (A) The level of brain activation can be defined as the average firing frequency of brainstem neurons as reflected by high and low frequency levels in the EEG. The high levels of A in REM sleep are a correlate of the mind’s ability to access and manipulate significant amounts of stored information from the brain during dream synthesis. (I) Input source is a measure of how much of the sensory data being processed are from external or internal sources. During REM sleep, internally generated PGO waves replace blocked external sensory input, activating sensory and affective centers that then prime the cortex for dream construction by stimulating the visual cortex with PGO waves. This can be estimated from the frequency of REMs in REM sleep, which is thought to reflect brainstem PGO and motor generator activity. (M) The brainstem neuromodulators that they release exert a broad chemical influence on the brain. It is well known that REM sleep is cholinergic potentiated and monoaminergic suppressed (Figure 3A).

Figure 3. Schematic representation of the AIM model. (A) During REM sleep dreams, cholinergic neurons in the LDT/PPT are involved in the generation of internal input PGO waves, while serotonergic dorsal raphe nucleus and noradrenergic locus coeruleus neurons and external inputs are inhibited. These internal inputs activate the LGN and occipital cortex, and produce dreams. The LDT/PPT–thalamus–cerebral cortex pathway causes the desynchronization of the cerebral cortex. The LDT/PPT–amygdala pathway may be involved in mood regulation during dreams. (B) The cubic 3-dimensional model shows normal transitions within the 3-dimensional parameters (activation, input, and modulation) from wakefulness to NREM and then to REM sleep. Abbreviations: 5–HT, serotonergic; ACh, cholinergic; DR, dorsal raphe nucleus; LC, locus coeruleus; LDT/PPT, laterodorsal tegmental and pedunculopontine nuclei; LGN, lateral geniculate nucleus; NA, noradrenergic; NREM, non-rapid eye movement; REM, rapid eye movement; W, wakefulness.
2.4. Neuronal Mechanisms of REM Sleep Regulation
REM sleep is generally thought to be mediated by a neural network located primarily in the brainstem. More recently, the concept of REM sleep regulation has evolved. Several hypothalamic and forebrain networks, including newly identified neuropeptides such as orexin and melanin-concentrating hormone (MCH), have been implicated in both the control and the final expression of this behavioral state [14][16][17][42][43][44][45][46][47]. Firstly, a reciprocal interaction between REM on and REM off states has been proposed to occur in the brainstem. In this model, the cholinergic laterodorsal and pedunculopontine tegmental neurons (LDT/PPT) are REM-on cells. The serotonergic dorsal raphe nucleus and noradrenergic locus coeruleus neurons are REM-off cells [22][48][49] (Figure 3A). However, this reciprocal interaction may not be sufficient to produce REM sleep, as suggested by experimental evidence over the past decade [16][46]. The glutamateric sublaterodorsal tegmental nucleus (SLD) and the GABAergic lateral paragigantocellular nucleus (LPGi) have been subsequently found to act as REM-on neurons, whereas GABAergic ventrolateral periaqueductal gray matter (vlPAG) and lateral pontine tegmentum (LPT) act as REM-off neurons [16][46].
2.5. Other Recent Mechanisms of Dreaming
Solms [50] has hypothesized that dreaming is controlled by forebrain mechanisms. It is suggested that the cholinergic brain stem mechanisms that control the REM state are only able to produce the psychological phenomena of dreaming through the mediation of a second, presumably dopaminergic, forebrain mechanism. The dopaminergic forebrain circuits arise from the neurons in the ventral tegmental area (VTA) and terminate in the amygdala, anterior cingulate gyrus, and frontal cortex. This neural circuit of the mesocortical–mesolimbic dopamine system has been implicated in dream generation, and has been described as the “SEEKING” or “wanting” command system to subserve emotional drive and motivation [50].
The amygdala, a limbic structure associated with emotions, memory, and dreams, receives the dopaminergic projection. It serves as a node to integrate the regulation of REM sleep and causes the intermittent appearance of REM sleep-related dreams and REM sleep behavior disorder (RBD) [51][52][53]. The amygdala is very active in REM sleep, especially in humans [54][55]. It can influence the frequency of PGO waves during REM sleep. This suggests that it also plays a key role in setting the “emotional tone” for PGO activity [56].
