
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhi-wei Li | -- | 6621 | 2023-10-30 09:34:13 | | | |
| 2 | Lindsay Dong | + 13 word(s) | 6634 | 2023-11-01 01:22:05 | | | | |
| 3 | Lindsay Dong | Meta information modification | 6634 | 2023-11-01 01:23:25 | | | | |
| 4 | Lindsay Dong | + 4 word(s) | 6638 | 2023-11-13 04:30:23 | | |
Video Upload Options
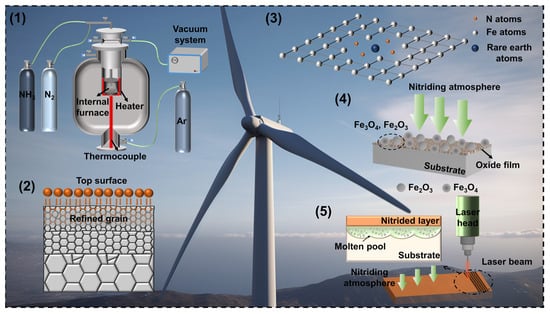
Gas nitriding, as a surface modification technology to improve the wear resistance of workpiece surfaces, is widely used in wind turbine gears, pressure vessel gears, high-precision die casting abrasives, and other areas. However, the gas nitriding time is too long, reaching 40–60 h, which reduces the efficiency of nitriding and hinders the development of gas nitriding. Therefore, various accelerating methods are born accordingly. There are five common accelerating methods are summarized: process parameter optimization, surface mechanical nano-crystallization, surface-active catalysis, surface pre-oxidation, and surface laser treatment.
1. Introduction
Gas nitriding is a chemical heat treatment process which makes nitrogen atoms penetrate into the surface of the workpiece at a certain temperature and in a certain medium [1][2]. It generally includes three processes [3][4][5]: generation of active nitrogen atoms, surface absorption, and diffusion of nitrogen atoms. The surface of the workpiece after nitriding treatment usually has the characteristics of high hardness [6], good wear resistance [7], high fatigue strength [8], and excellent corrosion resistance [1]. However, the traditional nitriding temperature is high and the nitriding time is long, which not only wastes energy and increases the manufacturing cost of the workpiece but also causes some performance reduction in the workpiece. For example, if the nitriding temperature of stainless steel is too high, it will cause a lack of chromium in the substrate and the degradation of corrosion resistance [9][10][11][12]. For aluminum alloy workpieces, if the nitriding temperature is too high, the matrix structure will change significantly, which will lead to poor inherent properties [13][14]. In the nitriding process, the temperature affects the nitriding speed, the decomposition efficiency of the nitriding medium, and the structure of the nitrided layer [15][16]. CrMo steel is usually used to manufacture parts with high strength and high-temperature operation, such as boilers, pressure vessels, steam turbine components, etc. Gas nitriding can improve the hardness and wear resistance of CrMo steel, making it more suitable for use in high-stress and high-temperature environments. It can also improve the corrosion resistance and prolong the life of the parts. Nickel-based alloys are widely used in high-temperature and corrosive environments, such as the aerospace, petrochemical, and energy industries. Gas nitriding can improve the surface hardness and wear resistance of nickel-based alloys and improve their performance in high-temperature and corrosive environments. This helps to extend the service life of alloy parts. Cobalt-based alloys are commonly used in the manufacture of high-temperature, high-strength, and corrosion-resistant parts, such as aero-engine parts and chemical equipment. Gas nitriding can increase the hardness and wear resistance of cobalt-based alloys and improve their stability in high-temperature and corrosive environments. This is very important for extending the life of alloy parts. Cemented carbides are commonly used for cutting, drilling, grinding, and other cutting tools as well as wear-resistant parts, such as mechanical seals and bearings. Gas nitriding can increase the surface hardness and wear resistance of cemented carbide tools, thereby prolonging their service life. This can improve the performance of cutting tools and reduce downtime. The application of gas nitriding on different materials has a common goal, that is, to improve the hardness, wear resistance, and corrosion resistance of the material so as to increase the life and performance of the parts. However, the specific effects may vary depending on the type of material and the environment in which it is used. Therefore, when choosing gas nitriding as a surface treatment method, it is necessary to adjust the nitriding parameters and processes according to the specific application requirements.
