Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Felisbina Luisa Queiroga and Version 2 by Lindsay Dong.
Mammary tumors are the most frequent neoplasia in female dogs. They develop spontaneous cancer and share several biological, clinical, pathological and molecular characteristics with cancer diagnosed in humans. Mammary cancer is also one of the leading causes of death in both species.
- mammary cancer
- canine
- dog
- breast cancer
- animal model
- comparative oncology
1. Introduction
Cancer is a heterogeneous group of diseases characterized by an uncontrolled proliferation of abnormal cells that can spread to the surrounding tissues. It is one of the most common causes of death in humans and dogs. In humans, around 10 million cancer-related deaths are reported and 19.3 million new cases are diagnosed annually, while in dogs, 4 million new cancer cases are diagnosed every year [1][2][3][1,2,3]. Cancer is the first cause of death in dogs over 10 years of age, with 50% of them developing this disease and one in four dying because of cancer [4]. Canines develop spontaneous cancer and share several biological, clinical, pathological and molecular features with humans [5][6][7][5,6,7]. Mammary tumors, affecting numerous mammal species, are the most common neoplasia diagnosed in female dogs and women, and they are considered to be a major problem in public health [5]. Gaining insight into the presentation and progression of breast cancer across different species will help us to better understand the pathogenesis of this complex disease [8].
2. Canine Mammary Tumors
Canine mammary tumors are an overly frequent condition in comparison to other types of cancer; they represent 50–70% of all neoplasia diagnosed in non-spayed female dogs, mainly affecting canines over 7 years of age. They appear as nodules of different sizes and are usually well-defined. The treatment regimen and prognosis of the patient can be established according to the physical characteristics, location, histological and molecular classification of the tumor. The incidence of canine mammary tumors varies depending on the geographic location of the study, and it is also affected by the age, hormonal exposition, breed and molecular features of the female dog, among other factors.2.1. Epidemiological Features
2.1.1. Incidence and Distribution
Information on the incidence of canine mammary tumors worldwide is very limited and only available for a few countries in Europe and North America. In Table 1, the incidence rate expressed per 100,000 and 10,000 dogs per year is shown, and in the text below there is additional epidemiological information. As seen in Table 1, the incidence of canine mammary tumors varies in every country and over time. This variation can be attributed to several factors, with spaying culture being one of them. Spaying is usually performed as a canine population control measure. However, castration at early ages also prevents mammary tumor development in the female dog [9][11] since estrogens and progesterone produced by the ovaries are mitogens for the mammary epithelium and can stimulate duct and lobe proliferation and growth [10][12].Table 1.
Canine mammary cancer epidemiology.
| Country (City/State) | Incidence | Year [Reference] |
|---|---|---|
| Italy | 193 per 100,000 | 2001–2008 [11][13] |
| Italy (Venice) | 250 per 100,000 | 2005–2013 [12][14] |
| Sweden | 111 per 10,000 | 1995–2002 [13][15] |
| United Kingdom | 205 per 100,000 | 1997–1998 [14][16] |
| Italy (Genoa) | 181.8 per 100,000 | 2000–2002 [15][17] |
| Italy (Genoa) | 196.6 per 100,00 | 1995–1999 [15][17] |
| Italy (Genoa) | 264 per 100,000 | 1990–1994 [15][17] |
| Italy (Genoa) | 119.2 per 100,000 | 1985–1989 [15][17] |
| USA (California) | 145 per 100,000 | 1963–1968 [16][18] |
2.1.2. Etiology and Risk Factors
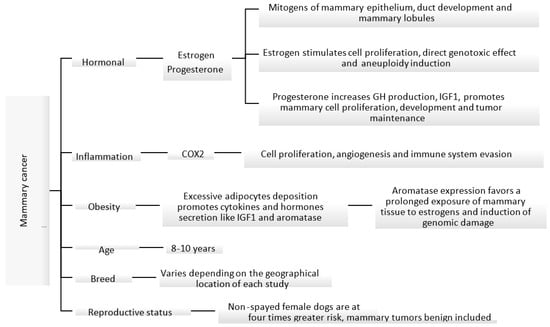
The etiology of canine mammary carcinoma is not fully understood; however, similarly to women’s breast cancer, its development is impacted by hormonal, genetic, nutritional and environmental factors [17][18][19][20][30,31,32,33]. Some of these factors are shown in Figure 1. Most malignant mammary tumors develop in middle-aged (5–7 years of age) and elderly (7–8 years of age) female dogs, with the median age of presentation ranging between 8 and 10 years [5][21][22][5,24,34]. In addition, the risk of developing mammary tumors increases with the delay in spaying [5][18][23][5,31,35]. Non-spayed female dogs are at four times greater risk of developing mammary tumors, compared to those spayed before two years of age [23][35].
