You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diego Franco | -- | 1233 | 2023-10-31 11:47:54 | | | |
| 2 | Jessie Wu | Meta information modification | 1233 | 2023-11-01 02:36:04 | | | | |
| 3 | Jessie Wu | -1 word(s) | 1232 | 2023-11-24 07:31:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carmona, R.; López-Sánchez, C.; Garcia-Martinez, V.; Garcia-López, V.; Muñoz-Chápuli, R.; Lozano-Velasco, E.; Franco, D. Origin and Derivates of the Embryonic Epicardium. Encyclopedia. Available online: https://encyclopedia.pub/entry/50984 (accessed on 21 December 2025).
Carmona R, López-Sánchez C, Garcia-Martinez V, Garcia-López V, Muñoz-Chápuli R, Lozano-Velasco E, et al. Origin and Derivates of the Embryonic Epicardium. Encyclopedia. Available at: https://encyclopedia.pub/entry/50984. Accessed December 21, 2025.
Carmona, Rita, Carmen López-Sánchez, Virginio Garcia-Martinez, Virginio Garcia-López, Ramón Muñoz-Chápuli, Estefanía Lozano-Velasco, Diego Franco. "Origin and Derivates of the Embryonic Epicardium" Encyclopedia, https://encyclopedia.pub/entry/50984 (accessed December 21, 2025).
Carmona, R., López-Sánchez, C., Garcia-Martinez, V., Garcia-López, V., Muñoz-Chápuli, R., Lozano-Velasco, E., & Franco, D. (2023, October 31). Origin and Derivates of the Embryonic Epicardium. In Encyclopedia. https://encyclopedia.pub/entry/50984
Carmona, Rita, et al. "Origin and Derivates of the Embryonic Epicardium." Encyclopedia. Web. 31 October, 2023.
Copy Citation
The embryonic epicardium originates from the proepicardium, an extracardiac primordium constituted by a cluster of mesothelial cells. In early embryos, the embryonic epicardium is characterized by a squamous cell epithelium resting on the myocardium surface. Subsequently, it invades the subepicardial space and thereafter the embryonic myocardium by means of an epithelial–mesenchymal transition. Within the myocardium, epicardial-derived cells present multilineage potential, later differentiating into smooth muscle cells and contributing both to coronary vasculature and cardiac fibroblasts in the mature heart.
epicardium
cardiac development
transcriptional regulation
post-transcriptional regulation
1. Origin of the Embryonic Epicardium
During cardiac development, the epicardium originates from an extracardiac primordium, the proepicardium (PE), which is constituted by a cluster of mesothelial cells located on the cephalic and ventral surfaces of the liver-sinus venosus limit in avian embryos [1][2][3][4][5][6][7][8] and the pericardial side of the septum transversum in mammalian embryos [9]. In early embryos, the epicardium acquires the form of a squamous cell epithelium that either rests directly on the surface of the myocardium or covers a subepicardial space that appears to be densely populated by mesenchymal cells [10]. It is also assumed that the epicardium is not a simple mesothelium but that it is made up of discrete clusters of heterogeneous cell types which include a hematopoietic contribution from distinct origins, encased by an extracellular matrix (ECM) that anatomically resembles a stem or progenitor cell ‘niche’ [11].
It was reported that PE originates in the periphery of the heart forming fields in the lateral plate mesoderm (LPM) as part of an early cardiac progenitor lineage [12]. A single PE bud is formed during zebrafish cardiogenesis [13] while in other fish, such as the sturgeons, bilateral primordia are formed which subsequently converge into a single PE structure in the embryonic midline [14]. Noticeably, in mice, bilateral PE anlage is also established and further develops similarly to sturgeons while in chick embryos only the right-side anlage develops [15]. Interestingly, chicken PE arises both from the splanchnic layer of the LPM and the somatic mesoderm, which also contributes to the mesothelial portion of the PE that forms the typical villous protrusions [16][17][18]. The above observations suggest that the embryonic left–right signal might play a significant role during PE development. Furthermore, while all proepicardial cells in a given species (e.g., in mice, zebrafish, and chicks) are morphologically similar, they exhibit a distinct differentiation potential due to various marker expressions [19][20][21]. Therefore, the detailed composition of the embryonic epicardium is not well known.
