Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yongjun Wei and Version 2 by Camila Xu.
Fermented vegetables are popular traditional foods known for their unique flavors and health benefits. The flavors of fermented vegetables can vary based on different vegetable materials and fermentation techniques used.
- fermented vegetables
- traditional fermentation
- probiotics
1. Introduction to Fermented Vegetable Foods
Fermented vegetables are popular traditional foods known for their unique flavors and health benefits [1]. The flavors of fermented vegetables can vary based on different vegetable materials and fermentation techniques used. A wide variety of fresh vegetables, such as cabbages, carrots, beets, cucumbers, celery, peppers, and green beans, can be used in the production of fermented vegetables [2]. Sauerkraut and pickles are the main types of fermented vegetables. Numerous studies have highlighted the health benefits of fermented vegetables, which provide nutritional products including vitamins, antioxidants, proteins, carbohydrates, and exopolysaccharides. Moreover, they exhibit anti-inflammatory, immunomodulatory, and gut-health-promoting properties [3][4][5][6][7][3,4,5,6,7].
The microbial metabolites produced during vegetable fermentation are essential in the formation of flavors. Microorganisms convert the fermentable substrates, mainly carbohydrates and proteins, into biologically active metabolites, such as short-chain fatty acids (SCFAs), sugar, organic acids, free amino acids (FAA), and volatiles. These metabolites contribute to the attractive flavors and desirable nutritional values of fermented vegetables [8][9][8,9]. Each type of fermented vegetables generally harbors a distinct population of microorganisms. Probiotics, especially lactic acid bacteria (LABs), are essential in fermented vegetables, for they optimize flavor characteristics, produce beneficial metabolites, inhibit undesirable microorganisms, and reduce harmful compounds [10][11][10,11]. LABs, known for their strong lactic acid production ability, become the dominant contributors during the later stage of vegetable fermentation [2][12][13][2,12,13].
Considering the contributions of vegetable fermentation and the metabolic activities of probiotic communities, fermented vegetables not only provide nutrition and appetizing healthy foods for humans but also offer various functional benefits. These benefits include antioxidant properties, cholesterol-lowering effects, and modulation of the gut microbiome [14][15][16][17][18][14,15,16,17,18]. Thus, it is critical to understand the interplay between the microbiota and physicochemical indices of fermented vegetables, in order to enhance the quality and safety of traditional fermented vegetable products.
2. Traditional Vegetable Fermentation
Due to different vegetable materials, environments, and production processes, traditional fermented vegetables can be roughly divided into sauerkraut and pickles. The cabbage is completely submerged in the brine containing a proper amount of salt, and the sauerkraut fermentation process occurs naturally [19]. Kimchi, similar to sauerkraut, includes a wider range of vegetables such as cabbages, peppers, daikon, long beans, garlic, and ginger. To shorten the fermentation process and improve fermentation quality, some vegetables would be fermented in salted brine in a certain fermentation process, with a circulating pump to maintain homogeneity [20]. Sauerkraut is compressed during fermentation and has a lower salt concentration compared to kimchi. Pickles are made by dehydrating various fresh vegetables under the sun for several hours. The semi-dried vegetables are then mixed with salt and placed in jars with water for spontaneous fermentation [2] (Figure 1).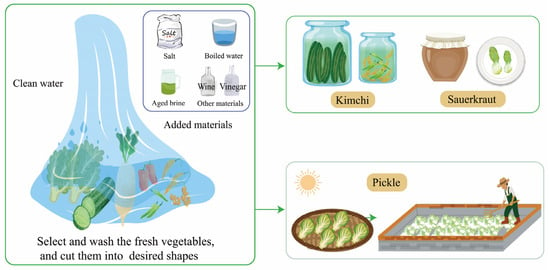
Figure 1.
The production process of traditional fermented vegetables.
