Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Felisbina Luisa Queiroga | -- | 3989 | 2023-10-31 10:38:58 | | | |
| 2 | Lindsay Dong | Meta information modification | 3989 | 2023-11-01 02:08:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vazquez, E.; Lipovka, Y.; Cervantes-Arias, A.; Garibay-Escobar, A.; Haby, M.M.; Queiroga, F.L.; Velazquez, C. Canine Mammary Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/50972 (accessed on 07 February 2026).
Vazquez E, Lipovka Y, Cervantes-Arias A, Garibay-Escobar A, Haby MM, Queiroga FL, et al. Canine Mammary Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/50972. Accessed February 07, 2026.
Vazquez, Eliza, Yulia Lipovka, Alejandro Cervantes-Arias, Adriana Garibay-Escobar, Michelle M. Haby, Felisbina Luisa Queiroga, Carlos Velazquez. "Canine Mammary Cancer" Encyclopedia, https://encyclopedia.pub/entry/50972 (accessed February 07, 2026).
Vazquez, E., Lipovka, Y., Cervantes-Arias, A., Garibay-Escobar, A., Haby, M.M., Queiroga, F.L., & Velazquez, C. (2023, October 31). Canine Mammary Cancer. In Encyclopedia. https://encyclopedia.pub/entry/50972
Vazquez, Eliza, et al. "Canine Mammary Cancer." Encyclopedia. Web. 31 October, 2023.
Copy Citation
Mammary tumors are the most frequent neoplasia in female dogs. They develop spontaneous cancer and share several biological, clinical, pathological and molecular characteristics with cancer diagnosed in humans. Mammary cancer is also one of the leading causes of death in both species.
mammary cancer
canine
dog
breast cancer
animal model
comparative oncology
1. Introduction
Cancer is a heterogeneous group of diseases characterized by an uncontrolled proliferation of abnormal cells that can spread to the surrounding tissues. It is one of the most common causes of death in humans and dogs. In humans, around 10 million cancer-related deaths are reported and 19.3 million new cases are diagnosed annually, while in dogs, 4 million new cancer cases are diagnosed every year [1][2][3]. Cancer is the first cause of death in dogs over 10 years of age, with 50% of them developing this disease and one in four dying because of cancer [4]. Canines develop spontaneous cancer and share several biological, clinical, pathological and molecular features with humans [5][6][7]. Mammary tumors, affecting numerous mammal species, are the most common neoplasia diagnosed in female dogs and women, and they are considered to be a major problem in public health [5]. Gaining insight into the presentation and progression of breast cancer across different species will help us to better understand the pathogenesis of this complex disease [8].
2. Canine Mammary Tumors
Canine mammary tumors are an overly frequent condition in comparison to other types of cancer; they represent 50–70% of all neoplasia diagnosed in non-spayed female dogs, mainly affecting canines over 7 years of age. They appear as nodules of different sizes and are usually well-defined. The treatment regimen and prognosis of the patient can be established according to the physical characteristics, location, histological and molecular classification of the tumor. The incidence of canine mammary tumors varies depending on the geographic location of the study, and it is also affected by the age, hormonal exposition, breed and molecular features of the female dog, among other factors.
2.1. Epidemiological Features
2.1.1. Incidence and Distribution
Information on the incidence of canine mammary tumors worldwide is very limited and only available for a few countries in Europe and North America. In Table 1, the incidence rate expressed per 100,000 and 10,000 dogs per year is shown, and in the text below there is additional epidemiological information. As seen in Table 1, the incidence of canine mammary tumors varies in every country and over time. This variation can be attributed to several factors, with spaying culture being one of them. Spaying is usually performed as a canine population control measure. However, castration at early ages also prevents mammary tumor development in the female dog [9] since estrogens and progesterone produced by the ovaries are mitogens for the mammary epithelium and can stimulate duct and lobe proliferation and growth [10].
Table 1. Canine mammary cancer epidemiology.
| Country (City/State) | Incidence | Year [Reference] |
|---|---|---|
| Italy | 193 per 100,000 | 2001–2008 [11] |
| Italy (Venice) | 250 per 100,000 | 2005–2013 [12] |
| Sweden | 111 per 10,000 | 1995–2002 [13] |
| United Kingdom | 205 per 100,000 | 1997–1998 [14] |
| Italy (Genoa) | 181.8 per 100,000 | 2000–2002 [15] |
| Italy (Genoa) | 196.6 per 100,00 | 1995–1999 [15] |
| Italy (Genoa) | 264 per 100,000 | 1990–1994 [15] |
| Italy (Genoa) | 119.2 per 100,000 | 1985–1989 [15] |
| USA (California) | 145 per 100,000 | 1963–1968 [16] |
2.1.2. Etiology and Risk Factors
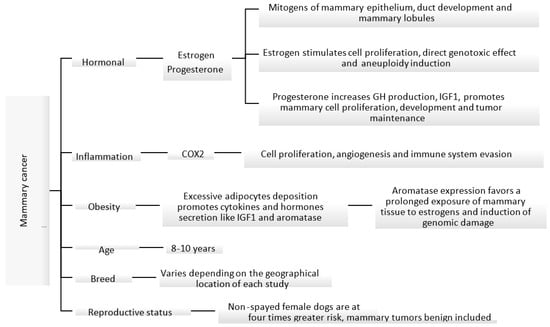
The etiology of canine mammary carcinoma is not fully understood; however, similarly to women’s breast cancer, its development is impacted by hormonal, genetic, nutritional and environmental factors [17][18][19][20]. Some of these factors are shown in Figure 1. Most malignant mammary tumors develop in middle-aged (5–7 years of age) and elderly (7–8 years of age) female dogs, with the median age of presentation ranging between 8 and 10 years [5][21][22]. In addition, the risk of developing mammary tumors increases with the delay in spaying [5][18][23]. Non-spayed female dogs are at four times greater risk of developing mammary tumors, compared to those spayed before two years of age [23].