3. The Physiological Functions of Dreaming: The Involvement of PGO Waves during REM Sleep
3.1. Memory Consolidation
Oneiric production is a form of mental sleep activity that appears to be closely related to memory processes and cognitive elaboration [57][58][59]. Converging evidence suggests that dreaming is influenced by memory consolidation during sleep [60]. A number of studies have reported that PGO waves during REM sleep in rodents have been repeatedly associated with memory consolidation [61]. For example, PGO waves increased during REM sleep following learning tasks [62][63][64][65][66]. Artificially enhancing PGO waves by injecting carbachol prevented avoidance memory deficits during a period of REM sleep deprivation [67], while suppressing PGO wave generation in rats impaired avoidance memory retention during sleep [68]. It appears that the density of PGO wave activity is directly related to memory processes. A number of studies have reported an increase in PGO wave density following fear memory training in rats, which predicted overnight memory consolidation [62][63][64][65]. The success of fear extinction was recently shown to be predicted by PGO wave density during REM sleep [66].
In general, PGO waves in the transition from NREM to REM sleep are considered to be the physiological signals that initiate and maintain REM sleep, constituting a state with characteristics distinct from both the preceding NREM sleep and the following REM sleep [69][70]. Pontine caudolateral parabrachial neuronal discharge has been found to contribute to the shift toward the two PGO-related states, the transition from NREM to REM sleep and REM sleep [34][69][71].
3.2. Unlearning
One of the hypothesized functions of REM sleep is a process of “unlearning” [20][72]. These authors proposed that the function of dream sleep is the removal of certain unwanted memories from the cerebral cortex. During REM sleep, the unconscious dream traces act to weaken rather than strengthen memory. It is noteworthy that “we dream to forget” [20] is not the same as normal forgetting. Dreams are not simply forgotten; they are actively unlearned [73]. The unlearning mechanism modifies the cerebral cortex by changing the strength of individual synapses. Because an increase in synaptic strength is necessary to consolidate memory, unlearning weakens synaptic strength.
The unlearning theory is supported by a growing number of studies. For example, during REM sleep, the postsynaptic dendritic spines of layer V pyramidal cells in the mouse motor cortex are eliminated during development and motor learning. On the other hand, critical spines are strengthened and maintained [74]. The hypothalamic MCH neurons are known to be involved in the control of REM sleep and mood [75]. An anatomical and functional study in cats found that MCH neurons project to cholinergic pontine neurons [76]. When MCH was microinjected into the nucleus pontis oralis, there was a significant decrease in latency to REM sleep and a significant increase in the amount of REM sleep, accompanied by increased PGO wave activity and its duration. This suggests that the MCH system is involved in the regulation of REM sleep by modulating neuronal activity in cholinergic pontine neurons [76]. However, it has recently been shown that MCH neurons are also involved in the unlearning mechanism of REM sleep [77]. The activation or inhibition of MCH neurons by optogenetics and chemogenetics impaired or improved hippocampal-dependent memory, respectively. The activation of MCH nerve terminals in vitro reduced the firing of hippocampal pyramidal neurons by increasing inhibitory inputs. These results strongly suggest that the activation of MCH projections to the hippocampus during REM sleep actively contributes to forgetting [77]. The activity of hippocampal neurons during REM sleep has been suggested to play a key role in unlearning [78]. REM sleep serves to maintain or strengthen memories until they are transferred out of the hippocampus, whereupon they should be erased from this space-restricted short-term memory factory so that these synapses can be used to encode new associative memories [78]. PGO waves that are phase-locked to the theta oscillation of the hippocampus during REM sleep may be involved in this process [79].
3.3. Brain Development and Plasticity
REM sleep is known to be particularly abundant during early development. At birth, half or more of our sleep time is occupied by REM sleep, compared to <20% of sleep time in adults [80]. PGO waves during REM sleep in development might be an important central nervous system (CNS) stimulator during a period when wakefulness is limited in time and scope and stimulation opportunities are few [79][81][82]. The ascending impulses emanating from the brainstem during REM sleep may be required to promote neuronal differentiation, maturation, and myelination in higher brain centers [17]; that is, REM sleep deprivation during the early life of animals has been used to understand some functional mechanisms of PGO waves in brain development.