2. Research Progress of Conventional Gas Nitriding
2.1. Mechanism of Gas Nitriding
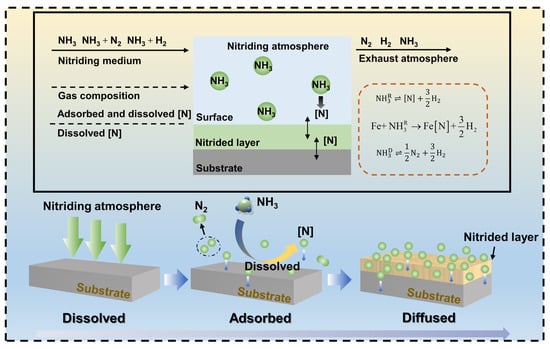
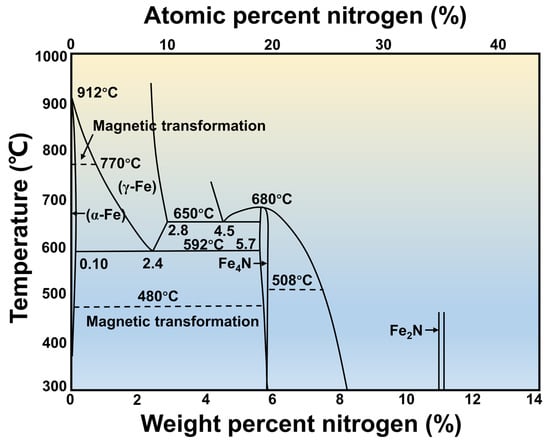
2.2. Nitrided Layer Structure of Gas Nitriding
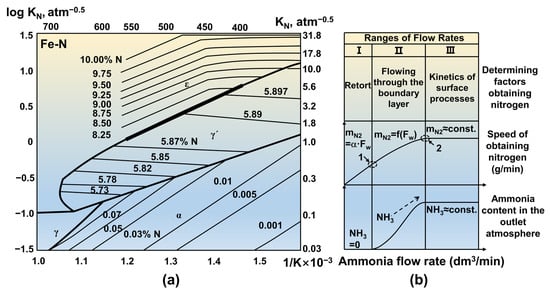
2.3. Process Parameters of Gas Nitriding
3. Effect of Accelerating Nitriding Methods on the Behavior and Efficiency of Gas Nitriding
3.1. Process Parameter Optimization
3.1.1. Accelerating Nitriding Mechanism of Optimizing Process Parameters
3.1.2. Effect of Process Parameter Optimization on Nitriding Behavior
Effect of Pressure on Nitriding Behavior
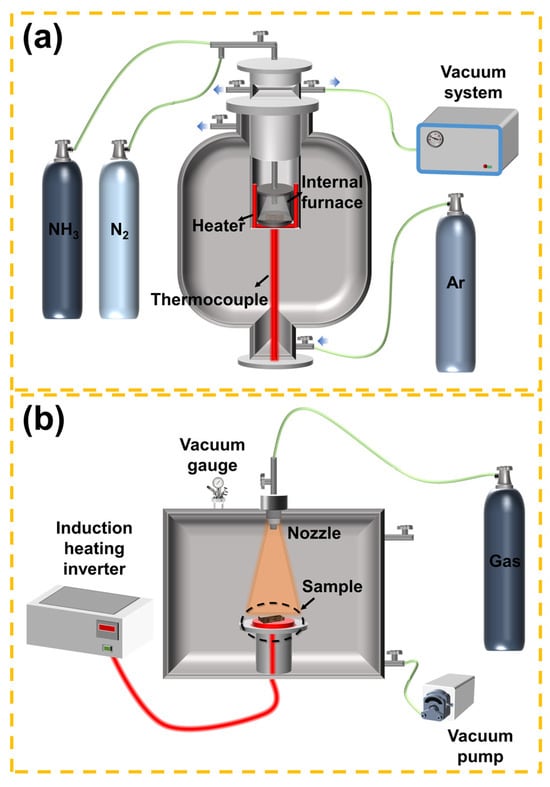
3.1.3. Effect of Process Parameter Optimization on Nitriding Efficiency
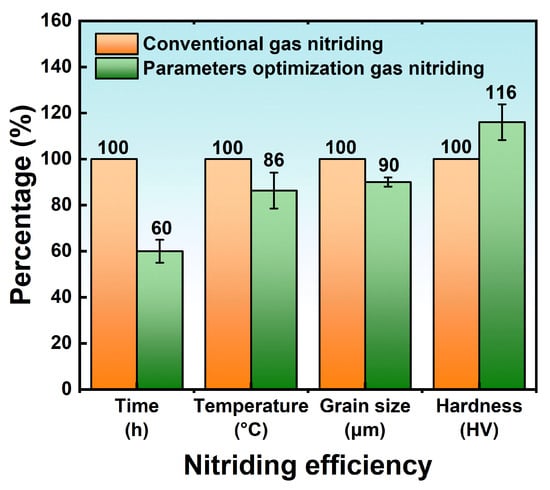
3.2. Surface Mechanical Nano-Crystallization
3.2.1. Accelerating the Nitriding Mechanism of Surface Mechanical Nano-Crystallization
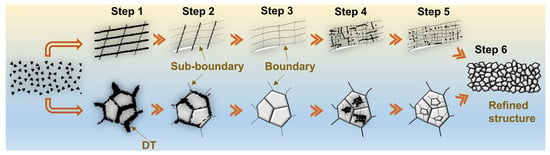
3.2.2. Effect of Surface Mechanical Nano-Crystallization on Nitriding Behavior
3.2.3. Effect of Surface Mechanical Nano-Crystallization on Nitriding Efficiency
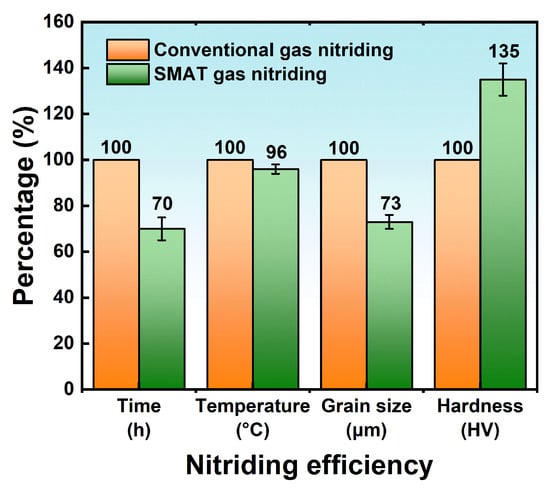
3.3. Surface-Active Catalytic Nitriding
3.3.1. Accelerating Nitriding Mechanism of Surface-Active Catalytic Nitriding
3.3.2. Effect of Surface-Active Catalytic on Nitriding Behavior
Effect of Alloying Elements (Ni, C, Ti, B, etc.) on Nitriding Behavior
3.3.3. Effect of Surface-Active Catalytic Nitriding on Nitriding Efficiency
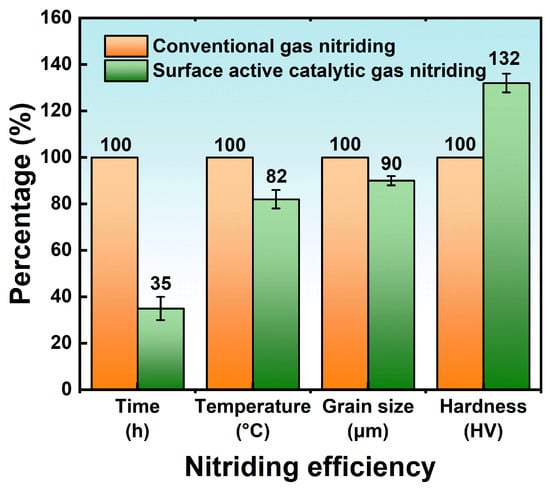
3.4. Surface Pre-Oxidized Nitriding