Figure 1. Known mechanisms and factors involved in the induction of canine mammary cancer [18][23][24][25][26][27].
In female dogs and women, ovarian steroids stimulate the normal growth of mammary tissue under physiologic conditions. However, the proliferative effect in the epithelium can create the perfect environment for neoplastic proliferation. Ovarian hormones, mainly estrogens and progesterone, play an important role in the development of mammary tumors [18][28]. During the luteal phase, mammary tissue is exposed to high levels of progesterone, which could lead to growth hormone (GH) up-regulation. This hormone is believed to stimulate the mammary stem cells as the first step of carcinogenesis [24]. GH increases insulin growing factor I (IGF-I) levels, which in turn stimulates the proliferation of mammary cells and acts as a local growing factor, promoting tumor development and maintenance [17][18][24]. Pseudopregnancy has no relationship with the development of mammary tumors [21][29]; however, the use of progesterone as a contraceptive can induce the development of benign mammary tumors in canines. Synthetic progestins, like medroxyprogesterone acetate, promote similar effects to endogenous progesterone in the mammary glands [30][31].
Estrogens can promote a pro-carcinogenic effect through inhibition of apoptosis and induce genetic/epigenetic changes that modulate the expression of genes involved in the regulation of cell proliferation and differentiation [28]. Estrogen-induced cell proliferation increases the incidence of genetic alterations. In addition, metabolites derived from the oxidative metabolism of estradiol can cause direct genotoxic effects [25][26]. High levels of steroidal hormones have been identified in serum and in mammary tissue in female dogs with malignant tumors in comparison to those with benign tumors, suggesting that steroidal hormones act as local growing factors, stimulating the proliferation of cells [18].
At the cellular level, obesity causes inflammation of the adipose tissue with activation of macrophages that produce inflammatory mediators such as tumor necrosis factor α and interleukin 6 and other substances like leptin, adiponectin, resistin and aromatase. This can lead to increased cell proliferation, inhibit apoptosis and induce angiogenesis [27]. In addition, adipose tissue and high cholesterol levels can be an important source of steroidal hormones including estrogens, progesterone and androgens. Peripheral aromatization of androgens to estrogens can lead to prolonged exposure of mammary tissue to estrogens [32]. In fact, one study found that aromatase expression increased in overweight female dogs with mammary carcinoma and therefore might impact its progression through hormonal receptor signaling [27].
Breed is another factor that can influence the incidence of mammary tumors in dogs. Several studies have shown a higher incidence in pure breeds than in mixed breeds; however, there is no consensus on which breeds are at the highest risk of developing mammary tumors [12]. This information varies greatly depending on the geographical location, study type and biases. A study conducted in Spain identified Retrievers, flushing dogs and water dogs as the breeds with a higher incidence of mammary tumors [22].
2.2. Histological and Molecular Classification
Mammary tumors represent the most frequent neoplasia diagnosed in non-spayed female dogs, and approximately 50% are malignant [5][20][33][34]. Malignant mammary tumors have the capacity to metastasize to regional lymph nodes and to distant organs like lungs; in some cases, they can migrate through blood vessels to abdominal organs, such as the liver, spleen and kidney [33]. Over the years, several systems for the histological classification of canine mammary tumors have been established. The first classification was published in 1974 [35], the second in 1999 [36], and, subsequently, a modification was made in 2011, which is the one currently used [37].
Canine mammary tumors are highly variable in their morphology and are generally composed of more than one cell type, including luminal epithelial cells, myoepithelial cells and mesenchymal cells, in combination or alone [36][37]. They can be of epithelial origin (simple adenoma or simple carcinoma) or mesenchymal (fibroadenoma, fibrosarcoma, osteosarcoma and other sarcomas); however, some present a combination of epithelial and myoepithelial tissue (benign mixed tumors or carcinosarcoma). Mesenchymal tumors and tumors with myoepithelial cell proliferation are frequent in canines, unlike in women, where they are hardly ever diagnosed [34][38][39].
The tumor type, nuclear and cellular pleomorphism, mitotic index, presence of necrotic areas, lymphatic and peritumoral invasion and regional metastatic lymph node are some criteria used in the diagnosis of malignant mammary tumors [37]. The histological grading system in canine mammary carcinoma consists of quantifying anaplasia, tubule formation, mitotic activity and nuclear pleomorphism.
The sum of all the individual values determines the histological grade of the malignancy (grade) [40]. The histological grade is considered to be a prognostic factor, where a higher level is associated with a poorer outcome and shorter survival rate [37][41][42][43].
In women, breast cancer tumors are classified into five molecular subtypes: luminal A, luminal B HER-2—(epidermal growth factor 2 negative), luminal B HER-2+ (epidermal growth factor 2 positive), HER-2 and triple-negative. This allows the selection of a specific targeted therapy, such as anti-estrogen drugs for the luminal A subtype, and monoclonal antibody-based immunotherapy like trastuzumab for HER-2 subtypes [44]. HER-2 is also considered an important tumor marker and is expressed in 30–35% of the canine mammary tumors [20][34]. In canine mammary cancer, multiple studies have been conducted using the same panel of markers; however, the results obtained so far, especially regarding incidence, have been highly variable and sometimes contradictory [45][46].
2.3. Carcinogenesis
The tumor microenvironment is composed of the extracellular matrix (ECM), cancer stem cells (CSC), adipocytes, nerves, tumor-associated stromal cells as fibroblast and endothelial cells, infiltrating immune cells like leukocyte and macrophages and their biological products as cytokines, growth factors and molecules that contribute to tumor progression [47][48].