Several lines of evidence have shown that PGO waves in REM sleep are associated with the regulation of neural plasticity [83][84][85]. Brain plasticity allows for the preservation of the ability to change, adapt, and learn in response to different environmental experiences and new demands. These processes occur with sleep cycles throughout life and begin in response to REM sleep in late fetal and early neonatal life [86]. PGO wave-associated cells are good candidates for generating or modulating plasticity in various brain structures [63], as they discharge high-frequency spike bursts during pre-REM and REM sleep [34]. Indeed, the activation of PGO wave-generating cells by cholinergic agonism induces changes to the electrical properties of PGO wave activity [87], accompanied by prominent behavioral effects [66][88].
3.4. Mood Regulation
Because REM sleep is thought to play an important role in emotional memory processing, disrupted REM sleep may be an important contributor to the pathophysiology of emotion-based disorders such as major depression and PTSD [89]. Major depression is extremely common and is one of the leading causes of disability worldwide. Sleep disturbances are typical of most patients with major depression and are a core symptom of the disorder. Polysomnographic indices document objective changes in sleep continuity, slow-wave activity reduction, and REM sleep alterations, such as a shortening of REM sleep latency, prolongation of the first REM sleep period, and increased REMs density [90][91].
It has been proposed that PGO waves enhance synaptic plasticity in the areas that they pass through [62]. This includes the hippocampus and the amygdala [61][92]. Several studies have found that high amygdala reactivity is associated with an increased risk for the development of major depression [93]. The pons receives amygdala axonal projections [94], and the electrical stimulation of the amygdala increases the density of the PGO wave during REM sleep [95]. The antidepressant drugs (norepinephrine or serotonin reuptake inhibitors) reduce the density of the PGO waves [96]. The inhibition of the generation of PGO waves may have an antidepressant effect [96]. The hippocampus, which plays a central role in mood dysregulation and neurogenesis, appears to be associated with the behavioral symptoms of major depression. The negative effects of REM sleep disruption on hippocampus-dependent cognitive functions may be due to a decrease in adult hippocampal neurogenesis in humans [97]. Decreased functional connectivity at limbic cortical levels, particularly in the prefrontal, anterior cingulate, and insula, altered amygdala microstructure, and decreased claustrum volume have been reported following major depression [98][99][100][101].
References
- Freud, S. The Interpretation of Dreams. In The Standard Edition of the Complete Psychological Works of Sigmund Freud; Strachey, J., Ed.; Hogarth Press: London, UK, 1953; Volume 4–5.
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274.
- Dement, W.; Kleitman, N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr. Clin. Neurophysiol. 1957, 9, 673–690.
- Dement, W.; Kleitman, N. The relation of eye movements during sleep to dream activity: An objective method for the study of dreaming. J. Exp. Psychol. 1957, 53, 339–346.
- Jouvet, M.; Michel, F. Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat. C. R. Seances Soc. Biol. Fil. 1959, 153, 422–425.
- Eiser, A.S. Physiology and psychology of dreams. Semin. Neurol. 2005, 25, 97–105.
- Fernández-Mendoza, J.; Lozano, B.; Seijo, F.; Santamarta-Liébana, E.; Ramos-Platón, M.J.; Vela-Bueno, A.; Fernández-González, F. Evidence of subthalamic PGO-like waves during REM sleep in humans: A deep brain polysomnographic study. Sleep 2009, 32, 1117–1126.
- Jouvet, M. Neurophysiology of the states of sleep. Physiol. Rev. 1967, 47, 117–177.
- Jouvet, M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb. Physiol. 1972, 64, 166–307.
- Negoescu, R.M.; Csiki, I.E. Autonomic control of the heart in some vagal maneuvers and normal sleep. Physiologie 1989, 26, 39–49.
- Monti, A.; Medigue, C.; Nedelcoux, H.; Escourrou, P. Autonomic control of the cardiovascular system during sleep in normal subjects. Eur. J. Appl. Physiol. 2002, 87, 174–181.
- Siegel, J. Clues to the functions of mammalian sleep. Nature 2005, 437, 1264–1271.
- Perogamvros, L.; Dang-Vu, T.T.; Desseilles, M.; Schwartz, S. Sleep and dreaming are for important matters. Front. Psychol. 2013, 4, 474.