3.4.1. Accelerating Nitriding Mechanism of Surface Pre-Oxidized Nitriding
3.4.2. Effect of Surface Pre-Oxidation on Nitriding Behavior
3.4.3. Effect of Surface Pre-Oxidation on Nitriding Efficiency
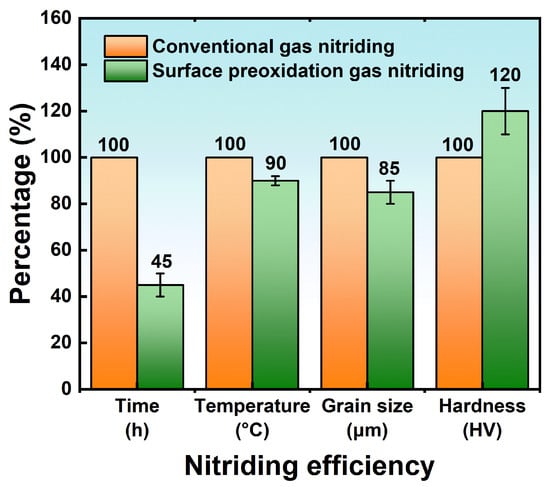
3.5. Surface Laser Treatment
3.5.1. Accelerating Nitriding Mechanism of Surface Laser Treatment
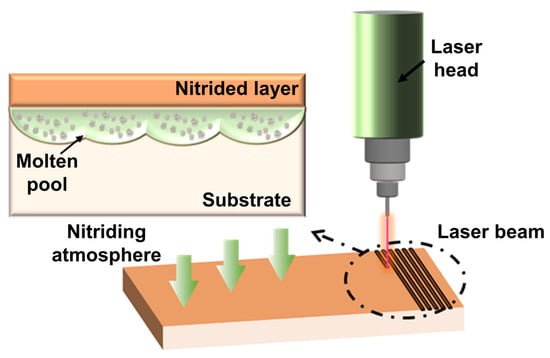
3.5.2. Effect of Surface Laser Treatment on Nitriding Behavior
3.5.3. Effect of Surface Laser Treatment on Nitriding Efficiency
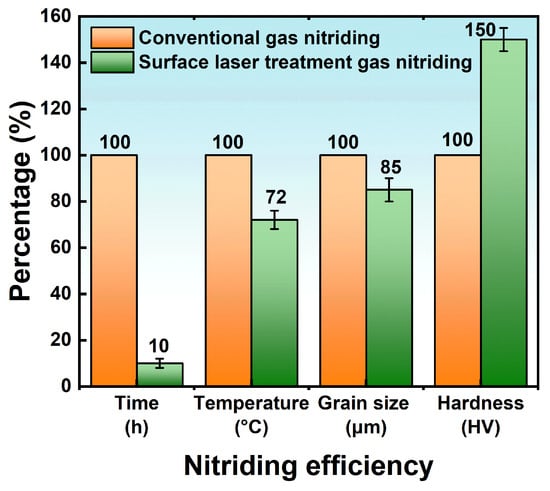
4. Conclusions
References
- Michalski, J.; Wach, P.; Tacikowski, J.; Betiuk, M.; Burdynski, K.; Kowalski, S.; Nakonieczny, A. Contemporary industrial application of nitriding and its modifications. Mater. Manuf. Process. 2009, 24, 855–858.
- Almeida, E.A.S.; Costa, C.E.; Milan, J.C.G. Study of the nitrided layer obtained by different nitriding methods. Matéria 2015, 20, 460–465.
- Tong, W.P.; Liu, C.Z.; Wang, W.; Tao, N.R.; Wang, Z.B.; Zuo, L.; He, J.C. Gaseous nitriding of iron with a nanostructured surface layer. Scr. Mater. 2007, 57, 533–536.
- Wang, B.; Fu, W.T.; Dong, F.; Jin, G.F.; Feng, W.W.; Wang, Z.H.; Sun, S.H. Significant acceleration of nitriding kinetics in pure iron by pressurized gas treatment. Mater. Des. 2015, 85, 91–96.
- Keddam, M.; Djeghlal, M.E.; Barrallier, L. A diffusion model for simulation of bilayer growth (ε/γ′) of nitrided pure iron. Mater. Sci. Eng. 2004, 378, 475–478.
- Alekseeva, M.S.; Gress, M.A.; Scherbakov, S.P.; Gerasimov, S.A.; Kuksenova, L.I. The influence of high-pressure gas nitriding on the properties of martensitic steels. Met. Sci. Heat Treat. 2017, 59, 524–528.