The extracellular matrix includes proteins that serve as a support to the tumor cells and facilitate cell–cell or cell–matrix interactions [49][50]. During the development of canine mammary tumors, the ECM suffers intense remodeling and degradation of its components and structure [50][51]. In canine mammary cancer, collagen fiber types I, III, IV and V are sparse and fibers ECM disorganized. In addition, collagen fibers are more aligned and shorter than normal tissue, which also correlates with shorter survival rates [49][50][51].
Cancer stem cells are subpopulations of tumor cells that are mainly characterized by their capacity for self-renewal and potential for differentiation and play an important role in cancer recurrence and metastasis [47][52]. Targeting cancer stem cells is used for the development of new treatments for cancer. Metastasis prognostic factors and cancer stem cell-related transcription factors that can be used to select therapeutic strategies have been identified in canine mammary tumors; these include ICAM-1, PRR14, Oct4 and Sox2 [53].
Another element that participates in the process of carcinogenesis is cancer-associated fibroblasts (CAFs). These cells are part of the stroma and participate in the epithelial-mesenchymal transition, secrete cytokines such as epidermal growth factor and transforming growth factor β and produce metalloproteinases that promote growth and tumor progression, invasiveness and metastasis [54][55][56]. In canine mammary cancer, there is an increased expression of periostin in CAFs compared to mammary adenomas, and this has a positive correlation with the histological malignancy grade [57].
The immune system plays an important dual role in cancer. It has the capacity to promote carcinogenesis but can also suppress tumor progression, depending on the subtypes of inflammatory cells, mostly lymphocytes and macrophages in the tumor microenvironment, e.g., T lymphocytes (T helper and T-FoxP3+) and macrophages subtype M2, which are in favor of tumor progression [48][58][59]. The inflammatory cells that are found in mammary tumors produce molecules, chemokines and cytokines that have proangiogenic and immunosuppressor activity. Female dogs with malignant mammary tumors that have a high level of inflammatory infiltrate, CD3+ T cells, CD4+ T cells or tumor-infiltrated macrophages have presented shorter survival times [60].
In any cell, a genetic or metabolic alteration can lead to a malignant transformation, but this is usually prevented by several molecular mechanisms that activate apoptosis. Under specific physiological conditions, DNA damage, alterations in DNA replication, poor regulation of the cell cycle, hypoxia or the accumulation of misfolded proteins, can all trigger pro-apoptotic pathways and/or anti-apoptotic suppression pathways. In cancer cells, these protective mechanisms are impaired. One of the best-described activators of apoptosis is tumor suppressor gene p53, also known as the genome guardian.
In women, p53 gene mutations have been reported in up to 30% of breast cancer cases and are generally associated with the most aggressive subtypes (e.g., triple-negative); high expression of p53 correlates with poor prognosis and shorter survival times [61]. Only a few studies have assessed p53 expression status in canine mammary cancer, and its role in progression is still unclear. In one study of 170 malignant mammary tumors in female dogs, only 0.5% (8/170) expressed p53. Tumors positive for p53 were high-grade and with high proliferative activity, suggesting that the p53 gene is involved in the progression of canine mammary cancer [62]. However, in another smaller study (40 tumor samples), a significant reduction in gene expression in eight samples, overexpression in two samples and normal expression in thirty samples was reported; a statistical analysis found no correlation between TP53 gene expression and tumor aggressiveness [63].
As mentioned previously, sex hormones participate in the initiation, promotion and progression of carcinogenesis of mammary tumors. Estrogen is mainly synthetized by the ovaries; however, it has been detected in high concentrations, along with some of its precursors, in malignant mammary tissue [64]. The exposure duration of mammary tissue to estrogens is key to tumor development. Benign mammary tumors and low-grade malignant tumors are usually ERα (estrogen receptor alpha) positive, while high-grade malignant tumors tend to be ERα negative by histology [62][65]. The ER1 (estrogen receptor 1) gene has a similar pattern of expression, as it is not expressed in high-grade carcinomas. Estrogen modulates gene expression and directly affects the phosphorylation (activation) of several protein kinases. As a result of these genomic and non-genomic pathways, estrogen can accelerate cell proliferation, which in turn increases the chances of acquiring new genetic errors [26].
HER-2 overexpression has been associated with poor prognosis, and HER-2 has functions in the regulation of tumor growth and cell differentiation and constitutes a marker for targeted treatment [66]. In women with breast cancer, HER-2 has been identified in 30% of the cases. In dogs, a positive correlation has been described between HER-2 expression, malignancy and high histological grade, suggesting a role in canine mammary carcinogenesis [67].
Prostaglandins (PG) are lipidic mediators involved in tumorigenic processes mainly controlled by a cyclooxygenase enzyme. PG can modulate the immune system and affect proliferative processes, apoptosis and angiogenesis [68]. Cyclooxygenases (Cox1, Cox2 and Cox3) are catalytic enzymes that are necessary for the conversion of arachidonic acid to prostaglandin G2 and subsequently to PGH2, a precursor of prostanoids (prostacyclins and thromboxanes). Isoenzyme Cox2 increases during inflammation and is implicated in the development and progression of different types of tumors, including canine mammary tumors [69][70]. Cox2 expression was associated with lymph node metastasis at the time of surgery and with the development of distant metastasis. It is also more frequent and intense in malignant (compared to benign) mammary tumors, has been reported in 56–100% of the malignant cells and is correlated with a shorter survival [70][71][72]. Cox2 modulates tumor progression through different mechanisms.
Genetic alterations are a part of mammary tumor development. The proto-oncogene epidermal growth factor receptor (EGFR) plays an important role in human breast cancer as expression of its phosphorylated form is associated with increased angiogenesis and metastasis [73]. In malignant canine mammary carcinomas, overexpression of EGFR is associated with tumor size, necrosis, mitotic grade, histological grade of malignancy, tumor relapse, distant metastasis and clinical stage.