2.1. The Fermentation Process of Traditional Fermented Vegetables
The traditional fermentation process involves several steps [21]. Firstly, fresh vegetables are selected and washed. Any rotten and moldy parts are removed to prevent fermentation issues and ensure food safety. Secondly, the vegetables are cut into desired shapes to facilitate the pickling process. Lastly, the vegetables are placed in a container, and salt water or aged brine is added. They are then marinated under specific conditions for a few days. During fermentation, the conditions, such as temperature, salt concentration, and fermentation time, are adjusted based on the specific vegetables being fermented. For example, white cabbages cultivated in South Tyrol are fermented for 42 days at 15 °C with 1.3–1.4% salt according to the traditional procedure [19]. Peppers, on the other hand, undergo spontaneous fermentation in sterile water (boiled water) with 0.5–3.5% salt for about 10 days [22]. Temperature plays a significant role in the growth of LABs and their acid production ability, which affects the pH and fermentation speed [23]. Salt concentration and pre-salting have a crucial impact on the quality of fermented vegetables [24], influencing microbial diversity and volatile compounds. Certain bacterial species, such as Pediococcus, Leuconostoc, Weissella, Sporobolomyces, Azospirillum, Klebsiella, Acinetobacter, and Cladosporium, have a close correlation with salt concentration during vegetable fermentation. However, Lactobacillus is not affected by different salt additions (0%, 3%, 6%, and 9%) and tends to dominate the fermentation process, especially in the later phase of fermentation [24]. In some vegetable fermentation procedures, aged brine is used while others simply use salt water. Aged brine can have a significant impact on microbial growth at the beginning of fermentation and contribute to the production of more aromatic compounds, higher organic acid content, and a lower pH value [25]. However, the fermentation functions of certain species in aged brine are not well understood, which limits our ability to regulate microorganisms and predict the quality of fermented vegetables. Traditional vegetable fermentation primarily relies on the naturally occurring bacteria and fungi presenting on the fresh vegetables or in the food processing environment. Based on metagenomic screening, highly diverse microorganisms have been revealed, which include fungi and bacteria [26]. The fungi have been reported to play a significant role in flavor formation in fermented chili pepper [27], broad bean paste [28], and fermented Da-jiang [29]. Diverse fungi, such as Cladosporium, Candida, Aspergillus, Pichia, Sporobolomyces, Debaromyces, Psathyrella, and Debaryomyces hansenii, have been observed in various fermented vegetables [4][24][30][31][32][4,24,30,31,32]. Both the bacterial and fungal species are strongly associated with increased concentrations of organic acids, amino acids, biogenic amines, and volatiles [31]. However, yeasts and molds can produce metabolites that result in undesirable taste and smell, and they can inhibit lactic acid production [33]. Bacteria, especially Lactobacillus, make up the majority of the microbiota involved in vegetable fermentation and gradually become dominant in the later stage of fermentation [34].2.2. Insight into the Microbiota of Traditional Fermented Vegetables
The physicochemical attributes of fermented vegetables, such as pH, acidity, nitrite, texture, and color, undergo constant changes during the fermentation process. Similarly, the flavor properties, such as sugar, organic acids, esters, terpenes, alcohols, and phenols, increase significantly [19][31][19,31]. As a result, the growth of certain microbial species was inhibited, leading to a decrease in the abundance of the initially dominant genera [35]. The Lactobacillus tends to dominate the fermented microbiota in the later stage of fermentation. In Jiangxi yancai, Sichuan paocai, and Dongbei suancai, the abundance of Levilactobacillus can be as high as 73.7% [2]. The dominant bacteria can vary depending on the type of vegetable, the production process, and