- Singh, S.K.; Naveen, C.; Sai, Y.V.; Satish, U.; Bandhavi, C.; Subbiah, R. Experimental study on wear resistance of AISI 347 treated with salt bath nitriding and gas nitriding processes-a review. Mater. Today Proc. 2019, 18, 2717–2722.
- Kim, Y.H.; Kim, H.G. Effect of Gas Nitriding Characteristics on the Mechanical Properties after Pre-Heat Treatment of Stainless Steels. J. Korean Soc. Heat Treat. 2010, 23, 142–149.
- Boztepe, E.; Alves, A.C.; Ariza, E.; Rocha, L.A.; Cansever, N.; Toptan, F. A comparative investigation of the corrosion and tribocorrosion behaviour of nitrocarburized, gas nitrided, fluidized-bed nitrided, and plasma nitrided plastic mould steel. Surf. Coat. Technol. 2018, 334, 116–123.
- Ichii, K. Structure of the ion-nitrided layer of 18-8 stainless steel. Technol. Rep. Kansai Univ. 1986, 27, 135.
- Kundalkar, D.; Mavalankar, M.; Tewari, A. Effect of gas nitriding on the thermal fatigue behavior of martensitic chromium hot-work tool steel. Mater. Sci. Eng. 2016, 651, 391–398.
- Pellizzari, M.; Molinari, A.; Straffelini, G. Thermal fatigue resistance of gas and plasma nitrided 41CrAlMo7 steel. Mater. Sci. Eng. 2003, 352, 186–194.
- Akhtar, S.S.; Arif, A.F.M.; Yilbas, B.S. Evaluation of gas nitriding process with in-process variation of nitriding potential for AISI H13 tool steel. Int. J. Adv. Manuf. Technol. 2010, 47, 687–698.
- Moradshahi, M.; Tavakoli, T.; Amiri, S.; Shayeganmehr, S. Plasma nitriding of Al alloys by DC glow discharge. Surf. Coat. Technol. 2006, 201, 567–574.
