Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yu Mikame | -- | 2245 | 2023-11-01 12:05:54 | | | |
| 2 | Wendy Huang | + 1 word(s) | 2246 | 2023-11-01 13:17:51 | | | | |
| 3 | Wendy Huang | Meta information modification | 2246 | 2023-11-03 10:45:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mikame, Y.; Yamayoshi, A. Peptide Nucleic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/51043 (accessed on 06 February 2026).
Mikame Y, Yamayoshi A. Peptide Nucleic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/51043. Accessed February 06, 2026.
Mikame, Yu, Asako Yamayoshi. "Peptide Nucleic Acid" Encyclopedia, https://encyclopedia.pub/entry/51043 (accessed February 06, 2026).
Mikame, Y., & Yamayoshi, A. (2023, November 01). Peptide Nucleic Acid. In Encyclopedia. https://encyclopedia.pub/entry/51043
Mikame, Yu and Asako Yamayoshi. "Peptide Nucleic Acid." Encyclopedia. Web. 01 November, 2023.
Copy Citation
Peptide nucleic acid (PNA) is an artificial DNA analog in which the negatively charged phosphodiester backbone is replaced by a charge-neutral pseudopeptide backbone. PNA exhibits several conformational flexibilities. It can adopt the A and B helical structures upon binding to target RNA and DNA, respectively, and form antiparallel and parallel duplexes. The antiparallel duplex is generally more stable than the parallel one.
peptide nucleic acid
PNA backbone
base modification
crosslinking
therapeutic application
genome editing
1. Outline of the Peptide Nucleic Acid Technology
PNA was first reported by Nielsen’s group in 1991 [1][2]. It comprises an electrically neutral aminoethyl glycine backbone. PNA is an artificial DNA analog in which the negatively charged phosphodiester backbone is replaced by a charge-neutral pseudopeptide backbone (Figure 1). PNA exhibits several conformational flexibilities. It can adopt the A and B helical structures upon binding to target RNA and DNA, respectively, and form antiparallel and parallel duplexes. The antiparallel duplex is generally more stable than the parallel one. The charge neutrality of PNA enabled its binding to the complementary DNA sequence target with increased affinity and sequence specificity, resulting in the unique mode of PNA action as follows. Considering the high affinity of PNA to DNA, single-stranded PNA (ssPNA) can invade dsDNA (Figure 1). Pseudocomplementary PNA (pcPNA) [3] was designed to form two PNA/DNA duplexes. pcPNA contains artificial nucleobases, where 2,6-diaminopurine (D) and 2-thiouracil (sU) are used instead of A and T, respectively, to avoid PNA/PNA self-duplex formation by the steric hindrance between them. The bifunctional PNA [4] and tail-clamp PNAs (tcPNAs) [5] were designed to “clamp” one DNA strand comprising two sections that are connected by a flexible linker that enables the invasion of the target DNA duplex. This invasion is initiated by the triplex formation of one section through Hoogsteen base pairing, and the other section forms a Watson–Crick base pairing with the same DNA strand, resulting in a PNA/DNA/PNA triplex. This “clamp” distorts the DNA structure, followed by the recruitment of endogenous repair factors that can be explored for genome editing. Further, tcPNA contains an extended Watson–Crick binding section to create a “tail” for distorting the target DNA for an extended stretch with increased affinity to DNA. The aforementioned PNA designs have their advantages, and their effectiveness has been demonstrated in antigene and genome editing.
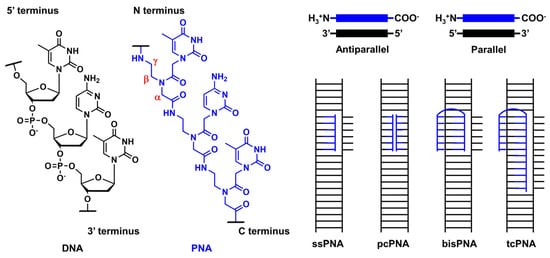
Figure 1. Structural features of PNA and unique mode of PNA actions.
2. Advancements in Peptic Nucleic Acid Engineering
The unique characteristics of PNA, the aforementioned charge neutrality, and structural flexibility, also cause several drawbacks, such as low water solubility, poor cellular uptake, self-aggregation, and orientational ambiguity, in target-sequence recognition. Many modifications have been explored to overcome these drawbacks to improve the application potential of PNAs. As many comprehensive and excellent reviews have described these modifications and applications [2][6][7][8][9], selected examples are only introduced, essential findings and recent advancements are focused on.