Figure 1. Known mechanisms and factors involved in the induction of canine mammary cancer [18][23][24][25][26][27].
Known mechanisms and factors involved in the induction of canine mammary cancer [31,35,36,37,38,39].
In female dogs and women, ovarian steroids stimulate the normal growth of mammary tissue under physiologic conditions. However, the proliferative effect in the epithelium can create the perfect environment for neoplastic proliferation. Ovarian hormones, mainly estrogens and progesterone, play an important role in the development of mammary tumors [18][28][31,40]. During the luteal phase, mammary tissue is exposed to high levels of progesterone, which could lead to growth hormone (GH) up-regulation. This hormone is believed to stimulate the mammary stem cells as the first step of carcinogenesis [24][36]. GH increases insulin growing factor I (IGF-I) levels, which in turn stimulates the proliferation of mammary cells and acts as a local growing factor, promoting tumor development and maintenance [17][18][24][30,31,36]. Pseudopregnancy has no relationship with the development of mammary tumors [21][29][24,41]; however, the use of progesterone as a contraceptive can induce the development of benign mammary tumors in canines. Synthetic progestins, like medroxyprogesterone acetate, promote similar effects to endogenous progesterone in the mammary glands [30][31][42,43].
Estrogens can promote a pro-carcinogenic effect through inhibition of apoptosis and induce genetic/epigenetic changes that modulate the expression of genes involved in the regulation of cell proliferation and differentiation [28][40]. Estrogen-induced cell proliferation increases the incidence of genetic alterations. In addition, metabolites derived from the oxidative metabolism of estradiol can cause direct genotoxic effects [25][26][37,38]. High levels of steroidal hormones have been identified in serum and in mammary tissue in female dogs with malignant tumors in comparison to those with benign tumors, suggesting that steroidal hormones act as local growing factors, stimulating the proliferation of cells [18][31].
At the cellular level, obesity causes inflammation of the adipose tissue with activation of macrophages that produce inflammatory mediators such as tumor necrosis factor α and interleukin 6 and other substances like leptin, adiponectin, resistin and aromatase. This can lead to increased cell proliferation, inhibit apoptosis and induce angiogenesis [27][39]. In addition, adipose tissue and high cholesterol levels can be an important source of steroidal hormones including estrogens, progesterone and androgens. Peripheral aromatization of androgens to estrogens can lead to prolonged exposure of mammary tissue to estrogens [32][48]. In fact, one study found that aromatase expression increased in overweight female dogs with mammary carcinoma and therefore might impact its progression through hormonal receptor signaling [27][39].
Breed is another factor that can influence the incidence of mammary tumors in dogs. Several studies have shown a higher incidence in pure breeds than in mixed breeds; however, there is no consensus on which breeds are at the highest risk of developing mammary tumors [12][14]. This information varies greatly depending on the geographical location, study type and biases. A study conducted in Spain identified Retrievers, flushing dogs and water dogs as the breeds with a higher incidence of mammary tumors [22][34].
