Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yongjun Wei | -- | 4202 | 2023-10-31 14:05:25 | | | |
| 2 | Camila Xu | Meta information modification | 4202 | 2023-11-01 06:27:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yuan, Y.; Yang, Y.; Xiao, L.; Qu, L.; Zhang, X.; Wei, Y. Probiotics during Vegetable Fermentation. Encyclopedia. Available online: https://encyclopedia.pub/entry/50992 (accessed on 12 March 2026).
Yuan Y, Yang Y, Xiao L, Qu L, Zhang X, Wei Y. Probiotics during Vegetable Fermentation. Encyclopedia. Available at: https://encyclopedia.pub/entry/50992. Accessed March 12, 2026.
Yuan, Yingzi, Yutong Yang, Lele Xiao, Lingbo Qu, Xiaoling Zhang, Yongjun Wei. "Probiotics during Vegetable Fermentation" Encyclopedia, https://encyclopedia.pub/entry/50992 (accessed March 12, 2026).
Yuan, Y., Yang, Y., Xiao, L., Qu, L., Zhang, X., & Wei, Y. (2023, October 31). Probiotics during Vegetable Fermentation. In Encyclopedia. https://encyclopedia.pub/entry/50992
Yuan, Yingzi, et al. "Probiotics during Vegetable Fermentation." Encyclopedia. Web. 31 October, 2023.
Copy Citation
Fermented vegetables are popular traditional foods known for their unique flavors and health benefits. The flavors of fermented vegetables can vary based on different vegetable materials and fermentation techniques used.
fermented vegetables
traditional fermentation
probiotics
1. Introduction to Fermented Vegetable Foods
Fermented vegetables are popular traditional foods known for their unique flavors and health benefits [1]. The flavors of fermented vegetables can vary based on different vegetable materials and fermentation techniques used. A wide variety of fresh vegetables, such as cabbages, carrots, beets, cucumbers, celery, peppers, and green beans, can be used in the production of fermented vegetables [2]. Sauerkraut and pickles are the main types of fermented vegetables. Numerous studies have highlighted the health benefits of fermented vegetables, which provide nutritional products including vitamins, antioxidants, proteins, carbohydrates, and exopolysaccharides. Moreover, they exhibit anti-inflammatory, immunomodulatory, and gut-health-promoting properties [3][4][5][6][7].
The microbial metabolites produced during vegetable fermentation are essential in the formation of flavors. Microorganisms convert the fermentable substrates, mainly carbohydrates and proteins, into biologically active metabolites, such as short-chain fatty acids (SCFAs), sugar, organic acids, free amino acids (FAA), and volatiles. These metabolites contribute to the attractive flavors and desirable nutritional values of fermented vegetables [8][9]. Each type of fermented vegetables generally harbors a distinct population of microorganisms. Probiotics, especially lactic acid bacteria (LABs), are essential in fermented vegetables, for they optimize flavor characteristics, produce beneficial metabolites, inhibit undesirable microorganisms, and reduce harmful compounds [10][11]. LABs, known for their strong lactic acid production ability, become the dominant contributors during the later stage of vegetable fermentation [2][12][13].
Considering the contributions of vegetable fermentation and the metabolic activities of probiotic communities, fermented vegetables not only provide nutrition and appetizing healthy foods for humans but also offer various functional benefits. These benefits include antioxidant properties, cholesterol-lowering effects, and modulation of the gut microbiome [14][15][16][17][18]. Thus, it is critical to understand the interplay between the microbiota and physicochemical indices of fermented vegetables, in order to enhance the quality and safety of traditional fermented vegetable products.
2. Traditional Vegetable Fermentation
Due to different vegetable materials, environments, and production processes, traditional fermented vegetables can be roughly divided into sauerkraut and pickles. The cabbage is completely submerged in the brine containing a proper amount of salt, and the sauerkraut fermentation process occurs naturally [19]. Kimchi, similar to sauerkraut, includes a wider range of vegetables such as cabbages, peppers, daikon, long beans, garlic, and ginger. To shorten the fermentation process and improve fermentation quality, some vegetables would be fermented in salted brine in a certain fermentation process, with a circulating pump to maintain homogeneity [20]. Sauerkraut is compressed during fermentation and has a lower salt concentration compared to kimchi. Pickles are made by dehydrating various fresh vegetables under the sun for several hours. The semi-dried vegetables are then mixed with salt and placed in jars with water for spontaneous fermentation [2] (Figure 1).
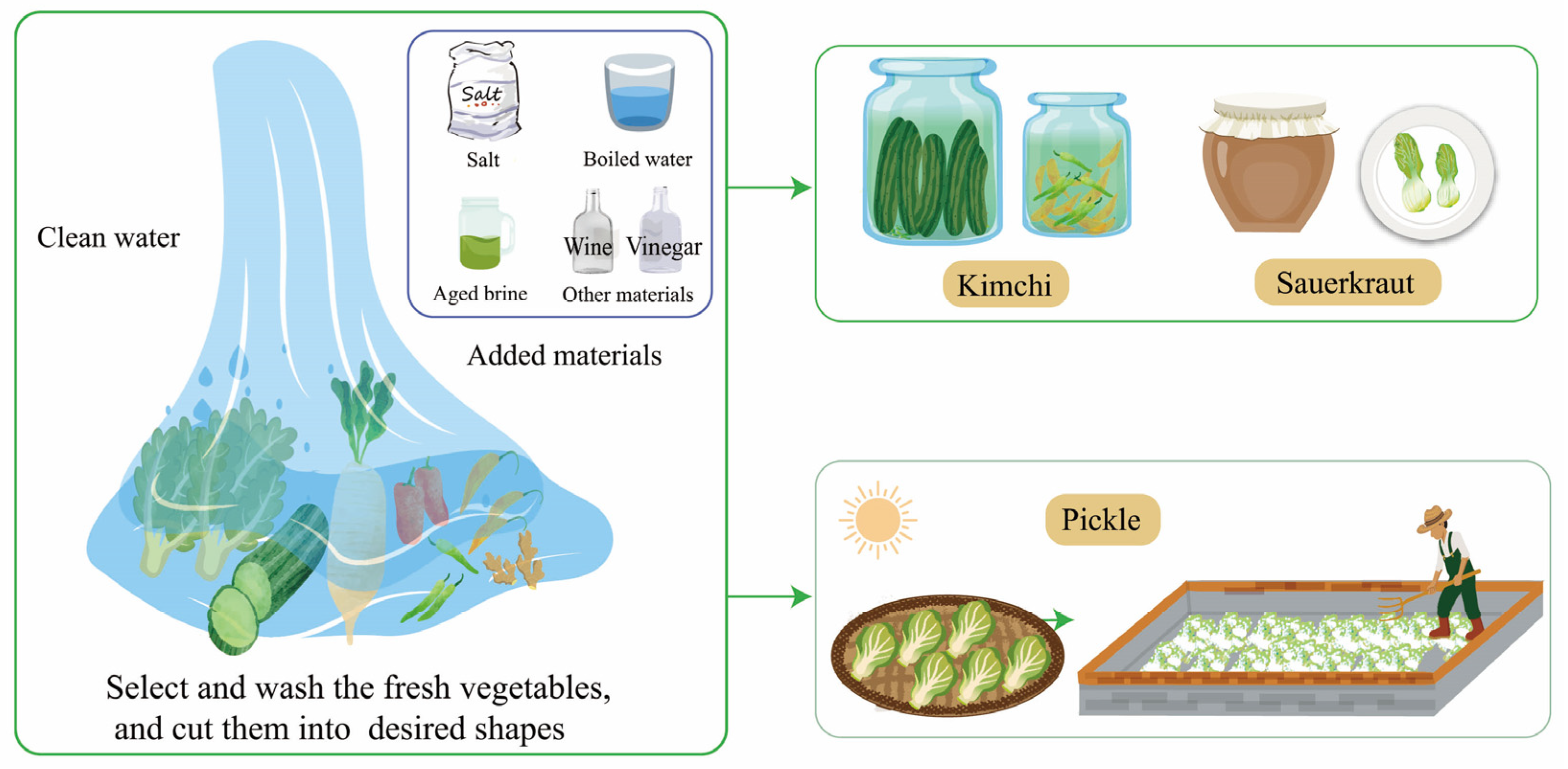
Figure 1. The production process of traditional fermented vegetables.
2.1. The Fermentation Process of Traditional Fermented Vegetables
The traditional fermentation process involves several steps [21]. Firstly, fresh vegetables are selected and washed. Any rotten and moldy parts are removed to prevent fermentation issues and ensure food safety. Secondly, the vegetables are cut into desired shapes to facilitate the pickling process. Lastly, the vegetables are placed in a container, and salt water or aged brine is added. They are then marinated under specific conditions for a few days.