2.1. Modification of the Peptic Nucleic Acid Backbone
Ly’s group reported that the introduction of simple substituents, such as methyl, or hydroxymethyl, at the γ-position induced the preorganized, right-handed helical structure of the PNA backbone (Figure 2, 30), resulting in strengthened binding to complementary DNA and RNA [10]. The same group demonstrated that the modification of the γ-position of PNA with guanidine, γ-GPNA (Figure 2, 31), greatly improved the cellular uptake of γ-GPNA [11]. They also developed miniPEG γ-modified PNA (Figure 2, 32) exhibiting superior nucleic acid binding due to the better preorganization of its PNA backbone. The miniPEG γ-modified PNA can invade any dsDNA sequence through only Watson–Crick base pairing to recognize the target [12]. Tahtinen’s group developed γ-guanidinylmethyl PNA (Figure 2, 33) for recognizing triplex-forming PNAs. Three consecutive incorporations of 33 in PNA achieved the best binding affinity and Hoogsteen-face selectivity of the oligomer with improved cellular uptake [13]. Presently, the aforementioned γ-GPNA and miniPEG γ-modified PNA are widely used PNA derivatives for therapeutic purposes.
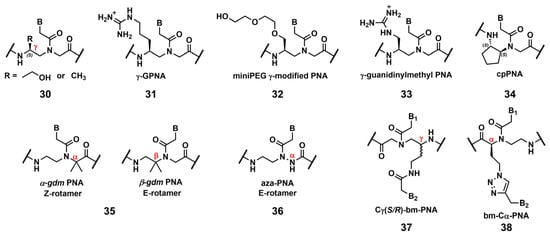
Figure 2. Chemical modifications of the PNA backbone. The position of the carbon is mentioned using α, β, γ (red).
Appella’s group reported the detailed biophysical and structural properties of S,S-cyclopentyl PNA, cpPNA (Figure 2, 34) [14][15]. The cyclopentane ring restricts the conformational flexibility of the PNA backbone, thus inducing a right-handed helix that favors binding to complementary DNA. Further, the affinity and selectivity improved with an increased amount of 34, which enabled the customization of the stability of the complex. Recently, Ganesh’s group reported that the introduction of the gem-dimethyl (gdm) group influenced the Z/E rotamer ratio of the tertiary amide. The α-gdm monomer exclusively exhibits the Z-rotamer, whereas the β-gdm monomer exhibits the E-rotamer (Figure 2, 35) [16][17]. Those E/Z-rotamers influenced the orientation preference of PNA in the formation of the complex. The same research group also reported aza-PNA bearing a nitrogen atom instead of a carbon atom at the α-position (Figure 2, 36) [18]. Interestingly, the aza-PNA monomer assumed the E-form via an eight-membered hydrogen-bonded ring with backbone folding. A future study will discuss how this modification impacts its target recognition.
A novel class of PNAs, bimodal PNA, Cγ(S/R)-bm-PNA (Figure 2, 37), was developed by Ganesh’s group [19][20][21][22]. Cγ(S/R)-bm-PNA contains an additional nucleobase at the γ-position of the PNA backbone, which enables bifacial recognition-forming duplexes at the B1 and B2 sides. The thermal stability of the DNA1/Cγ(S/R)-bm-PNA/DNA2 complexes was higher than those of their respective isolated duplexes [19][20]. The other bimodal PNA, bm-Cα-PNA (Figure 2, 38), contains an additional nucleobase at the α-position of the PNA backbone [21]. Additionally, 38, and 37 exhibited similar properties, although the target sequences were restricted to homothymine and homocytosine. These bimodal PNAs can be used to generate novel higher-order assemblies with DNAs and RNAs. For example, they reported a pentameric complex comprising a triplex and two duplexes, DNA1–Cγ(S/R)-bm-PNA/DNA2–Cγ(S/R)-bm-PNA/DNA3 [22]. Further investigations of these new bimodal PNAs, as well as their biological applications, are anticipated.