Other common genetic alterations found in canine mammary cancer are mutations in the genes encoding proteins of the PI3K/Akt/mTOR pathway. The PI3K/Akt/mTOR pathway is necessary for the regulation of proliferation, protein synthesis, apoptosis, cell motility and angiogenesis and is dysregulated in several canine mammary tumors [39][74]. Mutations in the PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), PTEN (phosphatase and tensin homolog), PIK3R1 (phosphoinositide-3-kinase regulatory subunit 1) and AKT1 (serine/threonine kinase 1) genes have been identified in canine mammary cancers at comparable frequencies to human breast cancers, indicating that they may be conserved across species [39]. The canine PIK3CA gene mutated in 55% and 38% of benign and malignant mammary tumors, respectively, encodes for a 1068 amino acid protein that shares 99% similarity with its human counterpart. Therefore, it is highly likely that a predisposing functional mutation of PIK3CA is comparable between humans and dogs [75]. Mutations in PIK3CA could over-activate this signaling pathway, promoting tumorigenesis [76].
2.4. Clinical Signs
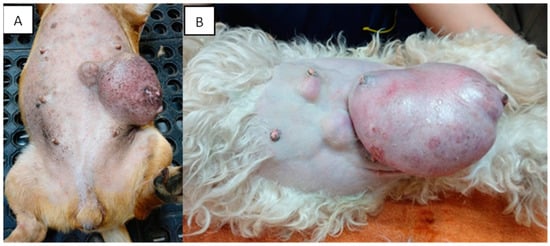
Mammary tumors are usually firm, well-defined nodules and their size can vary from millimeters to centimeters. They can occur in multiple glands at the same time and be of different histological types and grades. In addition, multiple tumors can coexist in the same mammary gland. The caudal abdominal glands are more frequently affected (up to 60% of cases) than the thoracic glands [77]. The skin in the affected area can be ulcerated or traumatized, as shown in Figure 2. Evaluation and palpation of regional lymph nodes are mandatory during diagnosis.

Figure 2. Canines with multiple mammary tumors localized in different glands. Tumor measurements larger than 5 cm in diameter with inflammation (A) and ulcerated skin (A,B) can be seen (own photo).
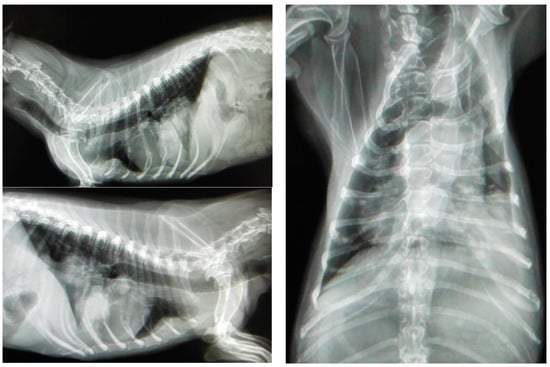
Most canines with mammary tumors are clinically healthy at the time of diagnosis [77]. However, patients with metastasis can present fatigue, lethargy, weight loss, dyspnea, cough, edema or lameness. Clinical signs depend on the extension and localization of metastasis [21][34]. Approximately 50% of mammary carcinomas metastasize to regional lymph nodes. Lymph node involvement is variable and can promote distant metastasis, most frequently to the lung (see Figure 3); metastatic bone lesions may also occur [21][34][78].

Figure 3. Three projections of thoracic radiographs, right and left lateral and ventrodorsal, with nodular interstitial pattern in a 12-year-old Dachshund patient with metastatic mammary carcinoma (own photo).
2.5. Diagnosis
Mammary tumor diagnosis is usually made either by an accidental finding during a physical exam or in patients who attend the veterinary consultation due to the presence of one or multiple nodules in the mammary glands. The definitive diagnosis and tumor grade are established based on histopathological analysis. Historically, mammary cancer classification consisted of establishing the type and histologic grade of the tumor. Nowadays, ERα, PR and HER-2 overexpression are also included, helping to better assess prognosis and establish appropriate treatment regimens in the medical practice [79].
An excisional biopsy is a good option for histopathological diagnosis of the tumor as it allows a complete histological evaluation and, in some cases, can be therapeutic. If the patient presents with multiple tumors, each tumor should be evaluated individually as different tumor types can be present in the same patient [21][80].
Fine needle aspiration (FNA) does not always lead to a diagnosis, mostly due to the heterogeneity of canine tumors and the inherent variability of cell morphology in different tumor areas. Therefore, the exact differentiation between benign and malignant tumors of epithelial origin often cannot be made. Although several studies in canines have reported low sensitivity and specificity rates of FNA cytology, Simon and collaborators demonstrated an 88% sensitivity and 96% specificity in malignant mammary tumor diagnosis by cytology (using histopathology as the gold standard). They collected at least four samples per tumor, which increased the probability of evaluating different areas of a heterogeneous tumor, and all samples were assessed by two observers [81].
Cytology can be useful to rule out differential diagnoses such as mastitis, lipomas, mast cell tumors and others. Although performing FNA of the tumor during clinical evaluation does not interfere with the surgical planning of the patients, the type of surgery is determined by the size of the lesion, the affected mammary glands and the lymphatic drainage [77].
2.6. Staging and Prognosis
Mammary tumors are staged using the tumor, lymph node, metastasis (TNM) system, established by the World Health Organization (WHO). According to this system, the patient is placed in one of five clinical stages based on tumor size, lymph node status and presence of metastasis. Stages I–III are assigned to non-metastatic patients (depending on their tumor size), while lymph node metastasis is classified as IV regardless of tumor size, and distant metastasis is classified as stage V.
Staging of all patients with mammary tumors is important because mammary carcinomas can metastasize through lymphatic vessels to lymph nodes and lungs (mainly). Lymphatic drainage should be assessed, and clinical exploration of the regional lymph nodes should be made in case they are palpable or enlarged. A tissue sample should be taken for cytology or histopathology evaluation to confirm or discard metastasis [82].