2.2. Histological and Molecular Classification
Mammary tumors represent the most frequent neoplasia diagnosed in non-spayed female dogs, and approximately 50% are malignant [5][20][33][34][5,33,51,52]. Malignant mammary tumors have the capacity to metastasize to regional lymph nodes and to distant organs like lungs; in some cases, they can migrate through blood vessels to abdominal organs, such as the liver, spleen and kidney [33][51]. Over the years, several systems for the histological classification of canine mammary tumors have been established. The first classification was published in 1974 [35][53], the second in 1999 [36][54], and, subsequently, a modification was made in 2011, which is the one currently used [37][55]. Canine mammary tumors are highly variable in their morphology and are generally composed of more than one cell type, including luminal epithelial cells, myoepithelial cells and mesenchymal cells, in combination or alone [36][37][54,55]. They can be of epithelial origin (simple adenoma or simple carcinoma) or mesenchymal (fibroadenoma, fibrosarcoma, osteosarcoma and other sarcomas); however, some present a combination of epithelial and myoepithelial tissue (benign mixed tumors or carcinosarcoma). Mesenchymal tumors and tumors with myoepithelial cell proliferation are frequent in canines, unlike in women, where they are hardly ever diagnosed [34][38][39][52,56,57]. The tumor type, nuclear and cellular pleomorphism, mitotic index, presence of necrotic areas, lymphatic and peritumoral invasion and regional metastatic lymph node are some criteria used in the diagnosis of malignant mammary tumors [37][55]. The histological grading system in canine mammary carcinoma consists of quantifying anaplasia, tubule formation, mitotic activity and nuclear pleomorphism. The sum of all the individual values determines the histological grade of the malignancy (grade) [40][58]. The histological grade is considered to be a prognostic factor, where a higher level is associated with a poorer outcome and shorter survival rate [37][41][42][43][55,59,60,61]. In women, breast cancer tumors are classified into five molecular subtypes: luminal A, luminal B HER-2—(epidermal growth factor 2 negative), luminal B HER-2+ (epidermal growth factor 2 positive), HER-2 and triple-negative. This allows the selection of a specific targeted therapy, such as anti-estrogen drugs for the luminal A subtype, and monoclonal antibody-based immunotherapy like trastuzumab for HER-2 subtypes [44][62]. HER-2 is also considered an important tumor marker and is expressed in 30–35% of the canine mammary tumors [20][34][33,52]. In canine mammary cancer, multiple studies have been conducted using the same panel of markers; however, the results obtained so far, especially regarding incidence, have been highly variable and sometimes contradictory [45][46][63,64].2.3. Carcinogenesis
The tumor microenvironment is composed of the extracellular matrix (ECM), cancer stem cells (CSC), adipocytes, nerves, tumor-associated stromal cells as fibroblast and endothelial cells, infiltrating immune cells like leukocyte and macrophages and their biological products as cytokines, growth factors and molecules that contribute to tumor progression [47][48][75,76]. The extracellular matrix includes proteins that serve as a support to the tumor cells and facilitate cell–cell or cell–matrix interactions [49][50][77,78]. During the development of canine mammary tumors, the ECM suffers intense remodeling and degradation of its components and structure [50][51][78,79]. In canine mammary cancer, collagen fiber types I, III, IV and V are sparse and fibers ECM disorganized. In addition, collagen fibers are more aligned and shorter than normal tissue, which also correlates with shorter survival rates [49][50][51][77,78,79]. Cancer stem cells are subpopulations of tumor cells that are mainly characterized by their capacity for self-renewal and potential for differentiation and play an important role in cancer recurrence and metastasis [47][52][75,80]. Targeting cancer stem cells is used for the development of new treatments for cancer. Metastasis prognostic factors and cancer stem cell-related transcription factors that can be used to select therapeutic strategies have been identified in canine mammary tumors; these include