2.2. Base Modification of Peptide Nucleic Acid
2.2.1. Base Modifications for Enhanced Triplex Formation
PNA forms a triplex structure via the Hoogsteen hydrogen-bond-forming T·A:T triad and C+·G:C triad base pairing. Therefore, the inability to form stable hydrogen bonds with the T:A and C:G base pairs, as well as the necessity of protonating C, limit their in vivo application, including parallel TFOs. Some of the designed base analogs for TFOs can be used for PNA. For example, the base analogs 2 [23] and 5 [24] (Figure 3) can be used for T:A and C:G base-pair recognition, and 5-mC, pseudoisocytosine (Figure 3, 12) [25] can be used to replace C. Several artificial bases were originally developed for PNA [26][27]. Recently, Rozner’s group systematically surveyed simple nitrogen heterocycles for C:G base-pair recognition and observed that 3-pyridazinyl nucleobase, PN (Figure 4, 39), forms more stable hydrogen bonds than the other heterocycles [27].
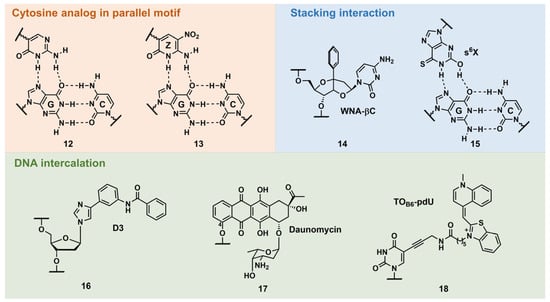
Figure 3. Selected examples of other types of triplex-stabilizing artificial nucleobases.
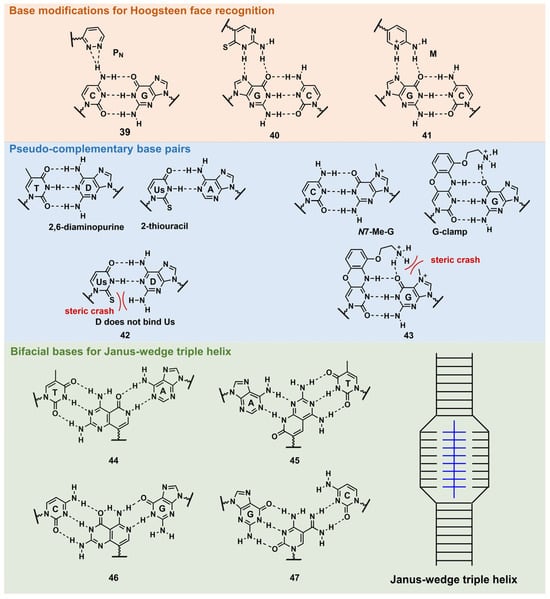
Figure 4. Nucleobase modifications of PNA.
Thio-pseudoisocytosine (Figure 4, 40) [28] was studied by Chen’s group as a replacement for C. Here, 40 forms stable base pairing through the synergistic effect of improved van der Waals contacts base stacking with hydrogen bond formation. Rozner’s group examined 2-aminopyridine M (Figure 4, 41) as a more basic C nucleobase [29][30]. The replacement of six pseudoisocytosines in 9-mer PNA comprising six Ms increased the affinity of PNA to dsDNA by ≈100-fold owing to the cationic character of M.
2.2.2. Base Modifications for Peptide Nucleic Acid Functionalization
As mentioned in Figure 1, D and sU were designed to avoid the formation of an unproductive PNA–PNA duplex for pcPNA via a steric clash between the 2-amino group of D and thiocarbonyl group of sU (Figure 4, 42) [31]. Hudson’s group reported an improved synthesis of sU, which eases the preparation of pcPNA and will accelerate future pcPNA studies [32]. Recently, Winssinger’s group reported a new pseudocomplementary G:C base pair for G-clamp-based dsDNA invasion (Figure 4, 43) [33].
G-clamp is a phenoxazine-derived tricyclic C analog that can strongly bind to G through additional interactions, such as π-stacking, the electrostatic attraction of a positively charged amine, and hydrogen bonding at the Hoogsteen face [34]. The introduction of G-clamp into PNA significantly improved the thermodynamic stability of the PNA/DNA duplex [35]. They developed N-7 methylguanine (N7-Me-G), which was designed to cause steric and electrostatic repulsions between the G-clamp. The modified PNAs were used in the detection of the dsDNA target, the RT-RPA amplicon, from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with single nucleotide resolution, discriminating between two SARS-CoV-2 strains. Each strand of modified PNA exhibited fast strand invasion in the physiological condition and formed stable complexes with low equivalents of PNA.