A retrospective case series study of 79 female dogs with malignant mammary tumors showed that patients with tumors in clinical stages IV and V, have a post-surgery survival of 6 months, unlike patients in early clinical stages (I, II or III) that survive for longer. If ovariohysterectomy is performed at the time of tumor removal, canines are more likely to live over 2 years; however, this could also be affected by the tumor type diagnosed by histopathology [83]. Another 2-year prospective study of 229 female dogs in Italy found a survival time of 18 months for canine patients diagnosed with adenosquamous carcinoma, 14 months for comedocarcinoma, 8 months for solid carcinoma and 3 months for anaplastic carcinoma and carcinosarcoma. These last two showed the highest rates of metastasis (89% and 100%, respectively) [41].
2.7. Treatment
The treatment of choice for mammary tumors in dogs is surgery, except for inflammatory carcinoma, where palliative medical treatment and chemotherapy are preferred [84]. The extent of surgery depends on the size and location of the tumor, as well as the presence of lymphatic drainage from the affected mammary gland [85]. Malignant tumors are significantly larger than benign ones, and 60% of patients have been reported to have multiple mammary tumors, which behave as independent primary tumors with different histopathological characteristics [86][87]. The goal of surgery is to remove all tumors with full surgical margins and/or prevent new mammary tumor formation. Canines with negative clinical or histopathological prognostic factors are not effectively treated with surgery alone and are at a higher risk of developing new mammary tumors [85][86].
An additional benefit of a surgical resection of mammary tumors is that it allows for histopathological examination of the tissue. Therefore, it has been associated with increased survival time and quality of life of patients. In addition, in some cases, it can be curative. This is especially the case for benign tumors, malignant low histological grade tumors or patients in early stages, except for inflammatory carcinoma or metastatic tumors [77].
Depending on the tumor size, location and number, surgery can be a simple mastectomy, regional mastectomy, radical mastectomy or a combination of these procedures. In patients with large tumors, lymph node metastases or unfavorable histopathological characteristics, local therapy is usually not effective and systemic treatment such as chemotherapy or hormonal therapy is required [82][88].
The lymphatic system is considered the main route of metastasis of canine mammary cancer. This is one of the reasons why the lymph node and the glands associated with lymphatic drainage are also removed during surgical excision of the mammary tumor. In healthy canines, the lymphatic vessels drain to the ipsilateral lymph nodes. While there is no drainage to the contralateral lymph node or gland, this can be altered by the presence of a mammary neoplasm [89][90].
Chemotherapy as an adjuvant or palliative therapy, or in cases of metastatic disease, is routinely used in women with breast cancer and has been shown to improve survival [21]. In veterinary medicine, several chemotherapeutic protocols have been used in dogs with malignant mammary tumors. However, additional prospective studies are required to verify their benefit in the survival of patients with mammary carcinoma [91]. Chemotherapy is recommended in patients at high risk of metastasis or recurrence characterized by regional lymph node metastasis, large tumors (>3 cm) and aggressive histopathological diagnosis such as high histological grade, vascular or lymphatic permeation [92]. As there is limited information on the efficacy of chemotherapy in canine patients with mammary cancer, a more in-depth assessment, including randomized controlled trials, is needed to establish guidelines for its use.
3. Conclusions
Mammary cancer is one of the most frequently diagnosed malignant neoplasms in canines, and it is the most frequent tumor in non-spayed female dogs. Similarities and differences have been demonstrated between mammary cancer in women and canines at the molecular level. These could serve as a basis for a better understanding of mammary cancer pathology, the development of new therapies and diagnostic tools, the establishment of classifications and meeting the concept of the One Health approach for the benefit of both species. However, epidemiological information in canines is limited, as few countries (and cities) have managed to properly document the clinical, pathological and epidemiological characteristics of mammary cancer in canines. Promoting the publication of research into different aspects of mammary cancer, establishing a collaborative network between different countries and determining the characteristics of dog populations will favor a better understanding of the disease. In addition, both surgical and chemotherapeutic procedures need to be standardized to improve response rates and survival of mammary cancer patients.
References
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140231.
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Davis, B.W.; Ostrander, E.A. Domestic Dogs and Cancer Research: A Breed-Based Genomics Approach. ILAR J. 2014, 55, 59–68.
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381.
- Raposo, T.P.; Arias-Pulido, H.; Chaher, N.; Fiering, S.N.; Argyle, D.J.; Prada, J.; Pires, I.; Queiroga, F.L. Comparative aspects of canine and human inflammatory breast cancer. Semin. Oncol. 2017, 44, 288–300.
- Zhang, H.; Pei, S.; Zhou, B.; Wang, H.; Du, H.; Zhang, D.; Lin, D. Establishment and characterization of a new triple-negative canine mammary cancer cell line. Tissue Cell 2018, 54, 10–19.
- Munson, L.; Moresco, A. Comparative Pathology of Mammary Gland Cancers in Domestic and Wild Animals. Breast Dis. 2007, 28, 7–21.
- Kustritz, M.V.R. Population Control in Small Animals. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, 721–732.
- Santos, M.; Marcos, R.; Faustino, A. Histological Study of Canine Mammary Gland During the Oestrous Cycle. Reprod. Domest. Anim. 2010, 45, e146–e154.
- Baioni, E.; Scanziani, E.; Vincenti, M.C.; Leschiera, M.; Bozzetta, E.; Pezzolato, M.; Desiato, R.; Bertolini, S.; Maurella, C.; Ru, G. Estimating canine cancer incidence: Findings from a population-based tumour registry in northwestern Italy. BMC Vet. Res. 2017, 13, 203.
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Vet. Med. 2016, 126, 183–189.
- Egenvall, A.; Bonnett, B.N.; Öhagen, P.; Olson, P.; Hedhammar, A.; von Euler, H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev. Vet. Med. 2005, 69, 109–127.