During fermentation, the conditions, such as temperature, salt concentration, and fermentation time, are adjusted based on the specific vegetables being fermented. For example, white cabbages cultivated in South Tyrol are fermented for 42 days at 15 °C with 1.3–1.4% salt according to the traditional procedure [19]. Peppers, on the other hand, undergo spontaneous fermentation in sterile water (boiled water) with 0.5–3.5% salt for about 10 days [22]. Temperature plays a significant role in the growth of LABs and their acid production ability, which affects the pH and fermentation speed [23]. Salt concentration and pre-salting have a crucial impact on the quality of fermented vegetables [24], influencing microbial diversity and volatile compounds. Certain bacterial species, such as Pediococcus, Leuconostoc, Weissella, Sporobolomyces, Azospirillum, Klebsiella, Acinetobacter, and Cladosporium, have a close correlation with salt concentration during vegetable fermentation. However, Lactobacillus is not affected by different salt additions (0%, 3%, 6%, and 9%) and tends to dominate the fermentation process, especially in the later phase of fermentation [24]. In some vegetable fermentation procedures, aged brine is used while others simply use salt water. Aged brine can have a significant impact on microbial growth at the beginning of fermentation and contribute to the production of more aromatic compounds, higher organic acid content, and a lower pH value [25]. However, the fermentation functions of certain species in aged brine are not well understood, which limits our ability to regulate microorganisms and predict the quality of fermented vegetables.
Traditional vegetable fermentation primarily relies on the naturally occurring bacteria and fungi presenting on the fresh vegetables or in the food processing environment. Based on metagenomic screening, highly diverse microorganisms have been revealed, which include fungi and bacteria [26]. The fungi have been reported to play a significant role in flavor formation in fermented chili pepper [27], broad bean paste [28], and fermented Da-jiang [29]. Diverse fungi, such as Cladosporium, Candida, Aspergillus, Pichia, Sporobolomyces, Debaromyces, Psathyrella, and Debaryomyces hansenii, have been observed in various fermented vegetables [4][24][30][31][32]. Both the bacterial and fungal species are strongly associated with increased concentrations of organic acids, amino acids, biogenic amines, and volatiles [31]. However, yeasts and molds can produce metabolites that result in undesirable taste and smell, and they can inhibit lactic acid production [33]. Bacteria, especially Lactobacillus, make up the majority of the microbiota involved in vegetable fermentation and gradually become dominant in the later stage of fermentation [34].
2.2. Insight into the Microbiota of Traditional Fermented Vegetables
The physicochemical attributes of fermented vegetables, such as pH, acidity, nitrite, texture, and color, undergo constant changes during the fermentation process. Similarly, the flavor properties, such as sugar, organic acids, esters, terpenes, alcohols, and phenols, increase significantly [19][31]. As a result, the growth of certain microbial species was inhibited, leading to a decrease in the abundance of the initially dominant genera [35]. The Lactobacillus tends to dominate the fermented microbiota in the later stage of fermentation. In Jiangxi yancai, Sichuan paocai, and Dongbei suancai, the abundance of Levilactobacillus can be as high as 73.7% [2].
The dominant bacteria can vary depending on the type of vegetable, the production process, and the fermentation conditions. For instance, pickled chili pepper, a traditional Chinese fermented vegetable, is fermented with aged brine in an open-air pickling tank for at least 3 months in Jianshui city, China. In this situation, Lactobacillus remains the main genus throughout the entire fermentation process [34]. In contrast, the spontaneous fermentation of pickled pepper in North China involves sterile water (boiled water) with salt in an oxygen-free environment, where the composition of Lactobacillus is 49.6% [22].
The flavor profiles of different fermented vegetables are closely related to specific bacterial species, which exhibit great diversity [36]. The microorganisms that are most adaptable to the fermentation process are responsible for producing the flavors [37]. Certain core bacterial species within the microbiota are believed to be the key contributors to the flavors. For example, Lactobacillus alimentarius may contribute fruity, sweet, and floral odors to pickled chayote, while other Lactobacillus species, Lactobacillus futsaii and Lactobacillus paralimentarius, in particular, generate a sour taste to the products [35]. The abundance of Lactobacillus alimentarius increased significantly during the fermentation of pickled chayote, suggesting it is essential [35]. Additionally, Secundilactobacillus malefermentans has been identified as a keystone species in the fermentation microbiota, especially during the last weeks of fermentation [19]. In pickled chili pepper, Lactobacillus versmoldensis and Lactobacillus brevis were identified as keystone microorganisms highly related to flavor production [34]. These bacterial species could potentially serve as starter cultures to optimize fermentation processes and achieve fermented vegetables with improved and standardized nutritional and sensory characteristics.
LABs during Vegetable Fermentation
LABs are Gram-positive and catalase-negative microorganisms. They are acid-resistant, facultatively anaerobic, morphologically globular, or rod-shaped, and they do not form spores [38][39]. LABs are widely distributed in various sources, such as food, plants, soil, animals, and the human body. The genera of LABs include Lactobacillus, Lactococcus, Pediococcus, Enterococcus, Streptococcus, Leuconostoc, Weissella, and others [14][38][40][41]. While there is a considerable diversity of LABs, food is one of the most common sources. Fermented foods, such as fermented dairy products, fermented vegetables, fermented meat, and fermented grains, are particularly rich in LABs. Fermented vegetables, being a common and easily accessible product, are often used as raw materials for isolating LABs. Certain isolated LAB strains derived from fermented vegetables exhibit probiotic effects. For example, Lactiplantibacillus pentosus CF2-10N isolated from fermented Aloreña green table olives can produce exopolysaccharide and vitamins and demonstrate an immunomodulatory effect [42]. W. koreensis SK isolated from kimchi can produce ornithine, which has an anti-obesity effect [43]. Lactiplantibacillus plantarum LRCC5314, isolated from kimchi, has anti-inflammatory effects [44]. L. pentosus LPG1 isolated from edible olives can produce bacteriocin and exopolysaccharides, exhibits an anti-inflammatory effect, reduces cholesterol levels, and inhibits food-borne pathogens [45].
LABs have a range of functions, including immune regulation, intestinal improvement, inhibition of food-borne pathogens, and an anti-inflammatory function [38][46][47]. Therefore, LABs have applications in various fields, including food and medical treatment, with the production of fermented food being one of the most common uses. During the fermentation process, LABs produce various metabolites, primarily lactic acid, bacterin, amino acids, exopolysaccharides, ornithine, aldehydes, and esters. When LABs are used as starter cultures for fermenting vegetables, they contribute to the production of beneficial fermented vegetable products. For example, inoculating cabbage with L. plantarum CGMCC No. 20193 and P. pentosaceus CGMCC No. 20192 increases the content of amino acids and other beneficial substances while reducing the nitrite content, making the product healthier [48]. Fermentation with L. plantarum ZJ316 increases the content of mustard, aldehydes, and esters, as well as the number of probiotics [49]. Lactobacillus paracasei HD1.7 produces bacteriocin Paracin 1.7, which helps inhibit the growth of pathogenic bacteria during cabbage fermentation [50]. Fermented carrot from L. plantarum 299v has been shown to have potential benefits in treating and preventing obesity and type 2 diabetes [51].
2.3. The Mechanisms of Traditional Fermented Vegetables
LABs play a leading role in fermented vegetable production. LABs can be classified according to their distribution environment of homofermenters, heterofermenters, and facultative fermenters [52]. Lactic fermentation is the primary process during the fermentation of vegetables and can be divided into homotypic fermentation and heterotypic fermentation [53] (Figure 2).