An investigation of the Janus–Wedge triple PNA helix was pioneered by McLaughlin’s group [36]. Therein, bifacial artificial PNA nucleobases form hydrogen bonds with the Watson–Crick faces of the two DNA target strands. In 2018, Thadke’s group first reported a complete set of bifacial nucleobases that can distinguish the T:A, A:T, C:G, and G:C base pairs (Figure 4, 44–47) [37][38]. The 6-mer miniPEG γ-modified PNA comprising these nucleobases efficiently and rapidly invaded target dsDNA with high sequence specificity under physiological conditions [38]. As 44–47 enable the targeting of any sequences, they could be novel gene-targeting tools, and their applications for therapeutic purposes will be investigated subsequently.
2.3. Recent Advancements in Crosslinkable Peptide Nucleic Acid for DNA Targeting
Glazer and Nielsen’s group developed psoralen-conjugated pcPNAs, demonstrating that they can be used to induce single-base substitutions and deletions within the target site [39][40]. The linker was only linked to the 8-position of psoralen in these PNAs, making the effect of the linker position on crosslinking formation elusive. Recently, the researchers developed novel psoralen NHS esters (Figure 5, 21 and 22), which enabled the easy conjugation of psoralen in the N-terminus of PNA and subsequent photo-crosslinking evaluation [41]. The yields of the adduct products of 5-Ps–PNA and trioxsalen–PNA prepared with 21 and 22 were 48% and 45%, respectively, after 30 s of irradiation; the yields were much higher than those of corresponding PNAs prepared using SPB (15%). The applications of these novel Ps–PNAs are being studied in the lab.
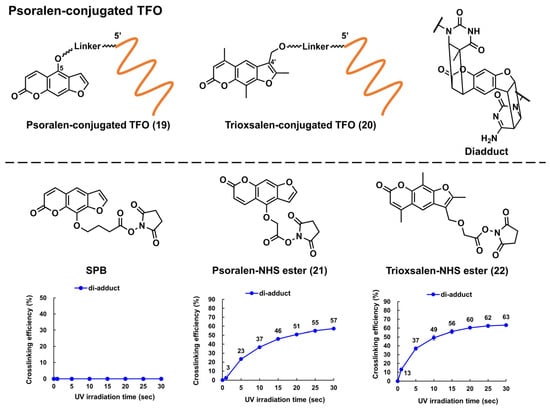
Figure 5. Structures of the psoralen-conjugated TFOs (Ps–TFOs) and psoralen N-hydroxysuccinimide (NHS) esters. The charts show the crosslinking efficiencies of the corresponding Ps–TFOs prepared using each NHS ester (the charts were quoted from [41]).
Crosslinkable furan-derived nucleobases were developed for PNA. The furan generates reactive species immediately after the activation of reactive oxygen species (ROS), which can be exploited for DNA crosslinking. Sequence-specific crosslinking was realized with γ-Lys-modified PNA [42][43]. Recently, Vilaivan’s group reported novel pyrrolidinyl PNA probes, exhibiting furan as a crosslinking formation with target DNA. The positional effect on crosslinking was examined, revealing that the external incorporation of the furan moiety improved the crosslinking. A future study will discuss its biological applications [44].
3. Recent Advancements in the Therapeutic Applications of Peptide Nucleic Acids
3.1. Modulation of DNA Expression
Recently, Tonelli’s group reported the therapeutic applications of PNAs in targeting the MYCN gene [45][46]. The PNA employed in these studies was named BGA002, which is conjugated with a nuclear localization signal peptide [12] and specific to the MYCN gene. Although the sequence information was not disclosed, BGA002 exhibited more potent biological activities than their previous MYCN-targeting PNA (BGA001), which also exhibited antitumor activity in mice with rhabdomyosarcomas [47]. With PNA BGA002, they targeted MYCN-amplified neuroblastoma (MNA–NB) cells, in which MYCN was associated with increased ROS, downregulated mitophagy, and a poor prognosis. BGA002 inhibited the expression of the MYCN gene, causing profound mitochondrial damage through the downregulation of the mitochondrial molecular chaperone TRAP1; ROS increased with the concomitant decrease in the MNA–NB xenograft tumor. This research first described the relevance of MYCN in MNA–NB in mitochondrial maintenance [45]. In other research [46], the combinational use of BGA002 and retinoic acid (RA) was found to be beneficial in the treatment of MNA–NB. RA has been used to treat MNA–NB patients. However, RA resistance was observed for some patients. The coadministration of BGA002 and RA mediated the therapeutic efficacy of RA by inhibiting BGA002 on the MYCN gene. The inhibition of the MYCN gene by BGA002 decreased the mTOR pathway activity, followed by the autophagy response of MNA–NB. The efficacy of BGA002 treatment with RA was also demonstrated in an MNA–NB mouse model.