After PE formation, cells translocate to the myocardial surface of the looping heart, where they adhere, migrate, and proliferate to form a squamous epithelial layer: the embryonic epicardium [22]. It has been described that PE translocation to the myocardium takes place through distinct mechanisms among species, including direct contact and/or the release of free-floating cell clusters (or cysts) into the pericardial cavity or PE cell migration from the sinus venosus towards the heart along the surface of the inflow tract [23][24][25][26]. After attachment to the myocardial surface, the cells start to migrate laterally until the complete heart is enveloped by the epicardium [5]. It has been described that the epicardium surrounding the arterial pole does not originate from the PE but from the coelomic/pericardial mesothelium at the area where the aortic sac leaves the pericardial cavity [27][28]. This cell population will contribute to the outer mesenchymal layer of the arterial pole within the pericardial cavity, contributing to the arterial epicardium formation, whereas the PE-derived epicardium will cover the myocardial outflow tract (Figure 1).
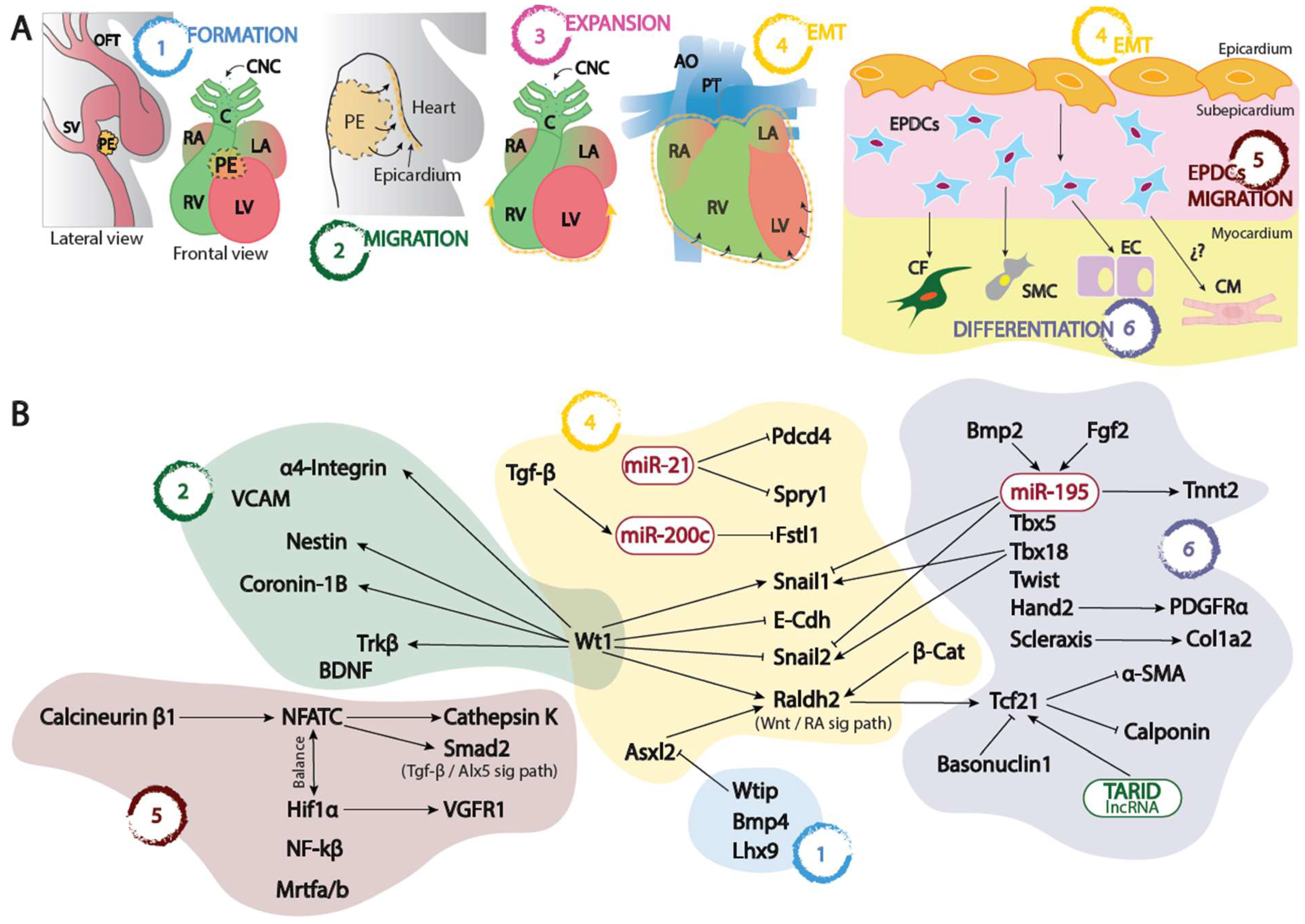
Figure 1. (A) Schematic representation of the proepicardium (PE) and embryonic epicardium formation since its origin at the sinus venosus–septum transversum (1), its migration (2) and expansion (3) into the naked myocardium, its epithelial to mesenchymal transition leading to the formation of the EPCDs (4), and finally the migration and invasion (5) of the embryonic myocardium differentiating into distinct cell types (6). (B) Schematic representation of the distinct transcriptional and post-transcriptional regulatory mechanisms involved in each of the distinct processes depicted in (A). OFT, outflow tract; SV, sinus venosus; PE, proepicardium; RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; CNC, cardiac neural crest, C, conus; AO, aorta; PT, pulmonary trunk; EPDCs, epicardial derived cells; CF, cardiac fibroblasts; SMC, smooth muscle cells; EC, endothelial cells; CM, cardiomyocytes; EMT, epithelial to mesenchymal transition.
2. Derivates of the Embryonic Epicardium
Once the epicardium is established, epicardial cells will be directly involved in the formation of the myocardium. A group of epithelial cells will undergo an epithelial–mesenchymal transition (EMT), giving rise to epicardium-derived cells (EPDCs), and then migrate into the matrix in the subepicardial layer to form the subepicardium [22]. The subepicardium thickness will eventually vary according to the underlying heart structure to be covered and may vary among species. In particular, in chick embryos, the subepicardium is relatively thin in the atrial and ventricular myocardium. However, in the atrioventricular sulcus, the subepicardium is thicker in order to provide those EPDCs needed for coronary formation [29][30].