ICAM-1, PRR14, Oct4 and Sox2 [53][81]. Another element that participates in the process of carcinogenesis is cancer-associated fibroblasts (CAFs). These cells are part of the stroma and participate in the epithelial-mesenchymal transition, secrete cytokines such as epidermal growth factor and transforming growth factor β and produce metalloproteinases that promote growth and tumor progression, invasiveness and metastasis [54][55][56][82,83,84]. In canine mammary cancer, there is an increased expression of periostin in CAFs compared to mammary adenomas, and this has a positive correlation with the histological malignancy grade [57][85]. The immune system plays an important dual role in cancer. It has the capacity to promote carcinogenesis but can also suppress tumor progression, depending on the subtypes of inflammatory cells, mostly lymphocytes and macrophages in the tumor microenvironment, e.g., T lymphocytes (T helper and T-FoxP3+) and macrophages subtype M2, which are in favor of tumor progression [48][58][59][76,86,87]. The inflammatory cells that are found in mammary tumors produce molecules, chemokines and cytokines that have proangiogenic and immunosuppressor activity. Female dogs with malignant mammary tumors that have a high level of inflammatory infiltrate, CD3+ T cells, CD4+ T cells or tumor-infiltrated macrophages have presented shorter survival times [60][88]. In any cell, a genetic or metabolic alteration can lead to a malignant transformation, but this is usually prevented by several molecular mechanisms that activate apoptosis. Under specific physiological conditions, DNA damage, alterations in DNA replication, poor regulation of the cell cycle, hypoxia or the accumulation of misfolded proteins, can all trigger pro-apoptotic pathways and/or anti-apoptotic suppression pathways. In cancer cells, these protective mechanisms are impaired. One of the best-described activators of apoptosis is tumor suppressor gene p53, also known as the genome guardian. In women, p53 gene mutations have been reported in up to 30% of breast cancer cases and are generally associated with the most aggressive subtypes (e.g., triple-negative); high expression of p53 correlates with poor prognosis and shorter survival times [61][91]. Only a few studies have assessed p53 expression status in canine mammary cancer, and its role in progression is still unclear. In one study of 170 malignant mammary tumors in female dogs, only 0.5% (8/170) expressed p53. Tumors positive for p53 were high-grade and with high proliferative activity, suggesting that the p53 gene is involved in the progression of canine mammary cancer [62][92]. However, in another smaller study (40 tumor samples), a significant reduction in gene expression in eight samples, overexpression in two samples and normal expression in thirty samples was reported; a statistical analysis found no correlation between TP53 gene expression and tumor aggressiveness [63][93]. As mentioned previously, sex hormones participate in the initiation, promotion and progression of carcinogenesis of mammary tumors. Estrogen is mainly synthetized by the ovaries; however, it has been detected in high concentrations, along with some of its precursors, in malignant mammary tissue [64][96]. The exposure duration of mammary tissue to estrogens is key to tumor development. Benign mammary tumors and low-grade malignant tumors are usually ERα (estrogen receptor alpha) positive, while high-grade malignant tumors tend to be ERα negative by histology [62][65][67,92]. The ER1 (estrogen receptor 1) gene has a similar pattern of expression, as it is not expressed in high-grade carcinomas. Estrogen modulates gene expression and directly affects the phosphorylation (activation) of several protein kinases. As a result of these genomic and non-genomic pathways, estrogen can accelerate cell proliferation, which in turn increases the chances of acquiring new genetic errors [26][38]. HER-2 overexpression has been associated with poor prognosis, and HER-2 has functions in the regulation of tumor growth and cell differentiation and constitutes a marker for targeted treatment [66][97]. In women with breast cancer, HER-2 has been identified in 30% of the cases. In dogs, a positive correlation has been described between HER-2 expression, malignancy and high histological