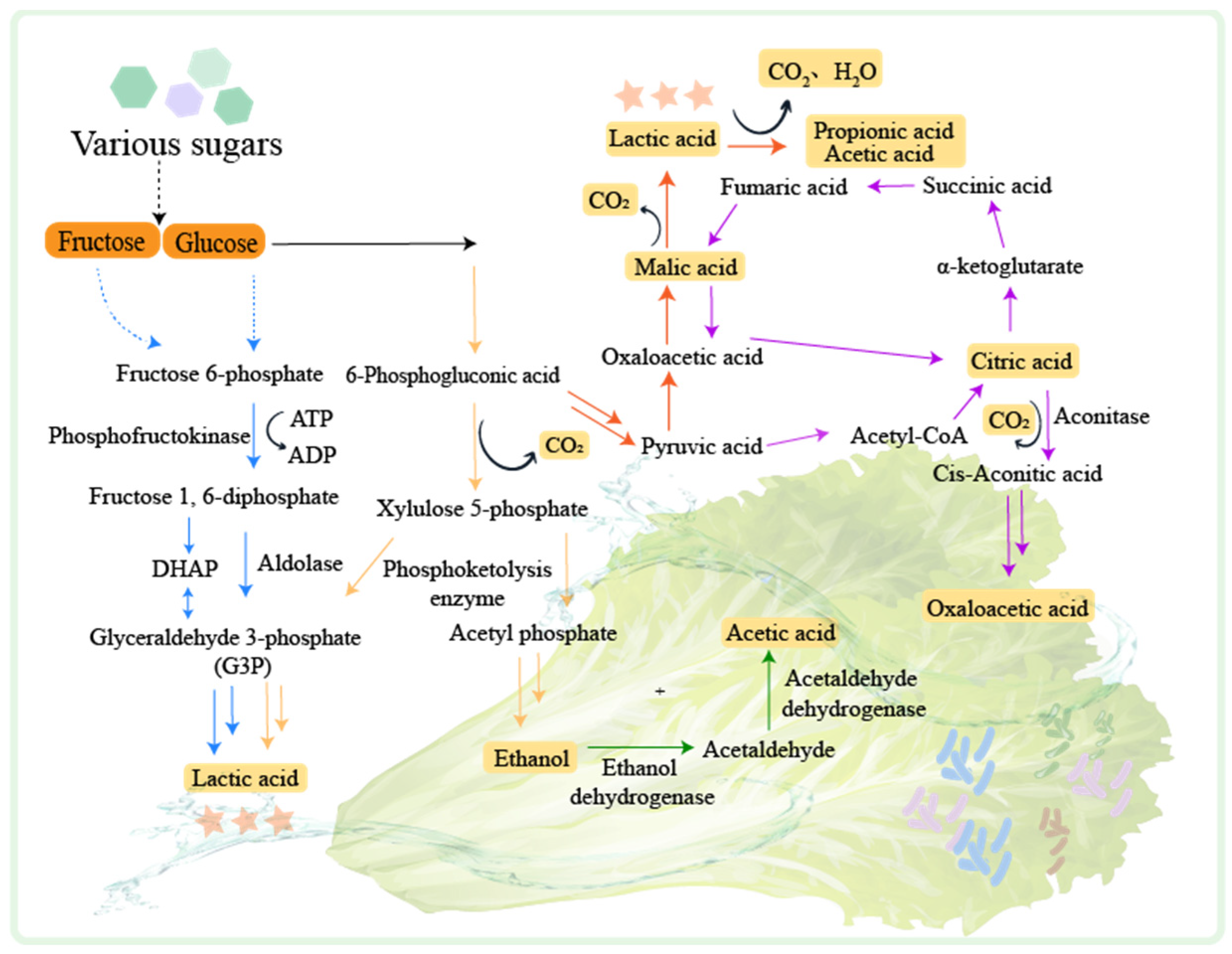
Figure 2. The mechanisms of fermented vegetables. PTS: Phosphoenolpyruvate-dependent sugar phosphotransferase system. The homotypic lactic fermentation pathway is indicated by the blue arrow. The heterotypic lactic fermentation pathway is indicated by the yellow arrow. The malate metabolic pathway is indicated by the red arrow. The citrate metabolic pathway is indicated by the purple arrow. The ethanol metabolic pathway is indicated by the green arrow.
In hetero-lactic fermentation, glucose is converted to 6-phosphogluconate by hexokinase and glucose dehydrogenase [54], and then to ribulose-5-phosphate by 6-phosphogluconate dehydrogenase. Xylulose-5-phosphate is generated by the epimerization of ribulose-3-epimerase, which is then decomposed to acetyl phosphate and glyceraldehyde-3-phosphate. Acetyl phosphate is converted to acetyl-CoA by phosphotransacetylase, which is then processed by aldehyde dehydrogenase and alcohol dehydrogenase to produce ethanol [55]. Glyceraldehyde-3-phosphate undergoes glycolysis to generate pyruvate, which is converted to lactate by lactate dehydrogenase. In homo-lactic fermentation, glucose is catalyzed by hexokinase and phosphohexose isomerase to produce fructose 6-phosphate and then decomposed into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P) by aldolase. G3P undergoes a series of reactions and is finally converted to pyruvate which is reduced to lactate [53], and lactic acid can be further converted to propionic acid and acetic acid by Propionibacterium [56].
Apart from lactic fermentation, vegetable fermentation also involves the production of ethanol, citric acid, and malic acid [52]. Ethanol can be converted to acetic acid by alcohol dehydrogenase and acetaldehyde dehydrogenase [57]. Citric acid generation is usually carried out in the presence of Aspergillus niger. The pyruvate generated by glycolysis is converted to acetyl-CoA and oxaloacetate. Oxaloacetate is reduced to malate, which participates in the tricarboxylic acid cycle to produce citrate. Citric acid can be converted to oxoacetic acid through the tricarboxylic acid cycle [58], and malic acid generated in this process can be fermented by Oenococcus oeni to produce lactic acid [59].
During vegetable fermentation, hetero-lactic fermentation is dominant at the early stage [60]. This process is carried out by microorganisms such as Bifidobacterium spp., Cryptococcus spp., and certain Lactobacillus such as Lactobacillus brevis and Lactobacillus buchneri [61]. Hetero-lactic fermentation reduces the pH of the environment, creating an unfavorable condition for the activity of LABs. At the middle stage of fermentation, homo-lactic fermentation occurs. This process is mainly performed by Lactobacillus delbrueckii, L. plantarum, and Streptococcus spp. These bacteria can convert more than 80% of glucose to lactic acid. At the later stage of fermentation, various compounds are produced by LAB fermentation. These compounds include organic acids, glycolyl, acetoin, acetic acid, ethanol, short peptides, and amino acids. They contribute to the flavor of fermented vegetables. At the later stage of vegetable fermentation, alcoholic and acetic fermentation processes also take place. The pH of the environment decreases, which slows down the LAB growth. In the meanwhile, the yeast and acetic acid bacteria become dominant. The yeast converts the sugars in vegetables into ethanol through alcohol fermentation. The acetic acid bacteria then convert the ethanol into acetic acid. Additionally, the acetic acid combines with alcohols to form esters, which further enhances the flavor of fermented vegetables.
3. The Effects of Probiotics on Fermented Vegetables
In recent times, probiotic starter cultures have become increasingly popular as essential contributors to vegetable fermentation due to their numerous benefits. These benefits include reducing harmful metabolic products, inhibiting pathogenic bacteria, and enhancing therapeutic effects. Probiotics play a significant role in promoting carbohydrate, amino acid, and nucleotide metabolisms, as well as reinforcing the biosynthesis of vitamins and bacteriocin. Moreover, they contribute to the generation of therapeutic products [62]. For example, a formulation that includes Lactobacillus acidophilus GL A-14, Lactobacillus rhamnosus HN001, and bovine lactoferrin can be used as an adjuvant therapy along with topical clotrimazole to treat vulvovaginal candidiasis. Individuals who used the adjuvant therapy experienced better improvement [63]. Additionally, in an in vivo study, Golden Syrian hamsters were orally administered a vaccine containing L. casei, and the result showed that the vaccine exhibited significant advantages when compared to the traditional vaccine [64]. L. plantarum CQPC02 isolated from the Sichuan pickle showed significant anti-fatigue and anti-oxidation effects in fatigue mice models, suggesting that it can be a potential microbiological therapeutic agent [65]. Organic acids produced by probiotics act as natural preservatives, prolonging the shelf life of fermented products and influencing flavor formation.
Probiotics possess metabolic capacity for various carbohydrates, including glucose, fructose, xylose, ribose, trehalose, maltose, and sucrose, and organic acids, including citrate, malate, and fumarate. The presence of fructose has been found to increase the expression of certain genes related to these metabolisms [66]. Phenolic derivatives derived from the microbial metabolism of probiotics significantly affect the sensory and health-promoting features of fermented vegetables. Lactobacilli and Pediococcus spp. are prominent producers of lactic acid and exhibit a high metabolic potential for the bioconversion of phenolics [19]. Bacteriocins produced by LABs have potential use as natural food preservatives due to their excellent antibacterial effects [38]. For instance, L. plantarum RUB1 has been found to produce a class IIb bacteriocin with strong antibacterial activity [67]. L. paracasei LS-6, which was isolated from a traditional fermented yogurt in Yunnan, China, can produce a bacteriocin LSX01 that exhibits activity against Staphylococcus aureus [68]. Additionally, nine strains isolated from home-made fermented vegetables from Northwest Bulgaria were found to be bacteriocin producers. These strains could potentially be utilized as starter cultures, reducing the need for chemical preservative additives in fermented vegetables [69]. Probiotics also have the ability to produce γ-aminobutyric acid (GABA), a major inhibitory neurotransmitter in the central nervous system. In addition, GABA exhibits anti-anxiety and tranquilizing effects [70][71]. Certain strains, such as Latilactobacillus curvatus K285 isolated from gat-kimchi [72], L. pentosus 9D3 isolated from Thai pickled weed [73], L. plantarum KB1253 [74], and Companilactobacillus allii WiKim39 and Lactococcus lactis WiKim0124 isolated from kimchi [75] have demonstrated a strong ability to produce GABA, making them potential starter cultures for producing functional foods. Mannitol, a 6-carbon sugar alcohol naturally present in microorganisms and plants, is slowly absorbed in intestinal tracts and does not increase blood sugar levels [70][76]. Strains such as Leuconostoc mesenteroides SKP88 and Leuconostoc citreum SKP92 isolated from pa (green onion)-kimchi can convert fructose to mannitol [76]. Ornithine, a non-proteinogenic amino acid converted from arginine, offers various functions such as anti-obesity properties, muscle growth promotion, anti-fatigue effects, and cirrhosis treatment [70]. Weissella koreensis DB1 can produce 15,059.65 mg/L ornithine. Safety evaluations have shown that it poses no health risk and can be used in fermented foods [77]. Fermented vegetables are rich in vitamins, which are essential for the proper functioning of the human body. The fermentation process helps retain the vitamin content from the raw materials [78]. In particular, co-fermentation of Pediococcus pentosaceus AL and Cyberlindnera rhodanensis J52 significantly increases the vitamin C concentration in fermented capsicum [79]. Additionally, certain LABs have the ability to produce vitamins. For example, Lactobacillus reuteri F2 has shown a strong ability for extracellular vitamin B12 production [80]. Therefore, fermenting vegetables with probiotic strains can serve as an effective method to enhance their beneficial properties.