- Funch, C.V.; Christiansen, T.L.; Somers, M.A.J. Gaseous nitriding of additively manufactured maraging steel; nitriding kinetics and microstructure evolution. Surf. Coat. Technol. 2022, 432, 128055.
- Li, G.M.; Liang, Y.L.; Sun, H.; Cao, Y.G. Nitriding behavior and mechanical properties of carburizing and nitriding duplex treated M50NiL steel. Surf. Coat. Technol. 2020, 384, 125315.
- Baranowska, J.; Arnold, B. Corrosion resistance of nitrided layers on austenitic steel. Surf. Coat. Technol. 2006, 200, 6623–6628.
- Somers, M.A.J.; Mittemeijer, E.J. Layer-growth kinetics on gaseous nitriding of pure iron: Evaluation of diffusion coefficients for nitrogen in iron nitrides. Metall. Mater. Trans. A 1995, 26, 57–74.
- Yang, S.; Yang, D.; Shi, W.; Deng, C.; Chen, C.; Feng, S. Global evaluation of carbon neutrality and peak carbon dioxide emissions: Current challenges and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 81725–81744.
- Karakan, M.; Alsaran, A.; Celik, A. Effect of process time on structural and tribological properties of ferritic plasma nitrocarburized AISI 4140 steel. Mater. Des. 2004, 25, 349–353.
- Michalski, J.; Tacikowski, J.; Wach, P.; Ratajski, J. Controlled gas nitriding of 40HM and 38HMJ steel grades with the formation of nitrided cases with and without the surface compound layer, composed of iron nitrides. Probl. Eksploat. 2006, 2, 43–52.
- Fattah, M.; Mahboubi, F. Comparison of ferritic and austenitic plasma nitriding and nitrocarburizing behavior of AISI 4140 low alloy steel. Mater. Des. 2010, 31, 3915–3921.
- Kumar, S.A.; Raman, S.G.S.; Narayanan, T.S.N.S.; Gnanamoorthy, R. Influence of counterbody material on fretting wear behaviour of surface mechanical attrition treated Ti–6Al–4V. Tribol. Int. 2013, 57, 107–114.
- Liu, B.; Wang, B.; Yang, X.D.; Zhao, X.F.; Qin, M.; Gu, J.F. Thermal fatigue evaluation of AISI H13 steels surface modified by gas nitriding with pre-and post-shot peening. Appl. Surf. Sci. 2019, 483, 45–51.
- Liu, J.; Suslov, S.; Vellore, A.; Ren, Z.C.; Amanov, A.; Pyun, Y.S.; Martini, A.; Dong, Y.L.; Ye, C. Surface nanocrystallization by ultrasonic nano-crystal surface modification and its effect on gas nitriding of Ti6Al4V alloy. Mater. Sci. Eng. 2018, 736, 335–343.
- Rodrigues, J.; Miranda, S.M.C.; Santos, N.F.; Neves, A.J.; Alves, E.; Lorenz, K.; Monteiro, T. Rare earth co-doping nitride layers for visible light. Mater. Chem. Phys. 2012, 134, 716–720.
- Zhang, C.; Wang, Y.; Chen, X.; Chen, H.T.; Wu, Y.Q.; Wang, Y.X.; Tang, L.N.; Cui, G.D.; Chen, D.Z. Catalytic behavior of LaFeO3 pervoskite oxide during low-pressure gas nitriding. Appl. Surf. Sci. 2020, 506, 145045.
- Zhang, C.S.; Yan, M.F.; Sun, Z. Experimental and theoretical study on interaction between lanthanum and nitrogen during plasma rare earth nitriding. Appl. Surf. Sci. 2013, 287, 381–388.
- Sueyoshi, H.; Hamaishi, K.; Kadomatsu, S.; Shiomizu, T.; Ohzono, Y. Effect of preheating in air on gas nitriding of SUS304. Nippon Kinzoku Gakkaishi (1952) 1996, 60, 616–623.
- Lutz, J.; Mändl, S. Effect of ion energy and chemistry on layer growth processes during nitriding of CoCr alloys. Nucl. Instrum. Methods Phys. Res. Sect. B 2009, 267, 1522–1525.
- Li, S.X.; Chen, L.; Wang, M.T.; Yan, Z.Q. Effect of pre-oxidation and rare earth cerium on plasma nitriding of 42CrMo steel. Heat Treat. Met. 2021, 46, 186–189.
- Schaaf, P. Laser nitriding of metals. Prog. Mater. Sci. 2002, 47, 1–161.
- Chen, X.K.; Wu, G.; Wang, R.; Guo, W.T.; Yang, J.P.; Cao, S.Z.; Wang, Y.L.; Han, W.H. Laser nitriding of titanium alloy in the atmosphere environment. Surf. Coat. Technol. 2007, 201, 4843–4846.