- Peever, J.; Fuller, P.M. The Biology of REM Sleep. Curr. Biol. 2017, 27, R1237–R1248.
- Dement, W.C.; Pelayo, R. Reminiscences of Michel Jouvet. Sleep Med. 2018, 49, 78–80.
- Wang, Y.Q.; Liu, W.Y.; Li, L.; Qu, W.M.; Huang, Z.L. Neural circuitry underlying REM sleep: A review of the literature and current concepts. Prog. Neurobiol. 2021, 204, 102106.
- Chen, H.L.; Gao, J.X.; Chen, Y.N.; Xie, J.F.; Xie, Y.P.; Spruyt, K.; Lin, J.S.; Shao, Y.F.; Hou, Y.P. Rapid Eye Movement Sleep during Early Life: A Comprehensive Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 13101.
- Arnulf, I.; Buda, C.; Sastre, J.P. Michel Jouvet: An explorer of dreams and a great storyteller. Sleep Med. 2018, 49, 4–9.
- Hobson, J.A.; McCarley, R.W. The brain as a dream state generator: An activation-synthesis hypothesis of the dream process. Am. J. Psychiatry 1977, 134, 1335–1348.
- Crick, F.; Mitchison, G. The function of dream sleep. Nature 1983, 304, 111–114.
- Hobson, J.A.; Pace-Schott, E.F.; Stickgold, R. Dreaming and the brain: Toward a cognitive neuroscience of conscious states. Behav. Brain Sci. 2000, 23, 793–842; discussion 904–1121.
- Hobson, J.A. REM sleep and dreaming: Towards a theory of protoconsciousness. Nat. Rev. Neurosci. 2009, 10, 803–813.
- Mikiten, T.; Niebyl, P.; Hendley, C. EEG desynchronization during behavioral sleep associated with spike discharges from the thalamus of the cat. Fed. Proc. 1961, 20, 327.
- Jeannerod, M.; Mouret, J.; Jouvet, M. The study of the ocular motor activity during the paradoxical phase of sleep in the cat. Electroencephalogr. Clin. Neurophysiol. 1965, 18, 554–566.
- Mouret, J.; Jeannerod, M.; Jouvet, M. Electrical activity of the visual system during the paradoxical phase of sleep in the cat. J. Physiol. 1963, 55, 305–306.
- Hobson, J.A.; Friston, K.J. Waking and dreaming consciousness: Neurobiological and functional considerations. Prog. Neurobiol. 2012, 98, 82–98.
- Cespuglio, R.; Laurent, J.P.; Jouvet, M. Relationships between ponto-geniculo-occipital (pgo) activity and ocular movements in reserpinised anaesthetised cat (author’s transl). Brain Res. 1975, 83, 319–335.
- Vanni-Mercier, G.; Debilly, G. A key role for the caudoventral pontine tegmentum in the simultaneous generation of eye saccades in bursts and associated ponto-geniculo-occipital waves during paradoxical sleep in the cat. Neuroscience 1998, 86, 571–585.
- Karashima, A.; Nakao, M.; Katayama, N.; Honda, K. Instantaneous acceleration and amplification of hippocampal theta wave coincident with phasic pontine activities during REM sleep. Brain Res. 2005, 1051, 50–56.
- Kaufman, L.S.; Morrison, A.R. Spontaneous and elicited PGO spikes in rats. Brain Res. 1981, 214, 61–72.
- Karashima, A.; Katayama, N.; Nakao, M. Enhancement of synchronization between hippocampal and amygdala theta waves associated with pontine wave density. J. Neurophysiol. 2010, 103, 2318–2325.
- Tsunematsu, T.; Patel, A.A.; Onken, A.; Sakata, S. State-dependent brainstem ensemble dynamics and their interactions with hippocampus across sleep states. Elife 2020, 9, e52244.
- Cohen, B.; Feldman, M. Relationship of electrical activity in pontine reticular formation and lateral geniculate body to rapid eye movements. J. Neurophysiol. 1968, 31, 806–817.
- Ramirez-Villegas, J.F.; Besserve, M.; Murayama, Y.; Evrard, H.C.; Oeltermann, A.; Logothetis, N.K. Coupling of hippocampal theta and ripples with pontogeniculooccipital waves. Nature 2021, 589, 96–102.