the fermentation conditions. For instance, pickled chili pepper, a traditional Chinese fermented vegetable, is fermented with aged brine in an open-air pickling tank for at least 3 months in Jianshui city, China. In this situation, Lactobacillus remains the main genus throughout the entire fermentation process [34]. In contrast, the spontaneous fermentation of pickled pepper in North China involves sterile water (boiled water) with salt in an oxygen-free environment, where the composition of Lactobacillus is 49.6% [22]. The flavor profiles of different fermented vegetables are closely related to specific bacterial species, which exhibit great diversity [36]. The microorganisms that are most adaptable to the fermentation process are responsible for producing the flavors [37]. Certain core bacterial species within the microbiota are believed to be the key contributors to the flavors. For example, Lactobacillus alimentarius may contribute fruity, sweet, and floral odors to pickled chayote, while other Lactobacillus species, Lactobacillus futsaii and Lactobacillus paralimentarius, in particular, generate a sour taste to the products [35]. The abundance of Lactobacillus alimentarius increased significantly during the fermentation of pickled chayote, suggesting it is essential [35]. Additionally, Secundilactobacillus malefermentans has been identified as a keystone species in the fermentation microbiota, especially during the last weeks of fermentation [19]. In pickled chili pepper, Lactobacillus versmoldensis and Lactobacillus brevis were identified as keystone microorganisms highly related to flavor production [34]. These bacterial species could potentially serve as starter cultures to optimize fermentation processes and achieve fermented vegetables with improved and standardized nutritional and sensory characteristics.LABs during Vegetable Fermentation
LABs are Gram-positive and catalase-negative microorganisms. They are acid-resistant, facultatively anaerobic, morphologically globular, or rod-shaped, and they do not form spores [38][39][38,39]. LABs are widely distributed in various sources, such as food, plants, soil, animals, and the human body. The genera of LABs include Lactobacillus, Lactococcus, Pediococcus, Enterococcus, Streptococcus, Leuconostoc, Weissella, and others [14][38][40][41][14,38,40,41]. While there is a considerable diversity of LABs, food is one of the most common sources. Fermented foods, such as fermented dairy products, fermented vegetables, fermented meat, and fermented grains, are particularly rich in LABs. Fermented vegetables, being a common and easily accessible product, are often used as raw materials for isolating LABs. Certain isolated LAB strains derived from fermented vegetables exhibit probiotic effects. For example, Lactiplantibacillus pentosus CF2-10N isolated from fermented Aloreña green table olives can produce exopolysaccharide and vitamins and demonstrate an immunomodulatory effect [42]. W. koreensis SK isolated from kimchi can produce ornithine, which has an anti-obesity effect [43]. Lactiplantibacillus plantarum LRCC5314, isolated from kimchi, has anti-inflammatory effects [44]. L. pentosus LPG1 isolated from edible olives can produce bacteriocin and exopolysaccharides, exhibits an anti-inflammatory effect, reduces cholesterol levels, and inhibits food-borne pathogens [45]. LABs have a range of functions, including immune regulation, intestinal improvement, inhibition of food-borne pathogens, and an anti-inflammatory function [38][46][47][38,46,47]. Therefore, LABs have applications in various fields, including food and medical treatment, with the production of fermented food being one of the most common uses. During the fermentation process, LABs produce various metabolites, primarily lactic acid, bacterin, amino acids, exopolysaccharides, ornithine, aldehydes, and esters. When LABs are used as starter cultures for fermenting vegetables, they contribute to the production of beneficial fermented vegetable products. For example, inoculating cabbage with L. plantarum CGMCC No. 20193 and P. pentosaceus CGMCC No. 20192 increases the content of amino acids and other beneficial substances while reducing the nitrite content, making the product healthier [48]. Fermentation with L. plantarum ZJ316 increases the content of mustard, aldehydes, and esters, as well as the number of probiotics [49]. Lactobacillus paracasei HD1.7 produces bacteriocin Paracin 1.7, which helps inhibit the growth of pathogenic bacteria during cabbage fermentation [50]. Fermented carrot from L. plantarum 299v has been shown to have potential benefits in treating and preventing obesity and type 2 diabetes [51].2.3. The Mechanisms of Traditional Fermented Vegetables