3.2. Genome Editing
In 2016, Glazer et al. reported an in vivo correction of a β-thalassemia mutation in mice by miniPEG γ-modified tcPNA (γtcPNA) [48]. An γtcPNA and donor-DNA injection with poly(lacto-glycolic acid) (PLGA) NPs, as well as a stem-cell-factor treatment, ameliorated the disease phenotype and collected the β-globin gene by up to 7% in hematopoietic stem cells. In utero experiments have also been performed using the same mouse model, achieving improvements in the disease phenotype in pups [49]. It seems that the tcPNA works by opening the target dsDNA via strand invasion (Figure 1), which permits the donor DNA to hybridize the target DNA. Finally, the homology-directed repair using this donor DNA as a template resulted in gene collection [50]. In 2022, Piotrowski-Daspit’s group further demonstrated the utility of PLGA NPs encapsulating PNA miniPEG γ-modified tcPNA and donor DNAs in cystic fibrosis (CF) treatment. CF patients experience multiorgan dysfunction, which is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. The in vivo correction of the CF mouse model using γtcPNA resulted in a partial gain of CFTR function and improved the phenotype [51]. Recently, Glazer’s group also applied the same system to genome editing in single-cell embryos containing mutated eGFP genes. The blastocysts from the embryos that were treated with γtcPNA exhibited the expression of corrected eGFP with high editing levels, up to 94%. The mice from the re-implanted embryos consistently exhibited editing. This research is the first example of embryonic-gene editing using PNA [52]. In these above experiments, gene editing was very site-specific, with considerably low levels of off-target sequence modification.
References
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500.
- Das, A.; Pradhan, B. Evolution of peptide nucleic acid with modifications of its backbone and application in biotechnology. Chem. Biol. Drug Des. 2021, 97, 865–892.
- Lonkar, P.; Kim, K.H.; Kuan, J.Y.; Chin, J.Y.; Rogers, F.A.; Knauert, M.P.; Kole, P.; Nielsen, P.E.; Glazer, P.M. Targeted correction of a thalassemia-associated β-globin mutation induced by pseudo-complementary peptide nucleic acids. Nucleic Acids Res. 2009, 37, 3635–3644.
- Rogers, F.A.; Vasquez, K.M.; Egholm, M.; Glazer, P.M. Site-directed recombination via bifunctional PNA-DNA conjugates. Proc. Natl. Acad. Sci. USA 2002, 99, 16695–16700.
- Schleifman, E.B.; Bindra, R.; Leif, J.; del Campo, J.; Rogers, F.A.; Uchil, P.; Kutsch, O.; Shultz, L.D.; Kumar, P.; Greiner, D.L.; et al. Targeted Disruption of the CCR5 Gene in Human Hematopoietic Stem Cells Stimulated by Peptide Nucleic Acids. Chem. Biol. 2011, 18, 1189–1198.
- Perera, J.D.R.; Carufe, K.E.W.; Glazer, P.M. Peptide nucleic acids and their role in gene regulation and editing. Biopolymers 2021, 112, e23460.
- Swenson, C.S.; Heemstra, J.M. Peptide nucleic acids harness dual information codes in a single molecule. Chem. Commun. 2020, 56, 1926–1935.
- Suparpprom, C.; Vilaivan, T. Perspectives on conformationally constrained peptide nucleic acid (PNA): Insights into the structural design, properties and applications. RSC Chem. Biol. 2022, 3, 648–697.
- Brodyagin, N.; Katkevics, M.; Kotikam, V.; Ryan, C.A.; Rozners, E. Chemical approaches to discover the full potential of peptide nucleic acids in biomedical applications. Beilstein. J. Org. Chem. 2021, 17, 1641–1688.
- Dragulescu-Andrasi, A.; Rapireddy, S.; Frezza, B.M.; Gayathri, C.; Gil, R.R.; Ly, D.H. A Simple γ-Backbone Modification Preorganizes Peptide Nucleic Acid into a Helical Structure. J. Am. Chem. Soc. 2006, 128, 10258–10267.
- Sahu, B.; Chenna, V.; Lathrop, K.L.; Thomas, S.M.; Zon, G.; Livak, K.J.; Ly, D.H. Synthesis of Conformationally Preorganized and Cell-Permeable Guanidine-Based γ-Peptide Nucleic Acids (γ-GPNAs). J. Org. Chem. 2009, 74, 1509–1516.