- Razavi, R.S.; Gordani, G.R.; Man, H.C. A review of the corrosion of laser nitrided Ti-6Al-4V. Anti-Corros. Methods Mater. 2011, 58, 140–154.
- Fang, B.Z.; Daniel, L.; Bonakdarpour, A.; Wilkinson, D.P. Upgrading the State-of-the-Art Electrocatalysts for Proton Exchange Membrane Fuel Cell Applications. Adv. Mater. Interfaces 2022, 9, 2200349.
- Zhao, M.M.; Huang, X.L.; Zhuang, D.M.; Sheng, L.; Xie, X.; Cao, M.; Pan, J.J.; Fan, H.Y.; He, J.P. Constructing porous nanosphere structure current collector by nitriding for lithium metal batteries. J. Energy Storage 2022, 47, 103665.
- Arabczyk, W.; Skulmowska, K.; Pelka, R.; Lendzion-Bielun, Z. Oscillatory Mechanism of α-Fe (N)↔ γ’-Fe4N Phase Transformations during Nanocrystalline Iron Nitriding. Materials 2022, 15, 1006.
- Sun, J.Q.; Wang, D.R.; Yang, J.; Li, F.J.; Zuo, L.L.; Ge, F.; Chen, Y.B. In Situ Preparation of Nano-Cu/Microalloyed Gradient Coating with Improved Antifriction Properties. Coatings 2022, 12, 1336.
- Wang, Y.; Lu, S.; Zheng, J.; Liang, L. Advances in Latest Application Status, Challenges, and Future Development Direction of Electrospinning Technology in the Biomedical. J. Nanomater. 2022, 2022, 3791908.
- Xu, W.C.; Cui, Z.D.; Zhu, S.L. Recent Advances in Open-Cell Porous Metal Materials for Electrocatalytic and Biomedical Applications. Acta Metall. Sin. 2022, 58, 1527–1544.
- Bell, T. Source Book on Nitriding; American Society for Metals: Metals Park, OH, USA, 1977.
- García Caballero, F.; Wang Fu, M.; Gao, M.C. Encyclopedia of Materials: Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2022.
- Chen, W.L.; Wu, C.L.; Liu, Z.R.; Ni, S.; Hong, Y.; Zhang, Y.; Chen, J.H. Phase transformations in the nitrocarburizing surface of carbon steels revisited by microstructure and property characterizations. Acta Mater. 2013, 61, 3963–3972.
- Wu, C.L.; Tian, L.; Hong, Y.; Wang, J.; Chen, X.Y. The effect of cooling methods and subsequent ageing on the nitrided layer of pure-iron by gas nitriding at 580 °C. J. Hunan Univ. 2015, 42, 33–39.
- Wu, C.L.; Luo, C.P.; Zou, G.F. Phase Transformations During Composite-Chromization of steel 20. Acta Metall. Sin. 2004, 40, 1074–1078.
- Dossett, J.L.; Totten, G.E. Introduction to steel heat treatment. Steel Heat Treat. Fundam. Process. 2013, 4, 3–25.
- Bell, T.; Mao, K.; Sun, Y. Surface engineering design: Modelling surface engineering systems for improved tribological performance. Surf. Coat. Technol. 1998, 108, 360–368.
- Gao, H.; Lysevych, M.; Tan, H.H.; Jagadish, C.; Zou, J. The effect of Sn addition on GaAs nanowire grown by vapor–liquid–solid growth mechanism. Nanotechnology 2018, 29, 465601.
- Wang, B.; Sun, S.H.; Guo, M.W.; Jin, G.F.; Zhou, Z.A.; Fu, W.T. Study on pressurized gas nitriding characteristics for steel 38CrMoAlA. Surf. Coat. Technol. 2015, 279, 60–64.
- Fare, S.; Lecis, N.; Brescia, E.; Mazzola, M. Role of grain boundaries in diffusional phenomena during gas nitriding of pure iron. Procedia Eng. 2011, 10, 2943–2948.
- Zhao, H.; Duan, L.; Chen, G.; Fan, H.Y.; Wang, J.; Zhou, C.C. High corrosion resistance performance of 304 stainless steel after liquid nitrocarburization. Compos. Part B 2018, 155, 173–177.
- Mirjani, M.; Mazrooei, J.; Karimzadeh, N.; Ashrafizadeh, F. Investigation of the effects of time and temperature of oxidation on corrosion behavior of plasma nitrided AISI 4140 steel. Surf. Coat. Technol. 2012, 206, 4389–4393.
- Li, Y.; Wang, L.; Zhang, D.D.; Shen, L. The effect of surface nanocrystallization on plasma nitriding behaviour of AISI 4140 steel. Appl. Surf. Sci. 2010, 257, 979–984.