LABs play a leading role in fermented vegetable production. LABs can be classified according to their distribution environment of homofermenters, heterofermenters, and facultative fermenters [52]. Lactic fermentation is the primary process during the fermentation of vegetables and can be divided into homotypic fermentation and heterotypic fermentation [53] (Figure 2).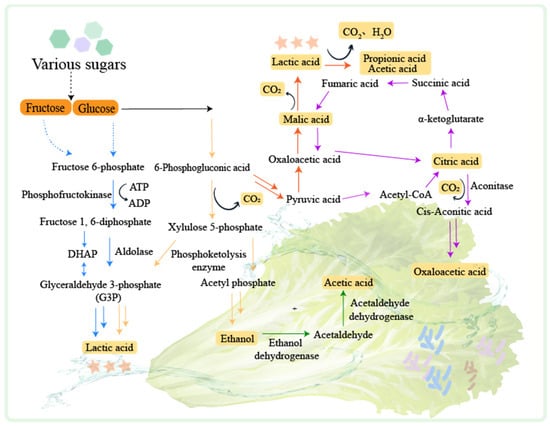
Figure 2. The mechanisms of fermented vegetables. PTS: Phosphoenolpyruvate-dependent sugar phosphotransferase system. The homotypic lactic fermentation pathway is indicated by the blue arrow. The heterotypic lactic fermentation pathway is indicated by the yellow arrow. The malate metabolic pathway is indicated by the red arrow. The citrate metabolic pathway is indicated by the purple arrow. The ethanol metabolic pathway is indicated by the green arrow.
3. The Effects of Probiotics on Fermented Vegetables
In recent times, probiotic starter cultures have become increasingly popular as essential contributors to vegetable fermentation due to their numerous benefits. These benefits include reducing harmful metabolic products, inhibiting pathogenic bacteria, and enhancing therapeutic effects. Probiotics play a significant role in promoting carbohydrate, amino acid, and nucleotide metabolisms, as well as reinforcing the biosynthesis of vitamins and bacteriocin. Moreover, they contribute to the generation of therapeutic products [62]. For example, a formulation that includes Lactobacillus acidophilus GL A-14, Lactobacillus rhamnosus HN001, and bovine lactoferrin can be used as an adjuvant therapy along with topical clotrimazole to treat vulvovaginal candidiasis. Individuals who used the adjuvant therapy experienced better improvement [63]. Additionally, in an in vivo study, Golden Syrian hamsters were orally administered a vaccine containing L. casei, and the result showed that the vaccine exhibited significant advantages when compared to the traditional vaccine [64]. L. plantarum CQPC02 isolated from the Sichuan pickle showed significant anti-fatigue and anti-oxidation effects in fatigue mice models, suggesting that it can be a potential microbiological therapeutic agent [65]. Organic acids produced by probiotics act as natural preservatives, prolonging the shelf life of fermented products and influencing flavor formation. Probiotics possess metabolic capacity for various carbohydrates, including glucose, fructose, xylose, ribose, trehalose, maltose, and sucrose, and organic acids, including citrate, malate, and fumarate. The presence of fructose has been found to increase the expression of certain genes related to these metabolisms [66]. Phenolic derivatives derived from the microbial metabolism of probiotics significantly affect the sensory and health-promoting features of fermented vegetables. Lactobacilli and Pediococcus spp. are prominent producers of lactic acid and exhibit a high metabolic potential for the bioconversion of phenolics [19]. Bacteriocins produced by LABs have potential use as natural food preservatives due to their excellent antibacterial effects [38]. For instance, L. plantarum RUB1 has been found to produce a class IIb bacteriocin with strong antibacterial activity [67]. L. paracasei LS-6, which was isolated from a traditional fermented yogurt in Yunnan, China, can produce a bacteriocin LSX01 that exhibits activity against Staphylococcus aureus [68]. Additionally, nine strains isolated from home-made fermented vegetables from Northwest Bulgaria were found to be bacteriocin producers. These strains could potentially be