
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Somashekar Krishna | -- | 2016 | 2023-10-27 19:23:23 | | | |
| 2 | Lindsay Dong | Meta information modification | 2016 | 2023-10-30 01:55:00 | | |
Video Upload Options
Despite the increasing rate of detection of incidental pancreatic cystic lesions (PCLs), current standard-of-care methods for their diagnosis and risk stratification remain inadequate. Intraductal papillary mucinous neoplasms (IPMNs) are the most prevalent PCLs. The existing modalities, including endoscopic ultrasound and cyst fluid analysis, only achieve accuracy rates of 65–75% in identifying carcinoma or high-grade dysplasia in IPMNs. Furthermore, surgical resection of PCLs reveals that up to half exhibit only low-grade dysplastic changes or benign neoplasms. To reduce unnecessary and high-risk pancreatic surgeries, more precise diagnostic techniques are necessary. A promising approach involves integrating existing data, such as clinical features, cyst morphology, and data from cyst fluid analysis, with confocal endomicroscopy and radiomics to enhance the prediction of advanced neoplasms in PCLs. Artificial intelligence and machine learning modalities can play a crucial role in achieving this goal.
1. Introduction
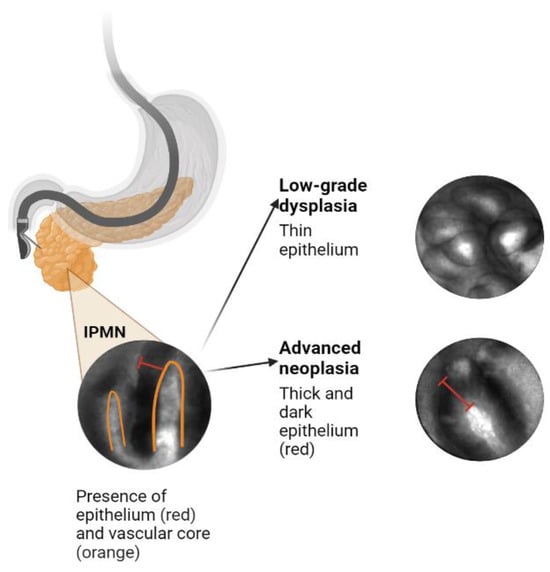
2. Artificial Intelligence Application to Pancreas Imaging
2.1. Utility and Accuracy of EUS-nCLE
2.2. CNN-AI Algorithm for nCLE Analysis
2.3. Improving and Prospectively Evaluating the Single-Center Algorithm
2.4. Creating an Integrative Predictive Algorithm
2.5. Prior Integrative Algorithms
References
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9.
- Ayoub, F.; Davis, A.M.; Chapman, C.G. Pancreatic Cysts-An Overview and Summary of Society Guidelines, 2021. JAMA 2021, 325, 391–392.
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479.
- Kaimakliotis, P.; Riff, B.; Pourmand, K.; Chandrasekhara, V.; Furth, E.E.; Siegelman, E.S.; Drebin, J.; Vollmer, C.M.; Kochman, M.L.; Ginsberg, G.G.; et al. Sendai and Fukuoka consensus guidelines identify advanced neoplasia in patients with suspected mucinous cystic neoplasms of the pancreas. Clin. Gastroenterol. Hepatol. 2015, 13, 1808–1815.
- Sakorafas, G.H.; Sarr, M.G.; van de Velde, C.J.; Peros, G. Intraductal papillary mucinous neoplasms of the pancreas: A surgical perspective. Surg. Oncol. 2005, 14, 155–178.
- Castellano-Megías, V.M.; Andrés, C.I.; López-Alonso, G.; Colina-Ruizdelgado, F. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J. Gastrointest. Oncol. 2014, 6, 311–324.
- Machado, N.O.; Al Qadhi, H.; Al Wahibi, K. Intraductal Papillary Mucinous Neoplasm of Pancreas. N. Am. J. Med. Sci. 2015, 7, 160–175.
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 824–848.e22.
- Marchegiani, G.; Pollini, T.; Andrianello, S.; Tomasoni, G.; Biancotto, M.; Javed, A.A.; Kinny-Köster, B.; Amini, N.; Han, Y.; Kim, H.; et al. Progression vs cyst stability of branch-duct intraductal papillary mucinous neoplasms after observation and surgery. JAMA Surg. 2021, 156, 654–661.
- Crist, D.W.; Sitzmann, J.V.; Cameron, J.L. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann. Surg. 1987, 206, 358–365.
- Chierici, A.; Intotero, M.; Granieri, S.; Paleino, S.; Flocchini, G.; Germini, A.; Cotsoglou, C. Timely synergic surgical and radiological aggressiveness improves perioperative mortality after hemorrhagic complication in Whipple procedure. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 387–390.
- Tanaka, M. Intraductal papillary mucinous neoplasm of the pancreas as the main focus for early detection of pancreatic adenocarcinoma. Pancreas 2018, 47, 544–550.
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753.
- Sharib, J.M.; Fonseca, A.L.; Swords, D.S.; Jaradeh, K.; Bracci, P.M.; Firpo, M.A.; Hatcher, S.; Scaife, C.L.; Wang, H.; Kim, G.E.; et al. Surgical overtreatment of pancreatic intraductal papillary mucinous neoplasms: Do the 2017 International Consensus Guidelines improve clinical decision making? Surgery 2018, 164, 1178–1184.