- Bahal, R.; Sahu, B.; Rapireddy, S.; Lee, C.M.; Ly, D.H. Sequence-Unrestricted, Watson-Crick Recognition of Double Helical B-DNA by (R)-MiniPEG-γPNAs. ChemBioChem 2012, 13, 56–60.
- Tähtinen, V.; Verhassel, A.; Tuomela, J.; Virta, P. γ-(S)-Guanidinylmethyl-Modified Triplex-Forming Peptide Nucleic Acids Increase Hoogsteen-Face Affinity for a MicroRNA and Enhanced Cellular Uptake. ChemBioChem 2019, 20, 3041–3051.
- Zheng, H.; Saha, M.; Appella, D.H. Synthesis of Fmoc-Protected (S,S)-trans-Cyclopentane Diamine Monomers Enables the Preparation and Study of Conformationally Restricted Peptide Nucleic Acids. Org. Lett. 2018, 20, 7637–7640.
- Zheng, H.; Botos, I.; Clausse, V.; Nikolayevskiy, H.; Rastede, E.E.; Fouz, M.F.; Mazur, S.J.; Appella, D.H. Conformational constraints of cyclopentane peptide nucleic acids facilitate tunable binding to DNA. Nucleic Acids Res. 2021, 49, 713–725.
- Kulkarni, P.; Datta, D.; Ramabhadran, R.O.; Ganesh, K.N. Gem-dimethyl peptide nucleic acid (α/β/γ-gdm-PNA) monomers: Synthesis and the role of gdm-substituents in preferential stabilization of Z/E-rotamers. Org. Biomol. Chem. 2021, 19, 6534–6545.
- Kulkarni, P.; Datta, D.; Ramabhadran, R.O.; Ganesh, K.N. Gemdimethyl Peptide Nucleic Acids (α/β/γ-gdm-PNA): E/Z-Rotamers Influence the Selectivity in the Formation of Parallel/Antiparallel gdm-PNA:DNA/RNA Duplexes. ACS Omega 2022, 7, 40558–40568.
- Shiraj, A.; Ramabhadran, R.O.; Ganesh, K.N. Aza-PNA: Engineering E-Rotamer Selectivity Directed by Intramolecular H-Bonding. Org. Lett. 2022, 24, 7421–7427.
- Bhingardeve, P.; Madhanagopal, B.R.; Ganesh, K.N. Cγ-(S/R)-Bimodal Peptide Nucleic Acids (Cγ-bm-PNA) Form Coupled Double Duplexes by Synchronous Binding to Two Complementary DNA Strands. J. Org. Chem. 2020, 85, 13680–13693.
- Gupta, M.K.; Madhanagopal, B.R.; Datta, D.; Ganesh, K.N. Structural Design and Synthesis of Bimodal PNA That Simultaneously Binds Two Complementary DNAs To Form Fused Double Duplexes. Org. Lett. 2020, 22, 5255–5260.
- Gupta, M.K.; Madhanagopal, B.R.; Ganesh, K.N. Peptide Nucleic Acid with Double Face: Homothymine-Homocytosine Bimodal Cα-PNA (bm-Cα-PNA) Forms a Double Duplex of the bm-PNA2:DNA Tripolex. J. Org. Chem. 2021, 86, 414–428.
- Bhingardeve, P.; Jain, P.; Ganesh, K.N. Molecular Assembly of Triplex of Duplexes from Homothyminyl-Homocytosinyl Cγ-(S/R)-Bimodal Peptide Nucleic Acids with dA/dG and the Cell Permeability of Bimodal Peptide Nucleic Acids. ACS Omega 2021, 6, 19757–19770.
- Ong, A.A.L.; Toh, D.F.K.; Patil, K.M.; Meng, Z.; Yuan, Z.; Krishna, M.S.; Devi, G.; Haruehanroengra, P.; Lu, Y.; Xia, K.; et al. General Recognition of U-G, U-A, and C-G Pairs by Double-Stranded RNA-Binding PNAs Incorporated with an Artificial Nucleobase. Biochemistry 2019, 58, 1319–1331.
- Toh, D.F.K.; Devi, G.; Patil, K.M.; Qu, Q.; Maraswami, M.; Xiao, Y.; Loh, T.P.; Zhao, Y.; Chen, G. Incorporating a guanidine-modified cytosine base into triplex-forming PNAs for the recognition of a C-G pyrimidine-purine inversion site of an RNA duplex. Nucleic Acids Res. 2016, 44, 9071–9082.