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246.
- Merlo, D.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.; Sestito, V.; Tanara, G.; et al. Cancer Incidence in Pet Dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984.
- Schneider, R. Comparison of age, sex, and incidence rates in human and canine breast cancer. Cancer 1970, 26, 419–426.
- Queiroga, F.L.; Raposo, T.; Carvalho, M.; Prada, J.; Pires, I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. In Vivo 2011, 25, 455–465.
- Benavente, M.; Bianchi, P.; Aba, M. Canine Mammary Tumors: Risk Factors, Prognosis and Treatments. J. Vet. Adv. 2016, 6, 1291–1300.
- Gentile, L.B.; Nagamine, M.K.; Biondi, L.R.; Sanches, D.S.; Toyota, F.; Giovani, T.M.; de Jesus, I.P.; da Fonseca, I.I.M.; Queiroz-Hazarbassanov, N.; Diaz, B.L.; et al. Establishment of primary mixed cell cultures from spontaneous canine mammary tumors: Characterization of classic and new cancer-associated molecules. PLoS ONE 2017, 12, e0184228.
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66.
- Sleeckx, N.; De Rooster, H.; Kroeze, E.V.; Van Ginneken, C.; Van Brantegem, L. Canine Mammary Tumours, an Overview. Reprod. Domest. Anim. 2011, 46, 1112–1131.
- Pastor, N.; Caballé, N.C.; Santella, M.; Ezquerra, L.J.; Tarazona, R.; Duran, E. Epidemiological study of canine mammary tumors: Age, breed, size and malignancy. Austral J. Vet. Sci. 2018, 50, 143–147.
- Schneider, R.; Dorn, C.R.; Taylor, D.O.N. Factors Influencing Canine Mammary Cancer Development and Postsurgical Survival2. JNCI J. Natl. Cancer Inst. 1969, 43, 1249–1261.
- Queiroga, F.L.; Pérez-Alenza, M.D.; Silvan, G.; Peña, L.; Lopes, C.S.; Illera, J.C. Crosstalk between GH/IGF-I axis and steroid hormones (progesterone, 17β-estradiol) in canine mammary tumours. J. Steroid Biochem. Mol. Biol. 2008, 110, 76–82.
- Russo, J.; Russo, I.H. The role of estrogen in the initiation of breast cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 89–96.
- Torres, C.G.; Iturriaga, M.P.; Cruz, P. Hormonal Carcinogenesis in Canine Mammary Cancer: Molecular Mechanisms of Estradiol Involved in Malignant Progression. Animals 2021, 11, 608.
- Lim, H.-Y.; Im, K.-S.; Kim, N.-H.; Kim, H.-W.; Shin, J.-I.; Yhee, J.-Y.; Sur, J.-H. Effects of Obesity and Obesity-Related Molecules on Canine Mammary Gland Tumors. Vet. Pathol. 2015, 52, 1045–1051.
- Kumaraguruparan, R.; Prathiba, D.; Nagini, S. Of humans and canines: Immunohistochemical analysis of PCNA, Bcl-2, p53, cytokeratin and ER in mammary tumours. Res. Vet. Sci. 2006, 81, 218–224.
- Veronesi, M.; Battocchio, M.; Rizzi, C.; Sironi, G. Relationship between dysplastic and neoplastic mammary lesions and pseudopregnancy in the bitch. Vet. Res. Commun. 2003, 27, 245–247.
- Rao, N.A.S.; Van Wolferen, M.; Gracanin, A.; Bhatti, S.F.M.; Król, M.; Holstege, F.C.; Mol, J.A. Gene expression profiles of progestin-induced canine mammary hyperplasia and spontaneous mammary tumors. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 1), 735–784.
- van Garderen, E.; de Wit, M.; Voorhout, W.F.; Rutteman, G.R.; Mol, J.A.; Nederbragt, H.; Misdorp, W. Expression of growth hormone in canine mammary tissue and mammary tumors. Evidence for a potential autocrine/paracrine stimulatory loop. Am. J. Pathol. 1997, 150, 1037–1047. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9060840 (accessed on 1 July 2023).
- Simpson, E.R.; Zhao, Y. Estrogen Biosynthesis in Adipose. Ann. N. Y. Acad. Sci. 1996, 784, 18–26.
- Karayannopoulou, M.; Lafionatis, S. Recent advances on canine mammary. Revue Méd. Vét. 2016, 167, 192–200.
- Gray, M.; Meehan, J.; Martínez-Pérez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617.
- Hampe, J.F.; Misdorp, W. Tumours and dysplasias of the mammary gland. Bull. World Health Organ. 1974, 50, 111–133.
- Misdorp, W.; Else, R.; Hellmen, E.; Lipscomb, T. Histologic Classification of Mammary Tumors of the Dog and Cat, 2nd ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1999; Volume 7.
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and Grading of Canine Mammary Tumors. Vet. Pathol. 2011, 48, 117–131.
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine Mammary Tumors: A review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet. Pathol. 2014, 51, 127–145.
- Kim, T.-M.; Yang, I.S.; Seung, B.-J.; Lee, S.; Kim, D.; Ha, Y.-J.; Seo, M.-K.; Kim, K.-K.; Kim, H.S.; Cheong, J.-H.; et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat. Commun. 2020, 11, 3616.
- Misdorp, W. Tumors of the mammary gland. In Tumors in Domestic Animals; Meuten, D., Ed.; Iowa State Press: Ames, IA, USA, 2002; pp. 575–588.
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic Significance of Canine Mammary Tumor Histologic Subtypes: An Observational Cohort Study of 229 Cases. Vet. Pathol. 2017, 54, 571–578.
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596.