- Dbouk, M.; Brewer Gutierrez, O.I.; Lennon, A.M.; Chuidian, M.; Shin, E.J.; Kamel, I.R.; Fishman, E.K.; He, J.; Burkhart, R.A.; Wolfgang, C.L.; et al. Guidelines on management of pancreatic cysts detected in high-risk individuals: An evaluation of the 2017 Fukuoka guidelines and the 2020 International Cancer of the Pancreas Screening (CAPS) consortium statements. Pancreatology 2021, 21, 613–621.
- Heckler, M.; Michalski, C.W.; Schaefle, S.; Kaiser, J.; Büchler, M.W.; Hackert, T. The Sendai and Fukuoka consensus criteria for the management of branch duct IPMN-A meta-analysis on their accuracy. Pancreatology 2017, 17, 255–262.
- Yu, S.; Takasu, N.; Watanabe, T.; Fukumoto, T.; Okazaki, S.; Tezuka, K.; Sugawara, S.; Hirai, I.; Kimura, W. Validation of the 2012 Fukuoka Consensus Guideline for Intraductal Papillary Mucinous Neoplasm of the Pancreas From a Single Institution Experience. Pancreas 2017, 46, 936–942.
- Kamboj, A.K.; Modi, R.M.; Swanson, B.; Conwell, D.L.; Krishna, S.G. A comprehensive examination of the novel techniques used for in vivo and ex vivo confocal laser endomicroscopy of pancreatic cystic lesions. VideoGIE 2016, 1, 6–7.
- Nakai, Y.; Iwashita, T.; Park, D.H.; Samarasena, J.B.; Lee, J.G.; Chang, K.J. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest. Endosc. 2015, 81, 1204–1214.
- Machicado, J.D.; Chao, W.L.; Carlyn, D.E.; Pan, T.Y.; Poland, S.; Alexander, V.L.; Maloof, T.G.; Dubay, K.; Ueltschi, O.; Middendorf, D.M.; et al. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest. Endosc. 2021, 94, 78–87.e2.
- Krishna, S.G.; Hart, P.A.; Malli, A.; Kruger, A.J.; McCarthy, S.T.; El-Dika, S.; Walker, J.P.; Dillhoff, M.E.; Manilchuk, A.; Schmidt, C.R.; et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin. Gastroenterol. Hepatol. 2020, 18, 432–440.e6.
- Ruder, S. An overview of multi-task learning in deep neural networks. arXiv 2017, arXiv:1706.05098.
- Zhang, K.; Chao, W.-L.; Sha, F.; Grauman, K. Video summarization with long short-term memory. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 11–14 October 2016; Springer: Berlin/Heidelberg, Germany, 2016.
- Gong, B.; Chao, W.-L.; Grauman, K.; Sha, F. Diverse sequential subset selection for supervised video summarization. Adv. Neural Inf. Process. Syst. 2014, 27, 2069–2077.
- Springer, S.; Wang, Y.; Dal Molin, M.; Masica, D.L.; Jiao, Y.; Kinde, I.; Blackford, A.; Raman, S.P.; Wolfgang, C.L.; Tomita, T.; et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015, 149, 1501–1510.
- Singhi, A.D.; McGrath, K.; E Brand, R.; Khalid, A.; Zeh, H.J.; Chennat, J.S.; E Fasanella, K.; I Papachristou, G.; Slivka, A.; Bartlett, D.L.; et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018, 67, 2131–2141.
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016.
- Li, W.; Yin, Y.; Quan, X.; Zhang, H. Gene expression value prediction based on XGBoost algorithm. Front. Genet. 2019, 10, 1077.
- Zhang, D.; Qian, L.; Mao, B.; Huang, C.; Huang, B.; Si, Y. A data-driven design for fault detection of wind turbines using random forests and XGboost. IEEE Access 2018, 6, 21020–21031.
- Rangwani, S.; Ardeshna, D.R.; Rodgers, B.; Melnychuk, J.; Turner, R.; Culp, S.; Chao, W.-L.; Krishna, S.G. Application of Artificial Intelligence in the Management of Pancreatic Cystic Lesions. Biomimetics 2022, 7, 79.
- Springer, S.; Masica, D.L.; Dal Molin, M.; Douville, C.; Thoburn, C.J.; Afsari, B.; Li, L.; Cohen, J.D.; Thompson, E.; Allen, P.J.; et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci. Transl. Med. 2019, 11, eaav4772.
- Tang, B.; Chen, Y.; Wang, Y.; Nie, J. A Wavelet-Based Learning Model Enhances Molecular Prognosis in Pancreatic Adenocarcinoma. BioMed. Res. Int. 2021, 2021, 7865856.
- He, T.; Puppala, M.; Ezeana, C.F.; Huang, Y.-S.; Chou, P.-H.; Yu, X.; Chen, S.; Wang, L.; Yin, Z.; Danforth, R.L.; et al. A deep learning–based decision support tool for precision risk assessment of breast cancer. JCO Clin. Cancer Inform. 2019, 3, 1–12.
- Cheng, J.; Zhang, J.; Han, Y.; Wang, X.; Ye, X.; Meng, Y.; Parwani, A.; Han, Z.; Feng, Q.; Huang, K. Integrative analysis of histopathological images and genomic data predicts clear cell renal cell carcinoma prognosis. Cancer Res. 2017, 77, e91–e100.
- Li, Y.; Ge, D.; Gu, J.; Xu, F.; Zhu, Q.; Lu, C. A large cohort study identifying a novel prognosis prediction model for lung adenocarcinoma through machine learning strategies. BMC Cancer 2019, 19, 886.