From the subepicardium, mesenchymal EPDCs will form migratory processes and invade the myocardium in a spatio-temporarily regulated fashion where several factors expressed in the underlying myocardium will define the permissiveness for EPDCs. These migrate into the underlying myocardium in a tangential pattern and most of them are retained directly underneath the local area of the subepicardium [5][30][31].
Within the myocardium, EPDCs present multi-lineage potential, differentiating into smooth muscle cells (SMCs) and contributing to the coronary vasculature and cardiac fibroblasts (CF) of the mature heart [22][26] in line with reports linking EMT and the acquisition of stem cell properties [32]. At present, it is still controversial as to whether these distinct cell lineages are already established in the proepicardium or if EPDCs acquire pluripotentiality during or after EMT [33].
Most EPDCs reach their final positions (i) around the coronary arteries as smooth muscle cells (SMCs) and adventitial fibroblasts [27][29][34][35][36]; (ii) in the atrioventricular cushions [27][31][35]; (iii) in the subendocardium of the ventricular trabeculae and atria [27][30][31]; and (iv) in the ventricular myocardium as interstitial fibroblasts [31]. Other contributions of EPDCs to cardiac endothelial cells (ECs) [37][38][39][40] and cardiomyocytes (CMs) [41][42] have also been described although this issue needs further research [43][44]. Therefore, both the sinus venosus and ventricular endocardium are considered major contributors to ECs [45] while EPDCs have a low contribution, if any [46]. With respect to epicardial-derived cardiomyocytes, lineage tracing studies, by using Scleraxis, WT1, and TBX18, have indicated possible epicardial-derived cardiomyocyte labeling, although their contribution is still controversial [41][43][44].
Additionally, after the embryonic epicardium has covered the developing heart, the epicardial cells and the EPDCs will produce cytokines and growth factors in order to induce the myocardial development [25]. These factors and the regulation of their expression are still rather unknown but a number of Fgfs, particularly Fgf-9 and Igf-2, have been proposed to be mitogenic factors for the developing cardiomyocytes [47][48][49]. In this sense, impaired embryonic epicardium development and/or cytokines and growth factor delivery results in deficient ventricular chamber maturation [47][50][51][52][53][54].