Probiotics, such as LABs, have the ability to inhibit the growth of pathogenic bacteria and reduce the negative effects of harmful substances due to high lactic acid generation capacity, thereby improving the quality and safety of fermented products [49][81]. LABs, such as L. brevis and L. plantarum ZJ316, can inhibit the growth of Ralstonia spp., Pseudomonas, Proteus, and Enterobacter in pickled chili pepper and pickled mustard [34][49]. Functional foods rich in probiotic LABs have the potential to combat accidentally ingested pesticides in the gastrointestinal tract directly [8]. The high lactic acid generation capacity of LABs, such as L. plantarum ZJ316, contributes to the reduction in nitrite residual levels [49]. Deltamethrin, dimethoate, and imidacloprid are common pesticides used during olive growth, which are harmful to human health. Natural black olive fermented with L. plantarum strains 112 and 123 showed higher degradation of these substances compared to crude olives [82] (Table 1). The consumption of probiotics in fermented vegetables can have a significant impact on the composition of gut microbiota. When mice were fed with green loofah fermented with L. plantarum Uruma-SU4, the level of Lactobacillus johnsonii, which is the predominant LAB in mice gut microbiota, was increased [73]. The Firmicutes/Bacteroidetes ratio (F/B ratio) can reflect the health of the gut microbiota, with a higher F/B ratio suggesting low gut microbiota diversity [74]. L. pentosus P2020 derived from the Chinese pickle can significantly lower the F/B ratio and restore the gut microbiota, thereby protecting against the development of hyperuricemia [75]. Similarly, L. plantarum TWK10 isolated from Taiwan pickled cabbage has been found to reduce the F/B ratio in aging mice and modulate the imbalance of gut microbiota, thereby attenuating aging-related disorders [76]. These findings suggest that vegetables fermented with probiotics have the potential to become valuable contributors to therapeutic interventions aimed at restoring gut microbiota.
Table 1. The metabolic products and health benefits of microbial strains in fermented vegetables.
| Microbial Strains | Isolation Source | Metabolic Products | Health Benefits | Study Type | Ref. |
|---|---|---|---|---|---|
| Leu. mesenteroides/L. plantarum | Kimchi | Benzyl isothiocyanate, indole compounds, thiocyanate, b-sitosterol, and dietary fiber |
Anticancer, anti-atherosclerotic, and cholesterol-lowering |
In vivo | [83] |
| L. plantarum JS19 | Shaanxi Jiang-shui | Ameliorate inflammatory bowel disease | In vivo | [84] | |
| W. paramesenteroides | Yan-dong-gua | Organic acids, hydrogen peroxide, and bacteriocins | Antibacterial | In vitro | [85] |
| B. subtilis natto | Natto | Nattokinase, dipicolinic acid, Menaquinone-7 | Anti-thrombotic, promote bone health | Clinical trial | [86] |
| L. plantarum/Lev. Brevis/Leu. fallax | Fermented cucumber | GABA | Antihypertensive, antidepressant, and anticancer | In vitro | [87] |
| L. plantarum/L. pentosus | Table olives | Bioactive compounds, dietary fibers, fatty acids, antioxidants | Antioxidant activity | In vivo | [88] |
| L. pentosus LPG1 | Table olive | Bacteriocin, exopolysaccharides | Anti-inflammatory, reduce cholesterol levels, and inhibit food-borne pathogens | Clinical trial | [45][89] |
| L. fermentum SHY10 | Chinese pickles | Antibacterial peptides | Antibacterial | In vitro | [90][91] |
| L. pentosus CF2-10N | Fermented Aloreña green table olives | Vitamin, exopolysaccharide | Immunomodulation | In vitro | [42] |
| L. plantarum Uruma-SU4 | Fermented loofah | Bile acid-lowering | In vivo | [92] | |
| L. plantarum LRCC5314 | Kimchi | Anti-inflammatory, inhibition of adipogenesis | In vitro | [44] | |
| Lactococcus lactis/Weissella cibaria | Fermented beetroot | Niacin, riboflavin | Antagonistic properties against pathogenic bacteria | In vitro | [93] |
| L. plantarum NPL 1258 P. pentosaceus NPL 1264 |
Fermented cucumber | Extracellular polymeric substances (EPS) | Shorten the fermentation cycle and reduce pathogenic organism populations | In vitro | [94] |
| L. pentosus P2020 | Chinese pickle | Lower serum uric acid | In vivo | [95] | |
| L. plantarum TWK10 | Pickled cabbage | Attenuate aging-related disorders | In vivo | [96] |
3.1. Application of Probiotic Starter Cultures in Vegetable Fermentation
Spontaneous fermentation often leads to poor-quality products, as they are susceptible to contamination by spoilage microorganisms and pathogenic bacteria, which poses a challenge for industrial production [97]. Additionally, there is a growing consumer demand for fresh-tasting, nutritionally rich, and health-promoting foods with pleasing sensory properties. To meet these demands, specific microbial species with desired properties can be isolated from fermented vegetables to be used as starter cultures in the production of functional food [93]. The use of probiotic starter cultures in fermentation offers effective approaches to standardize product quality, ensure safety, and optimize the benefits of the final products [98].
Probiotic starter cultures, mainly consisting of LABs, can be used in vegetable fermentation either as single-strain or mixed-strain cultures, depending on the specific fermented vegetable products. The selection of starter cultures significantly influences the physicochemical properties and aromatic qualities of the fermented vegetables [98]. Fermentation with a single bacterial species can accelerate the acidification process, resulting in faster conversion of fermented vegetables, reduced commercial losses, and lower production costs [20][81]. Fermentation with mixed probiotic starter cultures can enhance the fermentation abilities and shorten the maturation period. The mixed fermentation microbiota consists of dominant microorganisms, and the flavors of fermented vegetables are primarily derived from these dominant species. Kimchi inoculated with different starters exhibit high ratios of Leuconostoc, L. plantarum, and L. brevis [99]. A high inoculum of L. plantarum and P. pentosaceus strains (NPL 1258 and NPL 1259) has been found to effectively control the quality of fermented cucumbers [94]. Furthermore, the different mixing ratios of starter cultures lead to distinct metabolites in fermented vegetables. A high-level L. mesenteroides inoculation exhibits hetero-fermentative characteristics, resulting in higher mannitol and acetic acid levels but lower lactic acid levels compared to high-level homo-fermentative L. sakei inoculation [100].
By increasing the population of LABs and decreasing the undesirable microorganisms, probiotic starter cultures contribute to a shorter fermentation cycle and a reduction in the population of pathogenic organisms, thereby improving the safety and quality of fermented vegetables. Heterofermentative LABs can accelerate the growth of homofermentative LABs, leading to a rapid decrease in pH value [92]. Additionally, L. plantarum exhibits strong acid tolerance and performs well in the presence of Leu. mesenteroides. Therefore, the starter culture composed of L. plantarum and Leu. mesenteroides plays a significant role in producing the distinctive flavor of northeast sauerkraut [97].
3.2. Design of Robust, Stable, and Predictable Probiotic Microbiota
Designing a robust, stable, and predictable probiotic microbiota is crucial for ensuring the quality of fermented vegetable products. The presence of an adequate number of probiotics is essential for their health benefits, with a minimum of 106 CFU/mL being necessary [101]. To achieve this, supplementing the fermentation process with dietary fiber, such as cellulose or inulin, before fermentation, not only generates prebiotic carbohydrates but also creates a favorable growth environment for probiotic strains, thereby improving their viability in fermented vegetable foods [94][102][103] (Figure 3). Furthermore, establishing a microbial collection that encompasses various microbial strains and their corresponding genomic information can offer a valuable resource for developing effective probiotic strains and providing strains for designing probiotic microbiota [104][105].