utilized as starter cultures, reducing the need for chemical preservative additives in fermented vegetables [69]. Probiotics also have the ability to produce γ-aminobutyric acid (GABA), a major inhibitory neurotransmitter in the central nervous system. In addition, GABA exhibits anti-anxiety and tranquilizing effects [70][71][70,71]. Certain strains, such as Latilactobacillus curvatus K285 isolated from gat-kimchi [72], L. pentosus 9D3 isolated from Thai pickled weed [73], L. plantarum KB1253 [74], and Companilactobacillus allii WiKim39 and Lactococcus lactis WiKim0124 isolated from kimchi [75] have demonstrated a strong ability to produce GABA, making them potential starter cultures for producing functional foods. Mannitol, a 6-carbon sugar alcohol naturally present in microorganisms and plants, is slowly absorbed in intestinal tracts and does not increase blood sugar levels [70][76][70,76]. Strains such as Leuconostoc mesenteroides SKP88 and Leuconostoc citreum SKP92 isolated from pa (green onion)-kimchi can convert fructose to mannitol [76]. Ornithine, a non-proteinogenic amino acid converted from arginine, offers various functions such as anti-obesity properties, muscle growth promotion, anti-fatigue effects, and cirrhosis treatment [70]. Weissella koreensis DB1 can produce 15,059.65 mg/L ornithine. Safety evaluations have shown that it poses no health risk and can be used in fermented foods [77]. Fermented vegetables are rich in vitamins, which are essential for the proper functioning of the human body. The fermentation process helps retain the vitamin content from the raw materials [78]. In particular, co-fermentation of Pediococcus pentosaceus AL and Cyberlindnera rhodanensis J52 significantly increases the vitamin C concentration in fermented capsicum [79]. Additionally, certain LABs have the ability to produce vitamins. For example, Lactobacillus reuteri F2 has shown a strong ability for extracellular vitamin B12 production [80]. Therefore, fermenting vegetables with probiotic strains can serve as an effective method to enhance their beneficial properties. Probiotics, such as LABs, have the ability to inhibit the growth of pathogenic bacteria and reduce the negative effects of harmful substances due to high lactic acid generation capacity, thereby improving the quality and safety of fermented products [49][81][49,81]. LABs, such as L. brevis and L. plantarum ZJ316, can inhibit the growth of Ralstonia spp., Pseudomonas, Proteus, and Enterobacter in pickled chili pepper and pickled mustard [34][49][34,49]. Functional foods rich in probiotic LABs have the potential to combat accidentally ingested pesticides in the gastrointestinal tract directly [8]. The high lactic acid generation capacity of LABs, such as L. plantarum ZJ316, contributes to the reduction in nitrite residual levels [49]. Deltamethrin, dimethoate, and imidacloprid are common pesticides used during olive growth, which are harmful to human health. Natural black olive fermented with L. plantarum strains 112 and 123 showed higher degradation of these substances compared to crude olives [82] (Table 1). The consumption of probiotics in fermented vegetables can have a significant impact on the composition of gut microbiota. When mice were fed with green loofah fermented with L. plantarum Uruma-SU4, the level of Lactobacillus johnsonii, which is the predominant LAB in mice gut microbiota, was increased [73]. The Firmicutes/Bacteroidetes ratio (F/B ratio) can reflect the health of the gut microbiota, with a higher F/B ratio suggesting low gut microbiota diversity [74]. L. pentosus P2020 derived from the Chinese pickle can significantly lower the F/B ratio and restore the gut microbiota, thereby protecting against the development of hyperuricemia [75]. Similarly, L. plantarum TWK10 isolated from Taiwan pickled cabbage has been found to reduce the F/B ratio in aging mice and modulate the imbalance of gut microbiota, thereby attenuating aging-related disorders [76]. These findings suggest that vegetables fermented with probiotics have the potential to become valuable contributors to therapeutic interventions aimed at restoring gut microbiota.Table 1.