- Nunes, F.C.; Damasceno, K.A.; de Campos, C.B.; Bertagnolli, A.C.; Lavalle, G.E.; Cassali, G.D. Mixed tumors of the canine mammary glands: Evaluation of prognostic factors, treatment, and overall survival. Vet. Anim. Sci. 2019, 7, 100039.
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.H.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672.
- Gama, A.; Alves, A.; Schmitt, F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: Application of the human classification. Virchows Arch. 2008, 453, 123–132.
- Sassi, F.; Benazzi, C.; Castellani, G.; Sarli, G. Molecular-based tumour subtypes of canine mammary carcinomas assessed by immunohistochemistry. BMC Vet. Res. 2010, 6, 5.
- Michishita, M. Understanding of tumourigenesis in canine mammary tumours based on cancer stem cell research. Vet. J. 2020, 265, 105560.
- Carvalho, M.I.; Raposo, T.P.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Gregório, H.; Queiroga, F.L. The Dog as a Model to Study the Tumor Microenvironment. Tumor Microenviron. Nov. Concepts 2021, 1329, 123–152.
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397.
- Barreto, R.; Carvalho, H.; Matias, G.; Silva, M.; Ribeiro, R.; Campanelli, T.; Rigoglio, N.; Carreira, A.; Miglino, M. The extracellular matrix protein pattern in the canine neoplastic mammary gland. Tissue Cell 2023, 82, 102050.
- Garcia, A.P.V.; Reis, L.A.; Nunes, F.C.; Longford, F.G.J.; Frey, J.G.; de Paula, A.M.; Cassali, G.D. Canine mammary cancer tumour behaviour and patient survival time are associated with collagen fibre characteristics. Sci. Rep. 2021, 11, 5668.
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111.
- Kim, S.; Bok, E.; Lee, S.; Lee, H.-J.; Choe, Y.; Kim, N.-H.; Lee, W.-J.; Rho, G.-J.; Lee, S.-L. Metastasis prognostic factors and cancer stem cell-related transcription factors associated with metastasis induction in canine metastatic mammary gland tumors. J. Vet. Sci. 2021, 22, e62.
- Borecka, P.; Ciaputa, R.; Janus, I.; Piotrowska, A.; Ratajczak-Wielgomas, K.; Kmiecik, A.; Podhorska-Okolów, M.; Dzięgiel, P.; Nowak, M. Expression of Podoplanin in Mammary Cancers in Female Dogs. In Vivo 2020, 34, 213–223.
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434.
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics 2018, 18, e1700167.
- Borecka, P.; Ratajczak-Wielgomas, K.; Ciaputa, R.; Kandefer-Gola, M.; Janus, I.; Piotrowska, A.; Kmiecik, A.; Podhorska-Okolów, M.; Dzięgiel, P.; Nowak, M. Expression of Periostin in Cancer-associated Fibroblasts in Mammary Cancer in Female Dogs. In Vivo 2020, 34, 1017–1026.
- Carvalho, M.I.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Bianchini, R.; Jensen-Jarolim, E.; Queiroga, F.L. A Comparative Approach of Tumor-Associated Inflammation in Mammary Cancer between Humans and Dogs. BioMed Res. Int. 2016, 2016, 4917387.
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A Role for T-Lymphocytes in Human Breast Cancer and in Canine Mammary Tumors. BioMed Res. Int. 2014, 2014, 130894.
- Franzoni, M.S.; Brandi, A.; Prado, J.K.D.O.M.; Elias, F.; Dalmolin, F.; Lainetti, P.d.F.; Prado, M.C.M.; Leis-Filho, A.F.; Fonseca-Alves, C.E. Tumor-infiltrating CD4+ and CD8+ lymphocytes and macrophages are associated with prognostic factors in triple-negative canine mammary complex type carcinoma. Res. Vet. Sci. 2019, 126, 29–36.
- Bertheau, P.; Lehmann-Che, J.; Varna, M.; Dumay, A.; Poirot, B.; Porcher, R.; Turpin, E.; Plassa, L.-F.; de Roquancourt, A.; Bourstyn, E.; et al. p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast 2013, 22, S27–S29.
- Brunetti, B.; Bacci, B.; Angeli, C.; Benazzi, C.; Muscatello, L.V. p53, ER, and Ki67 Expression in Canine Mammary Carcinomas and Correlation with Pathological Variables and Prognosis. Vet. Pathol. 2021, 58, 325–331.
- Oliveira, T.F.; Maués, T.; Ramundo, M.S.; Figueiredo, A.M.S.; de Mello, M.F.V.; El-Jaick, K.B.; Ferreira, M.D.L.G.; Ferreira, A.M.R. TP53 gene expression levels and tumor aggressiveness in canine mammary carcinomas. J. Vet. Diagn. Investig. 2017, 29, 865–868.
- Blankenstein, M.; van de Ven, J.; Maitimu-Smeele, I.; Donker, G.; de Jong, P.; Daroszewski, J.; Szymczak, J.; Milewicz, A.; Thijssen, J. Intratumoral levels of estrogens in breast cancer. J. Steroid Biochem. Mol. Biol. 1999, 69, 293–297.
- Nieto, A.; Peña, L.; Pérez-Alenza, M.D.; Sánchez, M.A.; Flores, J.M.; Castaño, M. Immunohistologic Detection of Estrogen Receptor Alpha in Canine Mammary Tumors: Clinical and Pathologic Associations and Prognostic Significance. Vet. Pathol. 2000, 37, 239–247.
- Gutierrez, C.; Schiff, R. HER2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62.
- Silva, I.; Dias, A.; Bertagnolli, A.; Cassali, G.; Ferreira, E. Analysis of EGFR and HER-2 expressions in ductal carcinomas in situ in canine mammary glands. Arq. Bras. Med. Vet. Zootec. 2014, 66, 763–768.
- Wang, D.; DuBois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193.
- Saito, T.; Tamura, D.; Asano, R. Usefulness of selective COX-2 inhibitors as therapeutic agents against canine mammary tumors. Oncol. Rep. 2014, 31, 1637–1644.