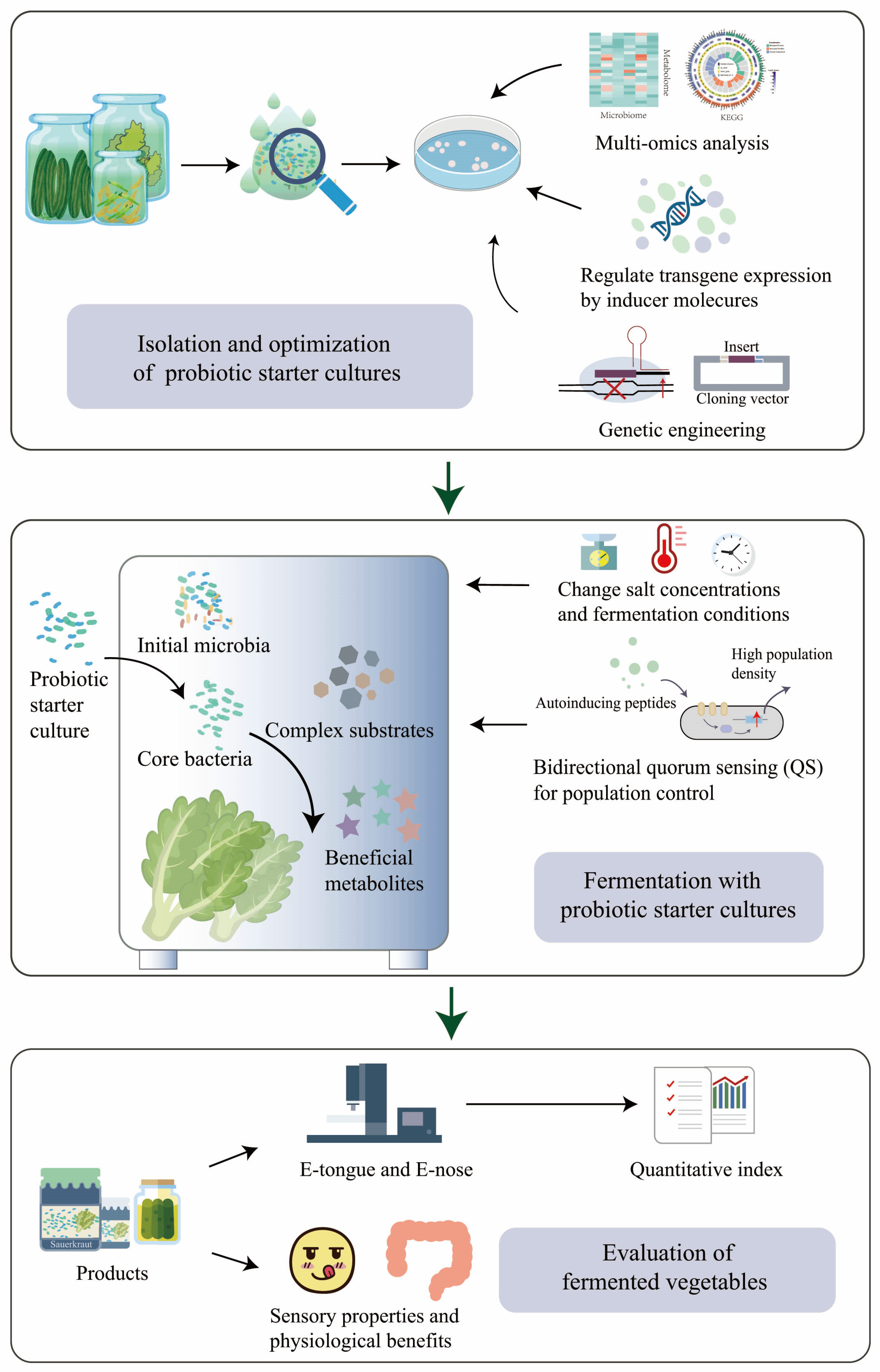
Figure 3. Design of robust, stable, and predictable vegetable fermentation with probiotic starter cultures.
Probiotics are living microorganisms that confer beneficial effects on the host [106]. By establishing communication systems through intercellular signaling and facilitating co-culture to create interdependent networks of microorganisms, it is possible to enhance the gastrointestinal environment tolerance of probiotics and enhance the beneficial properties of fermented vegetables [107][108]. Quorum sensing (QS) is a biological communication system that regulates various physiological and biochemical functions, including gene expression, biofilm formation, and bacteriocin production. This system operates through the use of specific signal molecules called autoinducers [109][110]. For example, under co-culture conditions, L. acidophilus can enhance its intestinal adhesion ability and promote the growth of other strains in the starter culture by the autoinducer-2 QS system [111] (Figure 3). As for LABs and Bifidobacteria, QS is essential for their resistance to harsh conditions and biological function regulation [112].
To improve the characteristics of fermented vegetables and achieve cost-effective industrial production with high quality, various strategies have been implemented. For example, the biotechnological strategy of roseoflavin induction was used to increase riboflavin content by 10 times in L. plantarum RYG-YYG-9049-M10 [113]. Additionally, the characterization of the pan-genome of L. sakei allows for the exploitation of its genomic diversity and the identification of marker genes, enabling the establishment of starter strain sets with complementary metabolic traits [114]. In the case of L. plantarum CGMCC 1.2437T, it has the capability to produce GABA. By using L-monosodium glutamate as a single inducing factor, GABA synthesis is facilitated while degradation is inhibited [115]. Another strategy involves co-culturing L. acidophilus and Bacillus subtilis, which can enhance the production of total SCFAs [116].
The advancements in metabolic engineering and synthetic biology have enabled wild-type microorganisms to acquire additional functionalities [117]. For instance, engineered yeast can now synthesize diverse plant natural products [118][119][120], while probiotic strains can be engineered to possess enhanced functional properties [121]. Furthermore, the design–build–test–learn cycle of synthetic biology has been employed to construct robust and stable probiotic microbiota [122]. These developments have great potential to improve the process of fermented vegetable production and offer ideal probiotic starter cultures for vegetable fermentation.
References
- Estruch, R.; Lamuela-Raventós, R.M. Cardiovascular Benefits of Fermented Foods and Beverages: Still up for Debate. Nat. Rev. Cardiol. 2023, 1–2.
- Xiao, M.; Huang, T.; Huang, C.; Hardie, J.; Peng, Z.; Xie, M.; Xiong, T. The Microbial Communities and Flavour Compounds of Jiangxi Yancai, Sichuan Paocai and Dongbei Suancai: Three Major Types of Traditional Chinese Fermented Vegetables. LWT 2020, 121, 108865.
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516.
- Leech, J.; Cabrera-Rubio, R.; Walsh, A.M.; Macori, G.; Walsh, C.J.; Barton, W.; Finnegan, L.; Crispie, F.; O’Sullivan, O.; Claesson, M.J.; et al. Fermented-Food Metagenomics Reveals Substrate-Associated Differences in Taxonomy and Health-Associated and Antibiotic Resistance Determinants. mSystems 2020, 5, e00522-20.
- Bousquet, J.; Anto, J.M.; Czarlewski, W.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; Vidal, A.; Sheikh, A.; Akdis, C.A.; et al. Cabbage and Fermented Vegetables: From Death Rate Heterogeneity in Countries to Candidates for Mitigation Strategies of Severe COVID-19. Allergy 2021, 76, 735–750.
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L.; Pedrolli, C.; Tuohy, K.M.; Fava, F. Microbial and Metabolic Characterization of Organic Artisanal Sauerkraut Fermentation and Study of Gut Health-Promoting Properties of Sauerkraut Brine. Front. Microbiol. 2022, 13, 929738.
- Nabavi-Rad, A.; Sadeghi, A.; Asadzadeh Aghdaei, H.; Yadegar, A.; Smith, S.M.; Zali, M.R. The Double-Edged Sword of Probiotic Supplementation on Gut Microbiota Structure in Helicobacter pylori Management. Gut Microbes 2022, 14, 2108655.
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99.
- Rajendran, S.; Silcock, P.; Bremer, P. Flavour Volatiles of Fermented Vegetable and Fruit Substrates: A Review. Molecules 2023, 28, 3236.
- Armenova, N.; Tsigoriyna, L.; Arsov, A.; Petrov, K.; Petrova, P. Microbial Detoxification of Residual Pesticides in Fermented Foods: Current Status and Prospects. Foods 2023, 12, 1163.
- Baralić, K.; Živančević, K.; Bozic, D.; Đukić-Ćosić, D. Probiotic Cultures as a Potential Protective Strategy against the Toxicity of Environmentally Relevant Chemicals: State-of-the-Art Knowledge. Food Chem. Toxicol. 2023, 172, 113582.
- Yasir, M.; Al-Zahrani, I.A.; Bibi, F.; Abd El Ghany, M.; Azhar, E.I. New Insights of Bacterial Communities in Fermented Vegetables from Shotgun Metagenomics and Identification of Antibiotic Resistance Genes and Probiotic Bacteria. Food Res. Int. 2022, 157, 111190.
- Zhang, S.; Zhang, Y.; Wu, L.; Zhang, L.; Wang, S. Characterization of Microbiota of Naturally Fermented Sauerkraut by High-Throughput Sequencing. Food Sci. Biotechnol. 2023, 32, 855–862.