The metabolic products and health benefits of microbial strains in fermented vegetables.
| Microbial Strains | Isolation Source | Metabolic Products | Health Benefits | Study Type | Ref. |
|---|---|---|---|---|---|
| Leu. mesenteroides/L. plantarum | Kimchi | Benzyl isothiocyanate, indole compounds, thiocyanate, b-sitosterol, and dietary fiber |
Anticancer, anti-atherosclerotic, and cholesterol-lowering |
In vivo | [83] |
| L. plantarum JS19 | Shaanxi Jiang-shui | Ameliorate inflammatory bowel disease | In vivo | [84] | |
| W. paramesenteroides | Yan-dong-gua | Organic acids, hydrogen peroxide, and bacteriocins | Antibacterial | In vitro | [85] |
| B. subtilis natto | Natto | Nattokinase, dipicolinic acid, Menaquinone-7 | Anti-thrombotic, promote bone health | Clinical trial | [86] |
| L. plantarum/Lev. Brevis/Leu. fallax | Fermented cucumber | GABA | Antihypertensive, antidepressant, and anticancer | In vitro | [87] |
| L. plantarum/L. pentosus | Table olives | Bioactive compounds, dietary fibers, fatty acids, antioxidants | Antioxidant activity | In vivo | [88] |
| L. pentosus LPG1 | Table olive | Bacteriocin, exopolysaccharides | Anti-inflammatory, reduce cholesterol levels, and inhibit food-borne pathogens | Clinical trial | [45][89][45,89] |
| L. fermentum SHY10 | Chinese pickles | Antibacterial peptides | Antibacterial | In vitro | [90][91][90,91] |
| L. pentosus CF2-10N | Fermented Aloreña green table olives | Vitamin, exopolysaccharide | Immunomodulation | In vitro | [42] |
| L. plantarum Uruma-SU4 | Fermented loofah | Bile acid-lowering | In vivo | [92] | |
| L. plantarum LRCC5314 | Kimchi | Anti-inflammatory, inhibition of adipogenesis | In vitro | [44] | |
| Lactococcus lactis/Weissella cibaria | Fermented beetroot | Niacin, riboflavin | Antagonistic properties against pathogenic bacteria | In vitro | [93] |
| L. plantarum NPL 1258 P. pentosaceus NPL 1264 |
Fermented cucumber | Extracellular polymeric substances (EPS) | Shorten the fermentation cycle and reduce pathogenic organism populations | In vitro | [94] |
| L. pentosus P2020 | Chinese pickle | Lower serum uric acid | In vivo | [95] | |
| L. plantarum TWK10 | Pickled cabbage | Attenuate aging-related disorders | In vivo | [96] |
3.1. Application of Probiotic Starter Cultures in Vegetable Fermentation
Spontaneous fermentation often leads to poor-quality products, as they are susceptible to contamination by spoilage microorganisms and pathogenic bacteria, which poses a challenge for industrial production [97]. Additionally, there is a growing consumer demand for fresh-tasting, nutritionally rich, and health-promoting foods with pleasing sensory properties. To meet these demands, specific microbial species with desired properties can be isolated from fermented vegetables to be used as starter cultures in the production of functional food [93]. The use of probiotic starter cultures in fermentation offers effective approaches to standardize product quality, ensure safety, and optimize the benefits of the final products [98]. Probiotic starter cultures, mainly consisting of LABs, can be used in vegetable fermentation either as single-strain