- Vuillon-Cacciuttolo, G.; Seri, B. Effects of optic nerve section in baboons on the geniculate and cortical spike activity during various states of vigilance. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 754–768.
- Lim, A.S.; Lozano, A.M.; Moro, E.; Hamani, C.; Hutchison, W.D.; Dostrovsky, J.O.; Lang, A.E.; Wennberg, R.A.; Murray, B.J. Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons. Sleep 2007, 30, 823–827.
- Hong, C.C.; Harris, J.C.; Pearlson, G.D.; Kim, J.S.; Calhoun, V.D.; Fallon, J.H.; Golay, X.; Gillen, J.S.; Simmonds, D.J.; van Zijl, P.C.; et al. fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum. Brain Mapp. 2009, 30, 1705–1722.
- Gott, J.A.; Liley, D.T.; Hobson, J.A. Towards a Functional Understanding of PGO Waves. Front. Hum. Neurosci. 2017, 11, 89.
- Andrillon, T.; Kouider, S. The vigilant sleeper: Neural mechanisms of sensory (de) coupling during sleep. Curr. Opin. Physiol. 2020, 15, 47–59.
- Van De Poll, M.N.; van Swinderen, B. Balancing Prediction and Surprise: A Role for Active Sleep at the Dawn of Consciousness? Front. Syst. Neurosci. 2021, 15, 768762.
- Jouvet, M. Research on the neural structures and responsible mechanisms in different phases of physiological sleep. Arch. Ital. Biol. 1962, 100, 125–206.
- Blumberg, M.S.; Dooley, J.C.; Sokoloff, G. The developing brain revealed during sleep. Curr. Opin. Physiol. 2020, 15, 14–22.
- Ferreira, J.G.P.; Bittencourt, J.C.; Adamantidis, A. Melanin-concentrating hormone and sleep. Curr. Opin. Neurobiol. 2017, 44, 152–158.
- Liu, D.; Dan, Y. A Motor Theory of Sleep-Wake Control: Arousal-Action Circuit. Annu. Rev. Neurosci. 2019, 42, 27–46.
- Luppi, P.H. Jouvet’s animal model of RBD, clinical RBD, and their relationships to REM sleep mechanisms. Sleep Med. 2018, 49, 28–30.
- Héricé, C.; Patel, A.A.; Sakata, S. Circuit mechanisms and computational models of REM sleep. Neurosci. Res. 2019, 140, 77–92.
- Park, S.H.; Weber, F. Neural and Homeostatic Regulation of REM Sleep. Front. Psychol. 2020, 11, 1662.
- Hobson, J.A.; McCarley, R.W.; Wyzinski, P.W. Sleep cycle oscillation: Reciprocal discharge by two brainstem neuronal groups. Science 1975, 189, 55–58.
- McCarley, R.W.; Hobson, J.A. Neuronal excitability modulation over the sleep cycle: A structural and mathematical model. Science 1975, 189, 58–60.
- Solms, M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav. Brain Sci. 2000, 23, 843–850; discussion 904–1121.
- Hasegawa, E.; Miyasaka, A.; Sakurai, K.; Cherasse, Y.; Li, Y.; Sakurai, T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science 2022, 375, 994–1000.
- Perogamvros, L.; Schwartz, S. Sleep and emotional functions. Curr. Top. Behav. Neurosci. 2015, 25, 411–431.
- Kumar Yadav, R.; Mallick, B.N. Dopaminergic- and cholinergic-inputs from substantia nigra and pedunculo-pontine tegmentum, respectively, converge in amygdala to modulate rapid eye movement sleep in rats. Neuropharmacology 2021, 193, 108607.
- Dang-Vu, T.T.; Schabus, M.; Desseilles, M.; Sterpenich, V.; Bonjean, M.; Maquet, P. Functional neuroimaging insights into the physiology of human sleep. Sleep 2010, 33, 1589–1603.
- Horne, J. Why REM sleep? Clues beyond the laboratory in a more challenging world. Biol. Psychol. 2013, 92, 152–168.
- Morrison, A.R.; Sanford, L.D.; Ross, R.J. The amygdala: A critical modulator of sensory influence on sleep. Biol. Signals Recept. 2000, 9, 283–296.