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679.
- Kim, C.-S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.-M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2020, 76, 32–40.
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107.
- Du, R.; Yu, L.; Yu, N.; Ping, W.; Song, G.; Ge, J. Characterization of Exopolysaccharide Produced by Levilactobacillus brevis HDE-9 and Evaluation of Its Potential Use in Dairy Products. Int. J. Biol. Macromol. 2022, 217, 303–311.
- Daliri, E.B.-M.; Kim, Y.; Do, Y.; Chelliah, R.; Oh, D.-H. In Vitro and In Vivo Cholesterol Reducing Ability and Safety of Probiotic Candidates Isolated from Korean Fermented Soya Beans. Probiotics Antimicrob. Proteins 2022, 14, 87–98.
- Tlais, A.Z.A.; Lemos Junior, W.J.F.; Filannino, P.; Campanaro, S.; Gobbetti, M.; Di Cagno, R. How Microbiome Composition Correlates with Biochemical Changes during Sauerkraut Fermentation: A Focus on Neglected Bacterial Players and Functionalities. Microbiol. Spectr. 2022, 10, e00168-22.
- Wang, D.; Chen, G.; Tang, Y.; Ming, J.; Huang, R.; Li, J.; Ye, M.; Fan, Z.; Chi, Y.; Zhang, Q.; et al. Study of Bacterial Community Succession and Reconstruction of the Core Lactic Acid Bacteria to Enhance the Flavor of Paocai. Int. J. Food Microbiol. 2022, 375, 109702.
- Yu, Y.; Yu, Y.; Xu, Z. Evaluation of Nitrite, Ethyl Carbamate, and Biogenic Amines in Four Types of Fermented Vegetables. Foods 2021, 10, 3150.
- Liang, T.; Xie, X.; Wu, L.; Li, L.; Li, H.; Xi, Y.; Feng, Y.; Xue, L.; Chen, M.; Chen, X.; et al. Microbial Communities and Physiochemical Properties of Four Distinctive Traditionally Fermented Vegetables from North China and Their Influence on Quality and Safety. Foods 2021, 11, 21.
- Wang, D.; Chen, G.; Tang, Y.; Li, H.; Shen, W.; Wang, M.; Liu, S.; Qin, W.; Zhang, Q. Effects of Temperature on Paocai Bacterial Succession Revealed by Culture-Dependent and Culture-Independent Methods. Int. J. Food Microbiol. 2020, 317, 108463.
- Liang, H.; He, Z.; Wang, X.; Song, G.; Chen, H.; Lin, X.; Ji, C.; Li, S. Effects of Salt Concentration on Microbial Diversity and Volatile Compounds during Suancai Fermentation. Food Microbiol. 2020, 91, 103537.
- Zhang, S.; Xiao, Y.; Jiang, Y.; Wang, T.; Cai, S.; Hu, X.; Yi, J. Effects of Brines and Containers on Flavor Production of Chinese Pickled Chili Pepper (Capsicum Frutescens L.) during Natural Fermentation. Foods 2023, 12, 101.
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally Fermented Pickles: How the Microbial Diversity Associated with Their Nutritional and Health Benefits? J. Funct. Foods 2020, 70, 103971.
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in Autochthonous Microbial Diversity and Volatile Metabolites during the Fermentation of Chili Pepper (Capsicum Frutescens L.). Food Chem. 2021, 335, 127512.
- Liu, P.; Xiang, Q.; Sun, W.; Wang, X.; Lin, J.; Che, Z.; Ma, P. Correlation between Microbial Communities and Key Flavors during Post-Fermentation of Pixian Broad Bean Paste. Food Res. Int. 2020, 137, 109513.
- Wu, J.; Tian, T.; Liu, Y.; Shi, Y.; Tao, D.; Wu, R.; Yue, X. The Dynamic Changes of Chemical Components and Microbiota during the Natural Fermentation Process in Da-Jiang, a Chinese Popular Traditional Fermented Condiment. Food Res. Int. 2018, 112, 457–467.
- Liu, L.; She, X.; Qian, Y.; Li, Y.; Tao, Y.; Che, Z.; Liu, G.; Rao, Y. Effect of Different Fermenting Containers on the Deterioration of Sichuan Pickle. LWT 2019, 111, 829–836.
- Zhang, C.; Zhang, J.; Liu, D. Biochemical Changes and Microbial Community Dynamics during Spontaneous Fermentation of Zhacai, a Traditional Pickled Mustard Tuber from China. Int. J. Food Microbiol. 2021, 347, 109199.
- Ruiz-Moyano, S.; Esperilla, A.; Hernández, A.; Benito, M.J.; Casquete, R.; Martín-Vertedor, D.; Pérez-Nevado, F. Application of ISSR-PCR as a Rapid Method for Clustering and Typing of Yeasts Isolated from Table Olives. LWT 2019, 109, 250–254.
- Satora, P.; Skotniczny, M.; Strnad, S.; Ženišová, K. Yeast Microbiota during Sauerkraut Fermentation and Its Characteristics. Int. J. Mol. Sci. 2020, 21, 9699.
- Ye, Z.; Shang, Z.; Zhang, S.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Dynamic Analysis of Flavor Properties and Microbial Communities in Chinese Pickled Chili Pepper (Capsicum Frutescens L.): A Typical Industrial-Scale Natural Fermentation Process. Food Res. Int. 2022, 153, 110952.
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of Microbial Communities, Flavor, and Physicochemical Properties of Pickled Chayote during an Industrial-Scale Natural Fermentation: Correlation between Microorganisms and Metabolites. Food Chem. 2022, 377, 132004.
- Zhang, S. Flavor Production in Fermented Chayote Inoculated with Lactic Acid Bacteria Strains: Genomics and Metabolomics Based Analysis. Food Res. Int. 2023, 163, 112224.
- Vaccalluzzo, A.; Pino, A.; Russo, N.; De Angelis, M.; Caggia, C.; Randazzo, C.L. FoodOmics as a New Frontier to Reveal Microbial Community and Metabolic Processes Occurring on Table Olives Fermentation. Food Microbiol. 2020, 92, 103606.
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of Lactic Acid Bacteria and Their Bacteriocins against Food Spoilage Microorganisms and Foodborne Pathogens. Molecules 2021, 26, 7055.
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Recent Update on Lactic Acid Bacteria Producing Riboflavin and Folates: Application for Food Fortification and Treatment of Intestinal Inflammation. J. Appl. Microbiol. 2021, 130, 1412–1424.
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, Characterization, and Assessment of Lactic Acid Bacteria toward Their Selection as Poultry Probiotics. BMC Microbiol. 2019, 19, 253.
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; Enshasy, H.E. Lactic Acid Bacteria: From Starter Cultures to Producers of Chemicals. FEMS Microbiol. Lett. 2018, 365, fny213.
- Abriouel, H.; Manetsberger, J.; Caballero Gómez, N.; Benomar, N. In Silico Genomic Analysis of the Potential Probiotic Lactiplantibacillus pentosus CF2-10N Reveals Promising Beneficial Effects with Health Promoting Properties. Front. Microbiol. 2022, 13, 989824.
- Mun, S.Y.; Chang, H.C. Characterization of Weissella koreensis SK Isolated from Kimchi Fermented at Low Temperature (around 0 °C) Based on Complete Genome Sequence and Corresponding Phenotype. Microorganisms 2020, 8, 1147.
- Yoon, S.; Cho, H.; Nam, Y.; Park, M.; Lim, A.; Kim, J.-H.; Park, J.; Kim, W. Multifunctional Probiotic and Functional Properties of Lactiplantibacillus plantarum LRCC5314, Isolated from Kimchi. J. Microbiol. Biotechnol. 2022, 32, 72–80.
- López-García, E.; Benítez-Cabello, A.; Arenas-de Larriva, A.P.; Gutierrez-Mariscal, F.M.; Pérez-Martínez, P.; Yubero-Serrano, E.M.; Garrido-Fernández, A.; Arroyo-López, F.N. Oral Intake of Lactiplantibacillus pentosus LPG1 Produces a Beneficial Regulation of Gut Microbiota in Healthy Persons: A Randomised, Placebo-Controlled, Single-Blind Trial. Nutrients 2023, 15, 1931.
- LeBlanc, J.G.; Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A. Application of Vitamin-Producing Lactic Acid Bacteria to Treat Intestinal Inflammatory Diseases. Appl. Microbiol. Biotechnol. 2020, 104, 3331–3337.
- Takahashi, K.; Orito, N.; Tokunoh, N.; Inoue, N. Current Issues Regarding the Application of Recombinant Lactic Acid Bacteria to Mucosal Vaccine Carriers. Appl. Microbiol. Biotechnol. 2019, 103, 5947–5955.
- Song, G.; He, Z.; Wang, X.; Zhao, M.; Cao, X.; Lin, X.; Ji, C.; Zhang, S.; Liang, H. Improving the Quality of Suancai by Inoculating with Lactobacillus plantarum and Pediococcus pentosaceus. Food Res. Int. 2021, 148, 110581.