- Raposo, T.; Beirão, B.; Pang, L.; Queiroga, F.; Argyle, D. Inflammation and cancer: Till death tears them apart. Vet. J. 2015, 205, 161–174.
- Queiroga, F.; Alves, A.; Pires, I.; Lopes, C. Expression of Cox-1 and Cox-2 in Canine Mammary Tumours. J. Comp. Pathol. 2007, 136, 177–185.
- Queiroga, F.L.; Pires, I.; Lobo, L.; Lopes, C.S. The role of Cox-2 expression in the prognosis of dogs with malignant mammary tumours. Res. Vet. Sci. 2010, 88, 441–445.
- Magkou, C.; Nakopoulou, L.; Zoubouli, C.; Karali, K.; Theohari, I.; Bakarakos, P.; Giannopoulou, I. Expression of the epidermal growth factor receptor (EGFR) and the phosphorylated EGFR in invasive breast carcinomas. Breast Cancer Res. 2008, 10, R49.
- Asproni, P.; Millanta, F.; Ressel, L.; Podestà, F.; Parisi, F.; Vannozzi, I.; Poli, A. An Immunohistochemical Study of the PTEN/AKT Pathway Involvement in Canine and Feline Mammary Tumors. Animals 2021, 11, 365.
- Kim, J.H. PIK3CA mutations matter for cancer in dogs. Res. Vet. Sci. 2020, 133, 39–41.
- Miller, T.W. Initiating breast cancer by PIK3CA mutation. Breast Cancer Res. 2012, 14, 301.
- Cassali, G.; Damasceno, K.; Bertagnolli, A.; Estrela-Lima, A.; Lavalle, G.; Santis, G.; Nardi, A.; Fernandes, C.; Cogliati, B.; Sobral, R.; et al. Consensus regarding the diagnosis, prognosis and treatment of canine mammary tumors: Benign mixed tumors, carcinomas in mixed tumors and carcinosarcomas. Braz. J. Vet. Pathol. 2017, 10, 153–180.
- Polton, G. Mammary tumours in dogs. Iran. Vet. J. 2009, 62, 50–56.
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat. 2018, 167, 459–468.
- Torres, G.; Mocha, E. Tumores mamarios en caninos. Orinoquia 2007, 11, 99–110.
- Simon, D.; Schoenrock, D.; Nolte, I.; Baumgã¤Rtner, W.; Barron, R.; Mischke, R. Cytologic examination of fine-needle aspirates from mammary gland tumors in the dog: Diagnostic accuracy with comparison to histopathology and association with postoperative outcome. Vet. Clin. Pathol. 2009, 38, 521–528.
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, Anatomy, Histology, Lymphatic Drainage, Clinical Features, and Cell Differentiation Markers of Canine Mammary Gland Neoplasms. Vet. Pathol. 2011, 48, 85–97.
- Chang, S.-C.; Chang, C.-C.; Chang, T.-J.; Wong, M.-L. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998–2002). J. Am. Vet. Med Assoc. 2005, 227, 1625–1629.
- Marconato, L.; Romanelli, G.; Stefanello, D.; Giacoboni, C.; Bonfanti, U.; Bettini, G.; Finotello, R.; Verganti, S.; Valenti, P.; Ciaramella, L.; et al. Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003–2008). J. Am. Vet. Med. Assoc. 2009, 235, 967–972.
- LaValle, G.E.; De Campos, C.B.; Bertagnolli, A.; Cassali, G.D. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. In Vivo 2012, 26, 375–379.
- Sorenmo, K.U.; Kristiansen, V.M.; Cofone, M.A.; Shofer, F.S.; Breen, A.-M.; Langeland, M.; Mongil, C.M.; Grondahl, A.M.; Teige, J.; Goldschmidt, M.H. Canine mammary gland tumours; a histological continuum from benign to malignant; clinical and histopathological evidence. Vet. Comp. Oncol. 2009, 7, 162–172.
- Petrov, E.A.; Ilievska, K.; Trojacanec, P.; Celeska, I.; Nikolovski, G.; Gjurovski, I.; Dovenski, T. Canine Mammary Tumours—Clinical Survey. Maced. Vet. Rev. 2014, 37, 129–134.
- Sorenmo, K.U.; Worley, D.R.; Zappulli, V. Tumors of the Mammary Gland. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; W.B. Saunders: Birmingham, AL, USA, 2019; pp. 604–625.
- Pereira, C.T.; Rahal, S.C.; de Carvalho Balieiro, J.C.; Ribeiro, A.A.C.M. Lymphatic Drainage on Healthy and Neoplasic Mammary Glands in Female Dogs: Can it Really be Altered? Anat. Histol. Embryol. 2003, 32, 282–290.
- Patsikas, M.N.; Karayannopoulou, M.; Kaldrymidoy, E.; Papazoglou, L.G.; Papadopoulou, P.L.; Tzegas, S.I.; Tziris, N.E.; Kaitzis, D.G.; Dimitriadis, A.S.; Dessiris, A.K. The Lymph Drainage of the Neoplastic Mammary Glands in the Bitch: A Lymphographic Study. Anat. Histol. Embryol. 2006, 35, 228–234.
- Tran, C.M.; Moore, A.S.; Frimberger, A.E. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet. Comp. Oncol. 2016, 14, 252–262.
- de Campos, C.B.; Lavalle, G.E.; Ligório, S.F.; Nunes, F.C.; Carneiro, R.A.; Amorim, R.L.; Cassali, G.D.; Msc, C.B.d.C.D.; Msc, S.F.L.B.; Msc, F.C.N.D.; et al. Absence of significant adverse events following thalidomide administration in bitches diagnosed with mammary gland carcinomas. Vet. Rec. 2016, 179, 514.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
01 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