grade, suggesting a role in canine mammary carcinogenesis [67][98]. Prostaglandins (PG) are lipidic mediators involved in tumorigenic processes mainly controlled by a cyclooxygenase enzyme. PG can modulate the immune system and affect proliferative processes, apoptosis and angiogenesis [68][99]. Cyclooxygenases (Cox1, Cox2 and Cox3) are catalytic enzymes that are necessary for the conversion of arachidonic acid to prostaglandin G2 and subsequently to PGH2, a precursor of prostanoids (prostacyclins and thromboxanes). Isoenzyme Cox2 increases during inflammation and is implicated in the development and progression of different types of tumors, including canine mammary tumors [69][70][100,101]. Cox2 expression was associated with lymph node metastasis at the time of surgery and with the development of distant metastasis. It is also more frequent and intense in malignant (compared to benign) mammary tumors, has been reported in 56–100% of the malignant cells and is correlated with a shorter survival [70][71][72][101,102,103]. Cox2 modulates tumor progression through different mechanisms. Genetic alterations are a part of mammary tumor development. The proto-oncogene epidermal growth factor receptor (EGFR) plays an important role in human breast cancer as expression of its phosphorylated form is associated with increased angiogenesis and metastasis [73][110]. In malignant canine mammary carcinomas, overexpression of EGFR is associated with tumor size, necrosis, mitotic grade, histological grade of malignancy, tumor relapse, distant metastasis and clinical stage. Other common genetic alterations found in canine mammary cancer are mutations in the genes encoding proteins of the PI3K/Akt/mTOR pathway. The PI3K/Akt/mTOR pathway is necessary for the regulation of proliferation, protein synthesis, apoptosis, cell motility and angiogenesis and is dysregulated in several canine mammary tumors [39][74][57,114]. Mutations in the PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), PTEN (phosphatase and tensin homolog), PIK3R1 (phosphoinositide-3-kinase regulatory subunit 1) and AKT1 (serine/threonine kinase 1) genes have been identified in canine mammary cancers at comparable frequencies to human breast cancers, indicating that they may be conserved across species [39][57]. The canine PIK3CA gene mutated in 55% and 38% of benign and malignant mammary tumors, respectively, encodes for a 1068 amino acid protein that shares 99% similarity with its human counterpart. Therefore, it is highly likely that a predisposing functional mutation of PIK3CA is comparable between humans and dogs [75][115]. Mutations in PIK3CA could over-activate this signaling pathway, promoting tumorigenesis [76][116].2.4. Clinical Signs
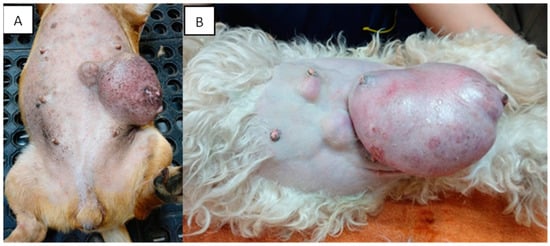
Mammary tumors are usually firm, well-defined nodules and their size can vary from millimeters to centimeters. They can occur in multiple glands at the same time and be of different histological types and grades. In addition, multiple tumors can coexist in the same mammary gland. The caudal abdominal glands are more frequently affected (up to 60% of cases) than the thoracic glands [77][130]. The skin in the affected area can be ulcerated or traumatized, as shown in Figure 2. Evaluation and palpation of regional lymph nodes are mandatory during diagnosis.
Figure 2. Canines with multiple mammary tumors localized in different glands. Tumor measurements larger than 5 cm in diameter with inflammation (A) and ulcerated skin (A,B) can be seen (own photo).
Most canines with mammary tumors are clinically healthy at the time of diagnosis [77][130]. However, patients with metastasis can present fatigue, lethargy, weight loss, dyspnea, cough, edema or lameness. Clinical signs depend on the extension and localization of metastasis [21][34][24,52]. Approximately 50% of mammary carcinomas metastasize to regional lymph nodes. Lymph node involvement is variable and can promote distant metastasis, most frequently to the lung (see Figure 3); metastatic bone lesions may also occur [21][34][78][24,52,131].
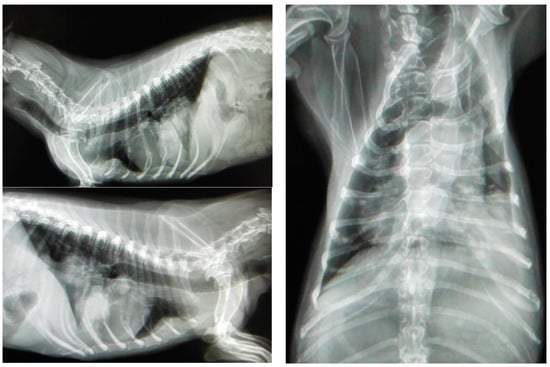

Figure 3. Three projections of thoracic radiographs, right and left lateral and ventrodorsal, with nodular interstitial pattern in a 12-year-old Dachshund patient with metastatic mammary carcinoma (own photo).