- Miyamoto, J.; Abraha, P. The effect of plasma nitriding treatment time on the tribological properties of the AISI H13 tool steel. Surf. Coat. Technol. 2019, 375, 15–21.
- Wang, J.; Lin, Y.H.; Yan, J.; Zen, D.Z.; Zhang, Q.A.; Huang, R.B.; Fan, H.Y. Influence of time on the microstructure of AISI 321 austenitic stainless steel in salt bath nitriding. Surf. Coat. Technol. 2012, 206, 3399–3404.
- Arabczyk, W.; Zamlynny, J. Study of the ammonia decomposition over iron catalysts. Catal. Lett. 1999, 60, 167–171.
- Wolowiec-Korecka, E.; Michalski, J.; Kucharska, B. Kinetic aspects of low-pressure nitriding process. Vacuum 2018, 155, 292–299.
- Michalski, J.; Wołowiec-Korecka, E. A study of parameters of nitriding processes. Part 1. Met. Sci. Heat Treat. 2019, 61, 183–190.
- Mitsui, H.; Kurihana, S. Solution nitriding treatment of Fe–Cr alloys under pressurized nitrogen gas. ISIJ Int. 2007, 47, 479–485.
- Lehrer, E. Über das Eisen-Wasserstoff-Ammoniak-Gleichgewicht. Z. Elektrochem. Angew. Phys. Chem. 1930, 36, 383–392.
- Michalski, J.; Tacikowski, J.; Wach, P.; Lunarska, E.; Baum, H. Formation of single-phase layer of γ′-nitride in controlled gas nitriding. Met. Sci. Heat Treat. 2005, 47, 516–519.
- Nakonieczny, A.; Senatorski, J.; Tacikowski, J.; Tymowski, G.; Liliental, W. Computer Controlled Gas Nitriding—A Viable Replacement for Carburising. Heat Treat. Met. 1998, 3, 46–51.
- Michalski, J. Characteristics and Calculations of Atmospheres for Controlled Gas Nitriding of Steel; Institute of Precision Mechanics: Warsaw, Poland, 2010.
- Takesue, S.; Kikuchi, S.; Misaka, Y.; Morita, T.; Komotori, J. Rapid nitriding mechanism of titanium alloy by gas blow induction heating. Surf. Coat. Technol. 2020, 399, 126160.
- Abrasonis, G.; Rivière, J.P.; Templier, C.; Muzard, S.; Pranevicius, L. Influence of surface preparation and ion flux on the nitriding efficiency of austenitic stainless steel. Surf. Coat. Technol. 2005, 196, 279–283.
- Baranowska, J. Importance of surface activation for nitrided layer formation on austenitic stainless steel. Surf. Eng. 2010, 26, 293–298.
- Priest, J.M.; Baldwin, M.J.; Fewell, M.P. The action of hydrogen in low-pressure rf-plasma nitriding. Surf. Coat. Technol. 2001, 145, 152–163.
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Lu, J.; Lu, K. Nitriding iron at lower temperatures. Science 2003, 299, 686–688.
- Lu, K.; Lu, J. Surface nanocrystallization (SNC) of metallic materials-presentation of the concept behind a new approach. J. Mater. Sci. Technol. 1999, 15, 193–197.
- Hu, G.; Sheng, G.M.; Han, J. Investigation and Application of Surface Self Nano-crystallization Induced by Severe Plastic Deformation. Mater. Rep. 2007, 21, 117–121.
- Azadmanjiri, J.; Berndt, C.C.; Kapoor, A.; Wen, C. Development of surface nano-crystallization in alloys by surface mechanical attrition treatment (SMAT). Crit. Rev. Solid State Mater. Sci. 2015, 40, 164–181.
- Bahl, S.; Suwas, S.; Ungar, T.; Chatterjee, K. Elucidating microstructural evolution and strengthening mechanisms in nanocrystalline surface induced by surface mechanical attrition treatment of stainless steel. Acta Mater. 2017, 122, 138–151.
- Jentzsch, W.D.; Böhmer, S. Investigations on nitride layer formation at the iron surface during gas nitriding. Krist. Tech. 1979, 14, 617–624.
- Tong, W.P.; He, C.S.; He, J.C.; Zuo, L.; Tao, N.; Wang, Z. Strongly enhanced nitriding kinetics by means of grain refinement. Appl. Phys. Lett. 2006, 89, 021918.
- Sun, J.; Tong, W.P.; Zhang, H.; Du, X.D.; Wu, Y.C. Enhanced strength and plasticity of gas nitrided iron by surface mechanical attrition pretreatment. Surf. Coat. Technol. 2016, 286, 279–284.