or mixed-strain cultures, depending on the specific fermented vegetable products. The selection of starter cultures significantly influences the physicochemical properties and aromatic qualities of the fermented vegetables [98]. Fermentation with a single bacterial species can accelerate the acidification process, resulting in faster conversion of fermented vegetables, reduced commercial losses, and lower production costs [20][81][20,81]. Fermentation with mixed probiotic starter cultures can enhance the fermentation abilities and shorten the maturation period. The mixed fermentation microbiota consists of dominant microorganisms, and the flavors of fermented vegetables are primarily derived from these dominant species. Kimchi inoculated with different starters exhibit high ratios of Leuconostoc, L. plantarum, and L. brevis [99]. A high inoculum of L. plantarum and P. pentosaceus strains (NPL 1258 and NPL 1259) has been found to effectively control the quality of fermented cucumbers [94]. Furthermore, the different mixing ratios of starter cultures lead to distinct metabolites in fermented vegetables. A high-level L. mesenteroides inoculation exhibits hetero-fermentative characteristics, resulting in higher mannitol and acetic acid levels but lower lactic acid levels compared to high-level homo-fermentative L. sakei inoculation [100]. By increasing the population of LABs and decreasing the undesirable microorganisms, probiotic starter cultures contribute to a shorter fermentation cycle and a reduction in the population of pathogenic organisms, thereby improving the safety and quality of fermented vegetables. Heterofermentative LABs can accelerate the growth of homofermentative LABs, leading to a rapid decrease in pH value [92]. Additionally, L. plantarum exhibits strong acid tolerance and performs well in the presence of Leu. mesenteroides. Therefore, the starter culture composed of L. plantarum and Leu. mesenteroides plays a significant role in producing the distinctive flavor of northeast sauerkraut [97].3.2. Design of Robust, Stable, and Predictable Probiotic Microbiota
Designing a robust, stable, and predictable probiotic microbiota is crucial for ensuring the quality of fermented vegetable products. The presence of an adequate number of probiotics is essential for their health benefits, with a minimum of 106 CFU/mL being necessary [101]. To achieve this, supplementing the fermentation process with dietary fiber, such as cellulose or inulin, before fermentation, not only generates prebiotic carbohydrates but also creates a favorable growth environment for probiotic strains, thereby improving their viability in fermented vegetable foods [94][102][103][94,102,103] (Figure 3). Furthermore, establishing a microbial collection that encompasses various microbial strains and their corresponding genomic information can offer a valuable resource for developing effective probiotic strains and providing strains for designing probiotic microbiota [104][105][104,105].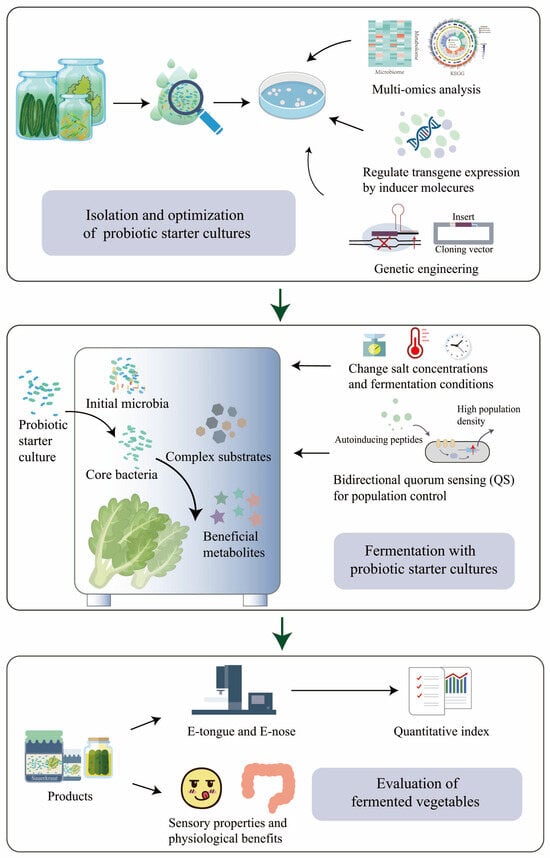
Figure 3.
Design of robust, stable, and predictable vegetable fermentation with probiotic starter cultures.