In contrast to the embryonic epicardium, the postnatal mammalian epicardium seems to be a dormant single-cell layer since most genes involved in epicardial activation, such as WT1, Tbx18, and Raldh2 are rapidly downregulated postnatally, being scarcely detectable only during the first three months in mice [55]. This is an interesting issue since regeneration of the injured myocardium in non-mammalian species is dependent on epicardial activation and re-expression of genes characteristic of the embryonic epicardium [56]. Even the murine heart, which has a very limited regenerative potential, shows a re-expression of the embryonic epicardial gene program and generation of Wt1-lineage positive subepicardial mesenchyme after myocardial infarction [57].
References
- Männer, J. The development of pericardial villi in the chick embryo. Anat. Embryol. 1992, 186, 379–385.
- Männer, J. Experimental study on the formation of the epicardium in chick embryos. Anat. Embryol. 1993, 187, 281–289.
- Männer, J.; Perez-Pomares, J.M.; Macias, D.; Munoz-Chapuli, R. The origin, formation and developmental significance of the epicardium: A review. Cells Tissues Organs 2001, 169, 89–103.
- Männer, J.; Schlueter, J.; Brand, T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev. Dyn. 2005, 233, 1454–1463.
- Lie-Venema, H.; van den Akker, N.M.; Bax, N.A.; Winter, E.M.; Maas, S.; Kekarainen, T.; Hoeben, R.C.; deRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. Sci. World J. 2007, 7, 1777–1798.
- Bax, N.A.; Lie-Venema, H.; Vicente-Steijn, R.; Bleyl, S.B.; Van Den Akker, N.M.; Maas, S.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Platelet-derived growth factor is involved in the differentiation of second heart field-derived cardiac structures in chicken embryos. Dev. Dyn. 2009, 238, 2658–2669.
- Carmona, R.; Guadix, J.A.; Cano, E.; Ruiz-Villalba, A.; Portillo-Sánchez, V.; Pérez-Pomares, J.M.; Muñoz-Chápuli, R. The embryonic epicardium: An essential element of cardiac development. J. Cell Mol. Med. 2010, 14, 2066–2072.
- Niderla-Bielińska, J.; Jankowska-Steifer, E.; Flaht-Zabost, A.; Gula, G.; Czarnowska, E.; Ratajska, A. Proepicardium: Current Understanding of its Structure, Induction, and Fate. Anat. Rec. 2019, 302, 893–903.
- Komiyama, M.; Ito, K.; Shimada, Y. Origin and development of the epicardium in the mouse embryo. Anat. Embryol. 1987, 176, 183–189.
- Muñoz-Chápuli, R.; Macías, D.; González-Iriarte, M.; Carmona, R.; Atencia, G.; Pérez-Pomares, J.M. The epicardium and epicardial-derived cells: Multiple functions in cardiac development. Rev. Esp. Cardiol. 2002, 55, 1070–1082. (In Spanish)
- Balmer, G.M.; Bollini, S.; Dubé, K.N.; Martinez-Barbera, J.P.; Williams, O.; Riley, P.R. Dynamic haematopoietic cell contribution to the developing and adult epicardium. Nat. Commun. 2014, 5, 4054.
- Mommersteeg, M.T.; Domínguez, J.N.; Wiese, C.; Norden, J.; de Gier-de Vries, C.; Burch, J.B.; Kispert, A.; Brown, N.A.; Moorman, A.F.; Christoffels, V.M. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc. Res. 2010, 87, 92–101.
- Serluca, F.C. Development of the proepicardial organ in the zebrafish. Dev. Biol. 2008, 315, 18–27.
- Icardo, J.M.; Guerrero, A.; Durán, A.C.; Colvee, E.; Domezain, A.; Sans-Coma, V. The development of the epicardiumin the sturgeon Acipenser naccarii. Anat. Rec. 2009, 292, 1593–1601.
- Schulte, I.; Schlueter, J.; Abu-Issa, R.; Brand, T.; Männer, J. Morphological and molecular left-right asymmetries in the development of the proepicardium: A comparative analysis on mouse and chick embryos. Dev. Dyn. 2007, 236, 684–695.