- Scarpelli, S.; Alfonsi, V.; Gorgoni, M.; De Gennaro, L. What about dreams? State of the art and open questions. J. Sleep Res. 2022, 31, e13609.
- Wamsley, E.J.; Stickgold, R. Dreaming and offline memory processing. Curr. Biol. 2010, 20, R1010–R1013.
- Mangiaruga, A.; Scarpelli, S.; Bartolacci, C.; De Gennaro, L. Spotlight on dream recall: The ages of dreams. Nat. Sci. Sleep 2018, 10, 1–12.
- Wamsley, E.J. Dreaming and offline memory consolidation. Curr. Neurol. Neurosci. Rep. 2014, 14, 433.
- Hutchison, I.C.; Rathore, S. The role of REM sleep theta activity in emotional memory. Front. Psychol. 2015, 6, 1439.
- Datta, S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J. Neurosci. 2000, 20, 8607–8613.
- Datta, S.; Saha, S.; Prutzman, S.L.; Mullins, O.J.; Mavanji, V. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J. Neurosci. Res. 2005, 80, 727–737.
- Ulloor, J.; Datta, S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: A mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J. Neurochem. 2005, 95, 418–428.
- Datta, S.; Li, G.; Auerbach, S. Activation of phasic pontine-wave generator in the rat: A mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. Eur. J. Neurosci. 2008, 27, 1876–1892.
- Datta, S.; O’Malley, M.W. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J. Neurosci. 2013, 33, 4561–4569.
- Datta, S.; Mavanji, V.; Ulloor, J.; Patterson, E.H. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: A mechanism for sleep-dependent plasticity. J. Neurosci. 2004, 24, 1416–1427.
- Mavanji, V.; Ulloor, J.; Saha, S.; Datta, S. Neurotoxic lesions of phasic pontine-wave generator cells impair retention of 2-way active avoidance memory. Sleep 2004, 27, 1282–1292.
- Datta, S. Neuronal activity in the peribrachial area: Relationship to behavioral state control. Neurosci. Biobehav. Rev. 1995, 19, 67–84.
- Carrera-Cañas, C.; Garzón, M.; de Andrés, I. The Transition Between Slow-Wave Sleep and REM Sleep Constitutes an Independent Sleep Stage Organized by Cholinergic Mechanisms in the Rostrodorsal Pontine Tegmentum. Front. Neurosci. 2019, 13, 748.
- Datta, S.; Hobson, J.A. Neuronal activity in the caudolateral peribrachial pons: Relationship to PGO waves and rapid eye movements. J. Neurophysiol. 1994, 71, 95–109.
- Hopfield, J.J.; Feinstein, D.I.; Palmer, R.G. ‘Unlearning’ has a stabilizing effect in collective memories. Nature 1983, 304, 158–159.
- Tsunematsu, T. What are the neural mechanisms and physiological functions of dreams? Neurosci. Res. 2023, 189, 54–59.
- Li, W.; Ma, L.; Yang, G.; Gan, W.B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017, 20, 427–437.
- Pascovich, C.; Lagos, P.; Urbanavicius, J.; Devera, A.; Rivas, M.; Costa, A.; López Hill, X.; Falconi, A.; Scorza, C.; Torterolo, P. Melanin-concentrating hormone (MCH) in the median raphe nucleus: Fibers, receptors and cellular effects. Peptides 2020, 126, 170249.
- Torterolo, P.; Sampogna, S.; Chase, M.H. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009, 1268, 76–87.
- Izawa, S.; Chowdhury, S.; Miyazaki, T.; Mukai, Y.; Ono, D.; Inoue, R.; Ohmura, Y.; Mizoguchi, H.; Kimura, K.; Yoshioka, M.; et al. REM sleep-active MCH neurons are involved in forgetting hippocampus-dependent memories. Science 2019, 365, 1308–1313.
- Poe, G.R. Sleep Is for Forgetting. J. Neurosci. 2017, 37, 464–473.
- Jouvet, M. Paradoxical sleep as a programming system. J. Sleep Res. 1998, 7 (Suppl. S1), 1–5.
- Roffwarg, H.P.; Muzio, J.N.; Dement, W.C. Ontogenetic development of the human sleep-dream cycle. Science 1966, 152, 604–619.