- Zhang, X.; Han, J.; Zheng, X.; Yan, J.; Chen, X.; Zhou, Q.; Zhao, X.; Gu, Q.; Li, P. Use of Lactiplantibacillus plantarum ZJ316 as a Starter Culture for Nitrite Degradation, Foodborne Pathogens Inhibition and Microbial Community Modulation in Pickled Mustard Fermentation. Food Chem. X 2022, 14, 100344.
- Zhao, D.; Du, R.P.; Ping, W.X.; Ge, J.P. Lactobacillus paracasei HD1.7 Used as a Starter Modulates the Bacterial Community and Metabolome Profile during Fermentation of Chinese Cabbage. Lett. Appl. Microbiol. 2018, 67, 411–419.
- Li, Y.; Ten, M.M.Z.; Zwe, Y.H.; Li, D. Lactiplantibacillus plantarum 299v as Starter Culture Suppresses Enterobacteriaceae More Efficiently than Spontaneous Fermentation of Carrots. Food Microbiol. 2022, 103, 103952.
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527.
- Martínez-Miranda, J.G.; Chairez, I.; Durán-Páramo, E. Mannitol Production by Heterofermentative Lactic Acid Bacteria: A Review. Appl. Biochem. Biotechnol. 2022, 194, 2762–2795.
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285.
- Eram, M.; Ma, K. Decarboxylation of Pyruvate to Acetaldehyde for Ethanol Production by Hyperthermophiles. Biomolecules 2013, 3, 578–596.
- Gonzalez-Garcia, R.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21.
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625.
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial Citric Acid: Production, Properties, Application, and Future Perspectives. Food Front. 2021, 2, 62–76.
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549.
- Moon, S.H.; Kim, C.R.; Chang, H.C. Heterofermentative Lactic Acid Bacteria as a Starter Culture to Control Kimchi Fermentation. LWT 2018, 88, 181–188.
- Okoye, C.O.; Wei, Z.; Jiang, H.; Wu, Y.; Wang, Y.; Gao, L.; Li, X.; Jiang, J. Metagenomics Analysis Reveals the Performance of Homo- and Heterofermentative Lactic Acid Bacteria in Alfalfa Silage Fermentation, Bacterial Community, and Functional Profiles. J. Anim. Sci. 2023, 101, skad163.
- Chung, H.-J.; Lee, H.; Na, G.; Jung, H.; Kim, D.-G.; Shin, S.-I.; Jung, S.-E.; Choi, I.; Lee, J.-H.; Sim, J.-H.; et al. Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules 2020, 10, 725.
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised Clinical Trial in Women with Recurrent Vulvovaginal Candidiasis: Efficacy of Probiotics and Lactoferrin as Maintenance Treatment. Mycoses 2019, 62, 328–335.
- Chau, E.C.T.; Kwong, T.C.; Pang, C.K.; Chan, L.T.; Chan, A.M.L.; Yao, X.; Tam, J.S.L.; Chan, S.W.; Leung, G.P.H.; Tai, W.C.S.; et al. A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529. Int. J. Mol. Sci. 2023, 24, 13931.
- Yi, R.; Feng, M.; Chen, Q.; Long, X.; Park, K.-Y.; Zhao, X. The Effect of Lactobacillus plantarum CQPC02 on Fatigue and Biochemical Oxidation Levels in a Mouse Model of Physical Exhaustion. Front. Nutr. 2021, 8, 641544.
- Sahin, A.W.; Rice, T.; Coffey, A. Genomic Analysis of Leuconostoc citreum TR116 with Metabolic Reconstruction and the Effects of Fructose on Gene Expression for Mannitol Production. Int. J. Food Microbiol. 2021, 354, 109327.
- Wu, A.; Fu, Y.; Kong, L.; Shen, Q.; Liu, M.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Production of a Class IIb Bacteriocin with Broad-Spectrum Antimicrobial Activity in Lactiplantibacillus plantarum RUB1. Probiotics Antimicrob. Proteins 2021, 13, 1820–1832.
- Jiang, Y.-H.; Xin, W.-G.; Yang, L.-Y.; Ying, J.-P.; Zhao, Z.-S.; Lin, L.-B.; Li, X.-Z.; Zhang, Q.-L. A Novel Bacteriocin against Staphylococcus Aureus from Lactobacillus paracasei Isolated from Yunnan Traditional Fermented Yogurt: Purification, Antibacterial Characterization, and Antibiofilm Activity. J. Dairy Sci. 2022, 105, 2094–2107.
- Rwubuzizi, R.; Carneiro, K.O.; Holzapfel, W.H.; Vaz-Velho, M.; Todorov, S.D. Bacteriocin and Antioxidant Production, a Beneficial Properties of Lactic Acid Bacteria Isolated from Fermented Vegetables of Northwest Bulgaria. Probiotics Antimicrob. Proteins, 2023; online ahead of print.
- Lee, S.-J.; Jeon, H.-S.; Yoo, J.-Y.; Kim, J.-H. Some Important Metabolites Produced by Lactic Acid Bacteria Originated from Kimchi. Foods 2021, 10, 2148.
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2742.
- Lee, S.J.; Jeon, H.S.; Yoo, J.Y.; Kang, Y.J.; Kim, M.J.; Kim, T.J.; Kim, J.H. Characterization of a Novel Glutamate Decarboxylase (GAD) from Latilactobacillus curvatus K285 Isolated from Gat-Kimchi. Food Sci. Biotechnol. 2022, 31, 69.
- Raethong, N.; Santivarangkna, C.; Visessanguan, W.; Santiyanont, P.; Mhuantong, W.; Chokesajjawatee, N. Whole-Genome Sequence Analysis for Evaluating the Safety and Probiotic Potential of Lactiplantibacillus pentosus 9D3, a Gamma-Aminobutyric Acid (GABA)-Producing Strain Isolated from Thai Pickled Weed. Front. Microbiol. 2022, 13, 969548.
- Nakatani, Y.; Fukaya, T.; Kishino, S.; Ogawa, J. Production of GABA-Enriched Tomato Juice by Lactiplantibacillus plantarum KB1253. J. Biosci. Bioeng. 2022, 134, 424–431.
- Lee, M.; Song, J.H.; Choi, E.J.; Yun, Y.-R.; Lee, K.W.; Chang, J.Y. UPLC-QTOF-MS/MS and GC-MS Characterization of Phytochemicals in Vegetable Juice Fermented Using Lactic Acid Bacteria from Kimchi and Their Antioxidant Potential. Antioxidants 2021, 10, 1761.
- Kang, Y.J.; Kim, M.J.; Kim, T.J.; Kim, J.H. Characterization of Two Mannitol-Producing Leuconostoc Strains from Pa-Kimchi and Their Application for Juice and Yogurt Fermentation. J. Microbiol. Biotechnol. 2023, 33, 780–787.
- Yeong, M.S.; Hee, M.S.; Choon, C.H. Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture. Foods 2020, 9, 1545.
- Janiszewska-Turak, E.; Witrowa-Rajchert, D.; Rybak, K.; Rolof, J.; Pobiega, K.; Woźniak, Ł.; Gramza-Michałowska, A. The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers. Molecules 2022, 27, 8637.
- Shi, Q.; Tang, X.; Liu, B.-Q.; Liu, W.-H.; Li, H.; Luo, Y.-Y. Correlation between Microbial Communities and Key Odourants in Fermented Capsicum Inoculated with Pediococcus pentosaceus and Cyberlindnera rhodanensis. J. Sci. Food Agric. 2023, 103, 1139–1151.
- Kumari, M.; Bhushan, B.; Kokkiligadda, A.; Kumar, V.; Behare, P.; Tomar, S.K. Vitamin B12 Biofortification of Soymilk through Optimized Fermentation with Extracellular B12 Producing Lactobacillus Isolates of Human Fecal Origin. Curr. Res. Food Sci. 2021, 4, 646–654.
- Liu, Y.; Chen, X.; Li, F.; Shi, H.; He, M.; Ge, J.; Ling, H.; Cheng, K. Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition. Foods 2023, 12, 1164.
- YildirimKumral, A.; Kumral, N.A.; Kolcu, A.; Maden, B.; Artik, B. Simulation Study for the Degradation of Some Insecticides during Different Black Table Olive Processes. ACS Omega 2020, 5, 14164–14172.
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W. Health Benefits of Kimchi (Korean Fermented Vegetables) as a Probiotic Food. J. Med. Food 2014, 17, 6–20.
- Ren, R.; Zhao, A.-Q.; Chen, L.; Wu, S.; Hung, W.-L.; Wang, B. Therapeutic Effect of Lactobacillus plantarum JS19 on Mice with Dextran Sulfate Sodium Induced Acute and Chronic Ulcerative Colitis. J. Sci. Food Agric. 2023, 103, 4143–4156.