- Egholm, M.; Christensen, L.; Deuholm, K.L.; Buchardt, O.; Coull, J.; Nielsen, P.E. Efficient pH-independent sequence-specific DNA binding by pseudoisocytosine-containing bis-PNA. Nucleic Acids Res. 1995, 23, 217–222.
- Kumpina, I.; Brodyagin, N.; MacKay, J.A.; Kennedy, S.D.; Katkevics, M.; Rozners, E. Synthesis and RNA-Binding Properties of Extended Nucleobases for Triplex-Forming Peptide Nucleic Acids. J. Org. Chem. 2019, 84, 13276–13298.
- Brodyagin, N.; Kumpina, I.; Applegate, J.; Katkevics, M.; Rozners, E. Pyridazine Nucleobase in Triplex-Forming PNA Improves Recognition of Cytosine Interruptions of Polypurine Tracts in RNA. ACS Chem. Biol. 2021, 16, 872–881.
- Devi, G.; Yuan, Z.; Lu, Y.; Zhao, Y.; Chen, G. Incorporation of thio-pseudoisocytosine into triplex-forming peptide nucleic acids for enhanced recognition of RNA duplexes. Nucleic Acids Res. 2014, 42, 4008–4018.
- Kotikam, V.; Kennedy, S.; MacKay, J.A.; Rozners, E. Synthetic, Structural, and RNA Binding Studies on 2-Aminopyridine-Modified Triplex-Forming Peptide Nucleic Acids. Chem. Eur. J. 2019, 25, 4367–4372.
- Ryan, C.A.; Brodyagin, N.; Lok, J.; Rozners, E. The 2-Aminopyridine Nucleobase Improves Triple-Helical Recognition of RNA and DNA When Used Instead of Pseudoisocytosine in Peptide Nucleic Acids. Biochemistry 2021, 60, 1919–1925.
- Haaima, G.; Hansen, H.F.; Christensen, L.; Dahl, O.; Nielsen, P.E. Increased DNA binding and sequence discrimination of PNA oligomers containing 2,6-diaminopurine. Nucleic Acids Res. 1997, 25, 4639–4643.
- Hudson, R.H.E.; Heidari, A.; Martin-Chan, T.; Park, G.; Wisner, J.A. On the Necessity of Nucleobase Protection for 2-Thiouracil for Fmoc-Based Pseudo-Complementary Peptide Nucleic Acid Oligomer Synthesis. J. Org. Chem. 2019, 84, 13252–13261.
- López-Tena, M.; Farrera-Soler, L.; Barluenga, S.; Winssinger, N. Pseudo-Complementary G:C Base Pair for Mixed Sequence dsDNA Invasion and Its Applications in Diagnostics (SARS-CoV-2 Detection). JACS Au 2023, 3, 449–458.
- Lin, K.Y.; Matteucci, M.D. A Cytosine Analogue Capable of Clamp-Like Binding to a Guanine in Helical Nucleic Acids. J. Am. Chem. Soc. 1998, 120, 8531–8532.
- Rajeev, K.G.; Maier, M.A.; Lesnik, E.A. High-Affinity Peptide Nucleic Acid Oligomers Containing Tricyclic Cytosine Analogues. Org. Lett. 2002, 4, 4395–4398.
- Chen, D.; Meena; Sharma, S.K.; McLaughlin, L.W. Formation and Stability of a Janus-Wedge Type of DNA Triple. J. Am. Chem. Soc. 2004, 126, 70–71.
- Thadke, S.A.; Perera, J.D.R.; Hridya, V.M.; Bhatt, K.; Shaikh, A.Y.; Hsieh, W.C.; Chen, M.; Gayathri, C.; Gil, R.R.; Rule, G.S.; et al. Design of Bivalent Nucleic Acid Ligands for Recognition of RNA-Repeated Expansion Associated with Huntington’s Disease. Biochemistry 2018, 57, 2094–2108.
- Thadke, S.A.; Hridya, V.M.; Perera, J.D.R.; Gil, R.R.; Mukherjee, A.; Ly, D.H. Shape selective bifacial recognition of double helical DNA. Commun. Chem. 2018, 1, 79.
- Kim, K.H.; Fan, X.J.; Nielsen, P.E. Efficient Sequence-Directed Psoralen Targeting Using Pseudocomplementary Peptide Nucleic Acids. Bioconjugate Chem. 2007, 18, 567–572.