- Jouvet, M. Paradoxical sleep: Is it the guardian of psychological individualism. Can. J. Psychol. 1991, 45, 148–168.
- Adrien, J. Neonatal sleep, a genetically-driven rehearsal before the show: An endless encounter with Michel Jouvet. Sleep Med. 2018, 49, 20–23.
- Ravassard, P.; Pachoud, B.; Comte, J.C.; Mejia-Perez, C.; Scoté-Blachon, C.; Gay, N.; Claustrat, B.; Touret, M.; Luppi, P.H.; Salin, P.A. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep 2009, 32, 227–240.
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34.
- Calais, J.B.; Ojopi, E.B.; Morya, E.; Sameshima, K.; Ribeiro, S. Experience-dependent upregulation of multiple plasticity factors in the hippocampus during early REM sleep. Neurobiol. Learn. Mem. 2015, 122, 19–27.
- Graven, S.N.; Browne, J.V. Sleep and Brain Development: The Critical Role of Sleep in Fetal and Early Neonatal Brain Development. Newborn Infant. Nurs. Rev. 2008, 8, 173–179.
- Datta, S.; Calvo, J.M.; Quattrochi, J.; Hobson, J.A. Cholinergic microstimulation of the peribrachial nucleus in the cat. I. Immediate and prolonged increases in ponto-geniculo-occipital waves. Arch. Ital. Biol. 1992, 130, 263–284.
- Mavanji, V.; Datta, S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: A mechanism for sleep-dependent plasticity. Eur. J. Neurosci. 2003, 17, 359–370.
- Murkar, A.L.A.; De Koninck, J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med. Rev. 2018, 41, 173–184.
- Casaglia, E.; Luppi, P.H. Is paradoxical sleep setting up innate and acquired complex sensorimotor and adaptive behaviours?: A proposed function based on literature review. J. Sleep Res. 2022, 31, e13633.
- Riemann, D.; Berger, M.; Voderholzer, U. Sleep and depression—Results from psychobiological studies: An overview. Biol. Psychol. 2001, 57, 67–103.
- Datta, S.; Siwek, D.F.; Patterson, E.H.; Cipolloni, P.B. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse 1998, 30, 409–423.
- Grogans, S.E.; Fox, A.S.; Shackman, A.J. The Amygdala and Depression: A Sober Reconsideration. Am. J. Psychiatry 2022, 179, 454–457.
- Hopkins, D.A.; Holstege, G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp. Brain Res. 1978, 32, 529–547.
- Calvo, J.M.; Badillo, S.; Morales-Ramirez, M.; Palacios-Salas, P. The role of the temporal lobe amygdala in ponto-geniculo-occipital activity and sleep organization in cats. Brain Res. 1987, 403, 22–30.
- Ross, R.J.; Ball, W.A.; Levitt, D.R.; Gresch, P.J.; Morrison, A.R. Effects of monoamine reuptake blockade on ponto-geniculo-occipital wave activity. Neuropharmacology 1990, 29, 965–968.
- Navarro-Sanchis, C.; Brock, O.; Winsky-Sommerer, R.; Thuret, S. Modulation of Adult Hippocampal Neurogenesis by Sleep: Impact on Mental Health. Front. Neural Circuits 2017, 11, 74.
- Helm, K.; Viol, K.; Weiger, T.M.; Tass, P.A.; Grefkes, C.; Del Monte, D.; Schiepek, G. Neuronal connectivity in major depressive disorder: A systematic review. Neuropsychiatr. Dis. Treat. 2018, 14, 2715–2737.
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181.
- Albert, K.M.; Potter, G.G.; Boyd, B.D.; Kang, H.; Taylor, W.D. Brain network functional connectivity and cognitive performance in major depressive disorder. J. Psychiatr. Res. 2019, 110, 51–56.
- Zhang, Y.; Yang, Y.; Zhu, L.; Zhu, Q.; Jia, Y.; Zhang, L.; Peng, Q.; Wang, J.; Liu, J.; Fan, W.; et al. Volumetric Deficit Within the Fronto-Limbic-Striatal Circuit in First-Episode Drug Naïve Patients with Major Depression Disorder. Front. Psychiatry 2020, 11, 600583.
More
Information
Subjects:
Physiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
06 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