- Sun, J.; Tong, W.P.; Zhang, H.; Zuo, L.; Wang, Z.B. Evaluation of surface-modified 20CrMo by plasma nitriding coupled with ion sputtering and SMAT. Surf. Coat. Technol. 2012, 213, 247–252.
- Tong, W.P.; Han, Z.; Wang, L.M.; Lu, J.; Lu, K. Low-temperature nitriding of 38CrMoAl steel with a nanostructured surface layer induced by surface mechanical attrition treatment. Surf. Coat. Technol. 2008, 202, 4957–4963.
- Balusamy, T.; Narayanan, T.S.N.S.; Ravichandran, K.; Park, I.S.; Lee, M.H. Plasma nitriding of AISI 304 stainless steel: Role of surface mechanical attrition treatment. Mater. Charact. 2013, 85, 38–47.
- Lin, Y.M.; Lu, J.; Wang, L.P.; Xu, T.; Xue, Q.J. Surface nanocrystallization by surface mechanical attrition treatment and its effect on structure and properties of plasma nitrided AISI 321 stainless steel. Acta Mater. 2006, 54, 5599–5605.
- Zhang, H.; Qin, H.F.; Ren, Z.C.; Zhao, J.Y.; Hou, X.N.; Doll, G.L.; Dong, Y.L.; Ye, C. Low-temperature nitriding of nanocrystalline Inconel 718 alloy. Surf. Coat. Technol. 2017, 330, 10–16.
- Zhang, C.S.; Wang, Y.; Chen, D.Z.; Wu, Y.Q.; Cui, G.D.; Yang, Y.; Wang, Y.X.; Chen, Y.X. Effect of elemental doping on the catalytic activity of ABO3 perovskite oxides during low-pressure gas nitriding. Appl. Surf. Sci. 2021, 542, 148706.
- Aleksandrov, V.A.; Ostaeva, G.Y.; Papisova, A.I.; Papisov, I.M.; Petrova, L.G.; Prikhodko, V.M.; Fatyukhin, D.S. Synthesis of copper–polymer nanocomposite on steel surface and composite-based catalyst for steel nitriding. Colloid J. 2015, 77, 556–560.
- Dossett, J.; Totten, G.E. Fundamentals of nitriding and nitrocarburizing. In ASM Handbook: Steel Heat Treating Fundamentals and Processes; ASM International: Materials Park, OH, USA, 2013; p. 619.
- Podgurski, H.H.; Davis, F.N. Thermochemistry and nature of nitrogen absorption in nitrogenated Fe Ti alloys. Acta Metall. 1981, 29, 1–9.
- Steiner, T.; Mittemeijer, E.J. Alloying element nitride development in ferritic Fe-based materials upon nitriding: A review. J. Mater. Eng. Perform. 2016, 25, 2091–2102.
- Steiner, T.; Meka, S.R.; Bischoff, E.; Waldenmaier, T.; Mittemeijer, E.J. Nitriding of ternary Fe–Cr–Mo alloys; role of the Cr/Mo-ratio. Surf. Coat. Technol. 2016, 291, 21–33.
- Steiner, T.; Meka, S.R.; Rheingans, B.; Bischoff, E.; Waldenmaier, T.; Yeli, G.; Martin, T.L.; Bagot, P.A.J.; Moody, M.P.; Mittemeijer, E.J. Continuous and discontinuous precipitation in Fe-1 at.% Cr-1 at.% Mo alloy upon nitriding; crystal structure and composition of ternary nitrides. Philos. Mag. 2016, 96, 1509–1537.
- Peng, T.T.; Dai, M.Y.; Cai, W.; Wei, W.; Wei, K.X.; Hu, J. The enhancement effect of salt bath preoxidation on salt bath nitriding for AISI 1045 steel. Appl. Surf. Sci. 2019, 484, 610–615.
- Li, J.C.; Sun, F.; Wang, S.K.; Yang, X.M.; Hu, J. Catalysis effect and mechanism of pre-oxidation on direct current plasma nitriding. Trans. Mater. Heat Treat. 2014, 7, 182–186.
- Zhen, J.Z.; Shi, Q.W.; Shi, J.; Liu, J.X. Research Progress of Low Temperature Surface Nitriding Technology for Steel Materials. Hot Work. Technol. 2019, 48, 35–40.
- Liu, H.; Li, J.C.; Sun, F.; Hu, J. Characterization and effect of pre-oxidation on DC plasma nitriding for AISI4140 steel. Vacuum 2014, 109, 170–174.
- Zhang, J.T.; Liu, Z.H.; Sun, J.X.; Zhao, H.L.; Shi, Q.Y.; Ma, D.W. Microstructure and mechanical property of electropulsing tempered ultrafine grained 42CrMo steel. Mater. Sci. Eng. 2020, 782, 139213.
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457.