- van Wijk, B.; van den Berg, G.; Abu-Issa, R.; Barnett, P.; van der Velden, S.; Schmidt, M.; Ruijter, J.M.; Kirby, M.L.; Moorman, A.F.; van den Hoff, M.J. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ. Res. 2009, 105, 431–441.
- Maya-Ramos, L.; Cleland, J.; Bressan, M.; Mikawa, T. Induction of the Proepicardium. J. Dev. Biol. 2013, 1, 82–91.
- Schlueter, J.; Brand, T. Subpopulation of proepicardial cells is derived from the somatic mesoderm in the chick embryo. Circ. Res. 2013, 113, 1128–1137.
- Torlopp, A.; Schlueter, J.; Brand, T. Role of fibroblast growth factor signaling during proepicardium formation in the chick embryo. Dev. Dyn. 2010, 239, 2393–2403.
- Liu, J.; Stainier, D.Y. Tbx5 and Bmp signaling are essential for proepicardium specification in zebrafish. Circ. Res. 2010, 106, 1818–1828.
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650.
- Smits, A.M.; Dronkers, E.; Goumans, M.J. The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol. Res. 2018, 127, 129–140.
- Plavicki, J.S.; Hofsteen, P.; Yue, M.S.; Lanham, K.A.; Peterson, R.E.; Heideman, W. Multiple modes of proepicardial cell migration require heartbeat. BMC Dev. Biol. 2014, 14, 18.
- Li, J.; Miao, L.; Zhao, C.; Shaikh Qureshi, W.M.; Shieh, D.; Guo, H.; Lu, Y.; Hu, S.; Huang, A.; Zhang, L.; et al. CDC42 is required for epicardial and pro-epicardial development by mediating FGF receptor trafficking to the plasma membrane. Development 2017, 144, 1635–1647.
- Cao, Y.; Duca, S.; Cao, J. Epicardium in Heart Development. Cold Spring Harb. Perspect. Biol. 2020, 12, a037192.
- Sanchez-Fernandez, C.; Rodriguez-Outeiriño, L.; Matias-Valiente, L.; Ramirez de Acuña, F.; Hernandez-Torres, F.; Lozano-Velasco, E.; Dominguez, J.N.; Franco, D.; Aranega, A.E. Regulation of Epicardial Cell Fate during Cardiac Development and Disease: An Overview. Int. J. Mol. Sci. 2022, 23, 3220.
- Männer, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate op the epicardial primordium. Anat. Rec. 1999, 255, 212–226.
- Perez-Pomares, J.M.; Phelps, A.; Sedmerova, M.; Wessels, A. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev. Dyn. 2003, 227, 56–68.
- Vrancken Peeters, M.-P.F.M.; Gittenberger-de Groot, A.C.; Mentink, M.M.T.; Poelmann, R.E. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat. Embryol. 1999, 199, 367–378.
- Lie-Venema, H.; Eralp, I.; Maas, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; DeRuiter, M.C. Myocardial heterogeneity in permissiveness for epicardium-derived cells and endothelial precursor cells along the developing heart tube at the onset of coronary vascularization. Anat. Rec. 2005, 282A, 120–129.
- Gittenberger-de Groot, A.C.; Vrancken Peeters, M.-P.F.M.; Mentink, M.M.T.; Gourdie, R.G.; Poelmann, R.E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 1998, 82, 1043–1052.
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715.
- Sanchez-Fernandez, C.; Rodriguez-Outeiriño, L.; Matias-Valiente, L.; de Acuña, F.R.; Franco, D.; Aránega, A.E. Understanding Epicardial Cell Heterogeneity during Cardiogenesis and Heart Regeneration. J. Cardiovasc. Dev. Dis. 2023, 10, 376.
- Poelmann, R.E.; Gittenberger-de Groot, A.C.; Mentink, M.M.T.; Bökenkamp, R.; Hogers, B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 1993, 73, 559–568.
- Poelmann, R.E.; Lie-Venema, H.; Gittenberger-de Groot, A.C. The role of the epicardium and neural crest as extracardiac contributors to coronary vascular development. Tex. Heart Inst. J. 2002, 29, 255–261.
- Mikawa, T.; Gourdie, R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996, 174, 221–232.
- Wessels, A.; van den Hoff, M.J.; Adamo, R.F.; Phelps, A.L.; Lockhart, M.M.; Sauls, K.; Briggs, L.E.; Norris, R.A.; van Wijk, B.; Perez-Pomares, J.M.; et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012, 366, 111–124.