- Kim, K.H.; Nielsen, P.E.; Glazer, P.M. Site-directed gene mutation at mixed sequence targets by psoralen-conjugated pseudo-complementary peptide nucleic acids. Nucleic Acids Res. 2007, 35, 7604–7613.
- Nakao, J.; Mikame, Y.; Eshima, H.; Yamamoto, T.; Dohno, C.; Wada, T.; Yamayoshi, A. Unique Crosslinking Properties of Psoralen-Conjugated Oligonucleotides Developed by Novel Psoralen N-Hydroxysuccinimide Esters. ChemBioChem 2023, 24, e202200789.
- Manicardi, A.; Gyssels, E.; Corradini, R.; Madder, A. Furan-PNA: A mildly inducible irreversible interstrand crosslinking system targeting single and double-stranded DNA. Chem. Commun. 2016, 52, 6930–6933.
- Elskens, J.; Manicardi, A.; Costi, V.; Madder, A.; Corradini, R. Synthesis and improved cross-linking properties of C5-modified furan bearing PNAs. Molecules 2017, 22, 2010.
- Muangkaew, P.; Vilaivan, T. Pyrrolidinyl Peptide Nucleic Acid Probes Capable of Crosslinking with DNA: Effects of Terminal and Internal Modifications on Crosslink Efficiency. ChemBioChem 2021, 22, 241–252.
- Montemurro, L.; Raieli, S.; Angelucci, S.; Bartolucci, D.; Amadesi, C.; Lampis, S.; Scardovi, A.L.; Venturelli, L.; Nieddu, G.; Cerisoli, L.; et al. A Novel MYCN-Specific Antigene Oligonucleotide Deregulates Mitochondria and Inhibits Tumor Growth in MYCN-Amplified Neuroblastome. Cancer Res. 2019, 79, 6166–6177.
- Lampis, S.; Raieli, S.; Montemurro, L.; Bartolucci, D.; Amadesi, C.; Bortolotti, S.; Angelucci, S.; Scardovi, A.L.; Nieddu, G.; Cerisoli, L.; et al. The MYCN inhibitor BGA002 restores the retinoic acid response leading to differentiation or apoptosis by the mTOR block in MYCN-amplified neuroblastoma. J. Exp. Clin. Cancer Res. 2022, 42, 160.
- Tonelli, R.; Mclntyre, A.; Camerin, C.; Walters, Z.S.; Leo, K.D.; Selfe, J.; Purgato, S.; Missiaglia, E.; Tortori, A.; Renshaw, J.; et al. Antitumor Activity of Sustauned N-Myc Reduction in Rhabdomyosarcomas and Transcriptional Block by Antigene Therapy. Clin. Cancer Res. 2012, 18, 796–807.
- Bahal, R.; Ali McNeer, N.; Quijano, E.; Liu, Y.; Sulkowski, P.; Turchick, A.; Lu, Y.C.; Bhunia, D.C.; Manna, A.; Greiner, D.L.; et al. In vivo correction of anaemia in β-thalassemic mice by γPNA-mediated gene editing with nanoparticle delivery. Nat. Commun. 2016, 7, 13304.
- Ricciardi, A.S.; Bahal, R.; Farrelly, J.S.; Quijano, E.; Bianchi, A.H.; Luks, V.L.; Putman, R.; López-Giráldez, F.; Coskun, S.; Song, E.; et al. In utero nanoparticle delivery for site-specific genome editing. Nat. Commun. 2018, 9, 2481.
- Chin, J.Y.; Glazer, P.M. Repair of DNA lesions associated with triplex-forming oligonucleotides. Mol. Carcinog. 2009, 48, 389–399.
- Piotrowski-Daspit, A.S.; Barone, C.; Lin, C.Y.; Deng, Y.; Wu, D.; Binns, T.C.; Xu, E.; Ricciardi, A.S.; Putman, R.; Garrison, A.; et al. In vivo correction of cystic fibrosis mediated by PNA nanoparticles. Sci. Adv. 2022, 8, eabo0522.
- Putman, R.; Ricciardi, A.S.; Carufe, K.E.W.; Quijano, E.; Bahal, R.; Glazer, P.M.; Saltzman, W.M. Nanoparticle-mediated genome editing in single-cell embryos via peptide nucleic acids. Bioeng. Transl. Med. 2023, 8, e10458.
More
Information
Subjects:
Critical Care Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
03 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