- Lan, W.-T.; Chen, Y.; Yanagida, F. Isolation and Characterization of Lactic Acid Bacteria from Yan-Dong-Gua (Fermented Wax Gourd), a Traditional Fermented Food in Taiwan. J. Biosci. Bioeng. 2009, 108, 484–487.
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of Soy-Based Fermented Foods: A Review. Biotechnol. Adv. 2019, 37, 223–238.
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Fermented Vegetables and Legumes vs. Lifestyle Diseases: Microbiota and More. Life 2023, 13, 1044.
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table Olives More than a Fermented Food. Foods 2020, 9, 178.
- López-García, E.; Benítez-Cabello, A.; Ramiro-García, J.; Ladero, V.; Arroyo-López, F.N. In Silico Evidence of the Multifunctional Features of Lactiplantibacillus pentosus LPG1, a Natural Fermenting Agent Isolated from Table Olive Biofilms. Foods 2023, 12, 938.
- Song, J.; Peng, S.; Yang, J.; Zhou, F.; Suo, H. Isolation and Identification of Novel Antibacterial Peptides Produced by Lactobacillus fermentum SHY10 in Chinese Pickles. Food Chem. 2021, 348, 129097.
- Yang, Y.; Lian, Y.; Yin, S.; Suo, H.; Zeng, F.; Wang, H.; Song, J.; Zhang, Y. Inhibition of Lactobacillus fermentum SHY10 on the White Membrane Production of Soaked Pickled Radish. Food Sci. Nutr. 2022, 10, 2236.
- Shikano, A.; Kuda, T.; Shibayama, J.; Toyama, A.; Ishida, Y.; Takahashi, H.; Kimura, B. Effects of Lactobacillus plantarum Uruma-SU4 Fermented Green Loofah on Plasma Lipid Levels and Gut Microbiome of High-Fat Diet Fed Mice. Food Res. Int. 2019, 121, 817–824.
- Maślak, E.; Złoch, M.; Arendowski, A.; Sugajski, M.; Janczura, I.; Rudnicka, J.; Walczak-Skierska, J.; Buszewska-Forajta, M.; Rafińska, K.; Pomastowski, P.; et al. Isolation and Identification of Lactococcus lactis and Weissella cibaria Strains from Fermented Beetroot and an Investigation of Their Properties as Potential Starter Cultures and Probiotics. Foods 2022, 11, 2257.
- Ahmed, S.; Ashraf, F.; Tariq, M.; Zaidi, A. Aggrandizement of Fermented Cucumber through the Action of Autochthonous Probiotic Cum Starter Strains of Lactiplantibacillus plantarum and Pediococcus pentosaceus. Ann. Microbiol. 2021, 71, 33.
- Wang, Z.; Song, L.; Li, X.; Xiao, Y.; Huang, Y.; Zhang, Y.; Li, J.; Li, M.; Ren, Z. Lactiplantibacillus pentosus P2020 Protects the Hyperuricemia and Renal Inflammation in Mice. Front. Nutr. 2023, 10, 1094483.
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Lin, K.-J.; Hsu, H.-Y.; Chiou, S.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Lactobacillus plantarum TWK10 Attenuates Aging-Associated Muscle Weakness, Bone Loss, and Cognitive Impairment by Modulating the Gut Microbiome in Mice. Front. Nutr. 2021, 8, 708096.
- Hu, W.; Yang, X.; Ji, Y.; Guan, Y. Effect of Starter Cultures Mixed with Different Autochthonous Lactic Acid Bacteria on Microbial, Metabolome and Sensory Properties of Chinese Northeast Sauerkraut. Food Res. Int. 2021, 148, 110605.
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Comparison of Northeast Sauerkraut Fermentation between Single Lactic Acid Bacteria Strains and Traditional Fermentation. Food Res. Int. 2020, 137, 109553.
- Park, S.-E.; Seo, S.-H.; Kim, E.-J.; Byun, S.; Na, C.-S.; Son, H.-S. Changes of Microbial Community and Metabolite in Kimchi Inoculated with Different Microbial Community Starters. Food Chem. 2019, 274, 558–565.
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.-H.; Lee, M.-A. Effects of Combining Two Lactic Acid Bacteria as a Starter Culture on Model Kimchi Fermentation. Food Res. Int. 2020, 136, 109591.
- Marinova, V.Y.; Rasheva, I.K.; Kizheva, Y.K.; Dermenzhieva, Y.D.; Hristova, P.K. Microbiological Quality of Probiotic Dietary Supplements. Biotechnol. Biotechnol. Equip. 2019, 33, 834–841.
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a Diet Based on Inulin-Rich Vegetables on Gut Health and Nutritional Behavior in Healthy Humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695.
- Güney, D.; Güngörmüşler, M. Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2021, 13, 495–505.
- Wei, Y.; Gao, X. Complete Genome Sequence of Curtobacterium sp. Strain YC1, Isolated from the Surface of Nostoc Flagelliforme Colonies in Yinchuan, Ningxia, China. Microbiol. Resour. Announc. 2021, 10, e01467-20.
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the Human Skin Microbiota and Its Roles in Cutaneous Diseases. Microb. Cell Factories 2022, 21, 176.
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live Biotherapeutic Products: The Importance of a Defined Regulatory Framework. Exp. Mol. Med. 2020, 52, 1397–1406.
- McCarty, N.S.; Ledesma-Amaro, R. Synthetic Biology Tools to Engineer Microbial Communities for Biotechnology. Trends Biotechnol. 2019, 37, 181.
- Özcan, E.; Seven, M.; Şirin, B.; Çakır, T.; Nikerel, E.; Teusink, B.; Toksoy Öner, E. Dynamic Co-culture Metabolic Models Reveal the Fermentation Dynamics, Metabolic Capacities and Interplays of Cheese Starter Cultures. Biotechnol. Bioeng. 2021, 118, 223–237.
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell. Infect. Microbiol. 2020, 10, 538077.
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum Sensing for Population-Level Control of Bacteria and Potential Therapeutic Applications. Cell. Mol. Life Sci. 2020, 77, 1319–1343.
- Wen, J.; Yu, Y.; Chen, M.; Cui, L.; Xia, Q.; Zeng, X.; Guo, Y.; Pan, D.; Wu, Z. Amino Acid-Derived Quorum Sensing Molecule Alanine on the Gastrointestinal Tract Tolerance of the Lactobacillus Strains in the Cocultured Fermentation Model. Microbiol. Spectr. 2022, 10, e00832-21.
- Kareb, O.; Aïder, M. Quorum Sensing Circuits in the Communicating Mechanisms of Bacteria and Its Implication in the Biosynthesis of Bacteriocins by Lactic Acid Bacteria: A Review. Probiotics Antimicrob. Proteins 2020, 12, 5–17.
- Ge, Y.-Y.; Zhang, J.-R.; Corke, H.; Gan, R.-Y. Screening and Spontaneous Mutation of Pickle-Derived Lactobacillus plantarum with Overproduction of Riboflavin, Related Mechanism, and Food Application. Foods 2020, 9, 88.
- Eisenbach, L.; Geissler, A.J.; Ehrmann, M.A.; Vogel, R.F. Comparative Genomics of Lactobacillus sakei Supports the Development of Starter Strain Combinations. Microbiol. Res. 2019, 221, 1–9.
- Zhuang, K.; Jiang, Y.; Feng, X.; Li, L.; Dang, F.; Zhang, W.; Man, C. Transcriptomic Response to GABA-Producing Lactobacillus plantarum CGMCC 1.2437T Induced by L-MSG. PLoS ONE 2018, 13, e0199021.
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475.
- Ko, Y.-S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and Strategies of Systems Metabolic Engineering for the Development of Microbial Cell Factories for Chemical Production. Chem. Soc. Rev. 2020, 49, 4615–4636.
- Wei, Y.; Bergenholm, D.; Gossing, M.; Siewers, V.; Nielsen, J. Expression of Cocoa Genes in Saccharomyces cerevisiae Improves Cocoa Butter Production. Microb. Cell Factories 2018, 17, 11.
- Wei, Y.; Ji, B.; Siewers, V.; Xu, D.; Halkier, B.A.; Nielsen, J. Identification of Genes Involved in Shea Butter Biosynthesis from Vitellaria paradoxa Fruits through Transcriptomics and Functional Heterologous Expression. Appl. Microbiol. Biotechnol. 2019, 103, 3727–3736.
- Wang, M.; Wei, Y.; Ji, B.; Nielsen, J. Advances in Metabolic Engineering of Saccharomyces cerevisiae for Cocoa Butter Equivalent Production. Front. Bioeng. Biotechnol. 2020, 8, 594081.
- Pramanik, S.; Venkatraman, S.; Vaidyanathan, V.K. Development of Engineered Probiotics with Tailored Functional Properties and Their Application in Food Science. Food Sci. Biotechnol. 2023, 32, 453–470.
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research Advances in Probiotic Fermentation of Chinese Herbal Medicines. iMeta 2023, 2, e93.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
863
Revisions:
2 times
(View History)
Update Date:
01 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