- Pérez-Pomares, J.M.; Carmona, R.; González-Iriarte, M.; Atencia, G.; Wessels, A.; Muñoz-Chápuli, R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 2002, 46, 1005–1013.
- Carmona, R.; Barrena, S.; López Gambero, A.J.; Rojas, A.; Muñoz-Chápuli, R. Epicardial cell lineages and the origin of the coronary endothelium. FASEB J. 2020, 34, 5223–5239.
- Cano, E.; Carmona, R.; Ruiz-Villalba, A.; Rojas, A.; Chau, Y.Y.; Wagner, K.D.; Wagner, N.; Hastie, N.D.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio- venous connections. Proc. Natl. Acad. Sci. USA 2016, 113, 656–661.
- Cai, C.L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008, 454, 104–108.
- Villa Del Campo, C.; Lioux, G.; Carmona, R.; Sierra, R.; Muñoz-Chápuli, R.; Clavería, C.; Torres, M. Myc overexpression enhances of epicardial contribution to the developing heart and promotes extensive expansion of the cardiomyocyte population. Sci. Rep. 2016, 6, 35366, Erratum in Sci. Rep. 2016, 6, 37880.
- Christoffels, V.M.; Grieskamp, T.; Norden, J.; Mommersteeg, M.T.; Rudat, C.; Kispert, A. Tbx18 and the fate of epicardial progenitors. Nature 2009, 458, E8–E9; discussion E9–E10.
- Rudat, C.; Kispert, A. Wt1 and epicardial fate mapping. Circ. Res. 2012, 111, 165–169.
- Red-Horse, K.; Ueno, H.; Weissman, I.L.; Krasnow, M.A. Coronary arteries form by Developmental reprogramming of venous cells. Nature 2010, 464, 549–553.
- Chen, H.I.; Sharma, B.; Akerberg, B.N.; Numi, H.J.; Kivelä, R.; Saharinen, P.; Aghajanian, H.; McKay, A.S.; Bogard, P.E.; Chang, A.H.; et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 2014, 141, 4500–4512.
- Lavine, K.J.; Ornitz, D.M. Fibroblast growth factors and Hedgehogs: At the heart of the epicardial signaling center. Trends Genet. 2008, 24, 33–40.
- Li, P.; Cavallero, S.; Gu, Y.; Chen, T.H.; Hughes, J.; Hassan, A.B.; Brüning, J.C.; Pashmforoush, M.; Sucov, H.M. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development 2011, 138, 1795–1805.
- Shen, H.; Cavallero, S.; Estrada, K.D.; Sandovici, I.; Kumar, S.R.; Makita, T.; Lien, C.L.; Constancia, M.; Sucov, H.M. Extracardiac control of embryonic cardiomyocyte proliferation and ventricular wall expansion. Cardiovasc. Res. 2015, 105, 271–278.
- Pennisi, D.J.; Ballard, V.L.; Mikawa, T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev. Dyn. 2003, 228, 161–172.
- Stuckmann, I.; Evans, S.; Lassar, A.B. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev. Biol. 2003, 255, 334–349.
- Merki, E.; Zamora, M.; Raya, A.; Kawakami, Y.; Wang, J.; Zhang, X.; Burch, J.; Kubalak, S.W.; Kaliman, P.; Izpisua Belmonte, J.C.; et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. USA 2005, 102, 18455–18460.
- Pennisi, D.J.; Mikawa, T. FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Dev. Biol. 2009, 328, 148–159.
- Cavallero, S.; Shen, H.; Yi, C.; Lien, C.L.; Kumar, S.R.; Sucov, H.M. CXCL12 Signaling Is Essential for Maturation of the Ventricular Coronary Endothelial Plexus and Establishment of Functional Coronary Circulation. Dev. Cell. 2015, 33, 469–477.
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644.
- Cao, J.; Poss, K.D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647.
- van Wijk, B.; Gunst, Q.D.; Moorman, A.F.M.; van den Hoff, M.J.B. Cardiac Regeneration from Activated Epicardium. PLoS ONE 2012, 7, e44692.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
536
Revisions:
3 times
(View History)
Update Date:
24 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




