
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dongbin Ahn | -- | 2545 | 2023-10-25 14:27:49 | | | |
| 2 | Lindsay Dong | Meta information modification | 2545 | 2023-10-26 03:35:03 | | |
Video Upload Options
The thyroglossal duct cyst (TGDC) is the most common congenital neck mass, accounting for 70–75% of all congenital neck masses. Although the Sistrunk operation has been used as a standard of treatment, it is accompanied by a considerable surgical burden, including the need for general anesthesia, a visible surgical scar on the neck surface, and postoperative complications. Ultrasound-guided ethanol ablation (US-EA) is a minimally invasive and office-based technique that is widely used as a non-surgical treatment for several benign cystic lesions, particularly benign thyroid cysts. US-EA has also been gaining popularity as a good alternative for TGDC treatment, which is associated with high feasibility, a high safety profile, and favorable treatment outcomes.
1. Introduction
2. Overall Practice of EA for TGDC
2.1. Pre-Procedural Evaluation
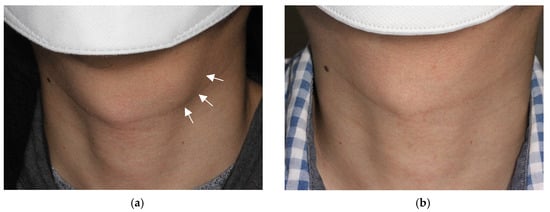
2.2. Aspiration of Internal Contents and Ethanol Injection
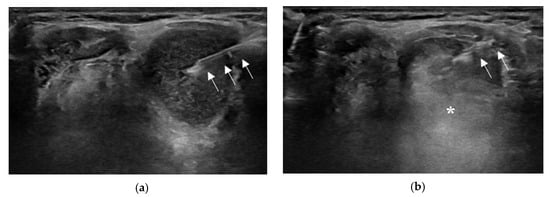
2.3. Technical Issues or Variations in EA for TGDC
2.3.1. Local Anesthesia
2.3.2. Needle Size Used for EA
2.3.3. Amount of Ethanol Injected
2.3.4. Retention or Aspiration of Injected Ethanol
2.3.5. EA for TGDC with Viscous Internal Contents
2.3.6. Number of Treatment Sessions
3. EA Results for TGDC
3.1. Treatment Efficacy
3.2. Outcome-Related Factors
3.3. Complications
3.4. Cost
4. Summary
4.1. Needs for Local Anesthesia
4.2. Needle Size Used for EA
4.3. Amount of Ethanol Injected
4.4. Retention or Aspiration of Injected Ethanol
4.5. Number of Treatment Sessions
4.6. Potential of EA as a Primary Treatment of TGDC in Terms of Feasibility, Safety, and Treatment Efficacy
4.7. Practical Limitations of EA for TGDC
References
- Flint, P.W.; Haughey, B.H.; Robbins, K.T.; Thomas, J.R.; Niparko, J.K.; Lund, V.J.; Lesperance, M.M. Cummings Otolaryngology-Head and Neck Surgery E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014.
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Kaleci, S.; Magliulo, G.; Presutti, L.; Re, M. Clinical presentation and treatment outcomes of thyroglossal duct cysts: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 119–126.
- Galluzzi, F.; Pignataro, L.; Gaini, R.M.; Hartley, B.; Garavello, W. Risk of recurrence in children operated for thyroglossal duct cysts: A systematic review. J. Pediatr. Surg. 2013, 48, 222–227.
- Goldsztein, H.; Khan, A.; Pereira, K.D. Thyroglossal duct cyst excision—The Sistrunk procedure. Oper. Tech. Otolaryngol.-Head. Neck Surg. 2009, 20, 256–259.
- Shah, R.; Gow, K.; Sobol, S.E. Outcome of thyroglossal duct cyst excision is independent of presenting age or symptomatology. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1731–1735.
- Thompson, L.D.R.; Herrera, H.B.; Lau, S.K. Thyroglossal Duct Cyst Carcinomas: A Clinicopathologic Series of 22 Cases with Staging Recommendations. Head. Neck Pathol. 2017, 11, 175–185.
- Ahn, D.; Kwak, J.H.; Lee, G.J.; Sohn, J.H. Ultrasound-Guided Ethanol Ablation as a Primary Treatment for Thyroglossal Duct Cyst: Feasibility, Characteristics, and Outcomes. Otolaryngol. Head. Neck Surg. 2023, 168, 1381–1388.
- Rayess, H.M.; Monk, I.; Svider, P.F.; Gupta, A.; Raza, S.N.; Lin, H.-S. Thyroglossal duct cyst carcinoma: A systematic review of clinical features and outcomes. Otolaryngol.–Head. Neck Surg. 2017, 156, 794–802.
- Malka Yosef, L.; Lahav, Y.; Hazout, C.; Zloczower, E.; Halperin, D.; Cohen, O. Impact of age on surgical outcomes and failure rates in patients with thyroglossal duct cysts. Am. J. Otolaryngol. 2021, 42, 102902.
- Ross, J.; Manteghi, A.; Rethy, K.; Ding, J.; Chennupati, S.K. Thyroglossal duct cyst surgery: A ten-year single institution experience. Int. J. Pediatr. Otorhinolaryngol. 2017, 101, 132–136.
- Aculate, N.R.; Jones, H.B.; Bansal, A.; Ho, M.W. Papillary carcinoma within a thyroglossal duct cyst: Significance of a central solid component on ultrasound imaging. Br. J. Oral Maxillofac. Surg. 2014, 52, 277–278.
- Lee, D.K.; Seo, J.W.; Park, H.S.; Kang, M.K.; Jang, A.L.; Lee, J.H.; Hong, J.C. Efficacy of ethanol ablation for thyroglossal duct cyst. Ann. Otol. Rhinol. Laryngol. 2015, 124, 62–67.
- Park, S.I.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H.; Kim, T.Y.; Lee, Y.M.; Baek, S.M. Ethanol ablation for the treatment of thyroglossal duct cysts: Follow-up results for longer than 2 years. Eur. Radiol. 2022, 32, 3525–3531.
- Karatay, E.; Javadov, M. The effectiveness of ethanol ablation in the treatment of thyroglossal duct cysts in adult cases and evaluation with cosmetic scoring. Jpn. J. Radiol. 2021, 39, 994–999.
- Kim, S.M.; Baek, J.H.; Kim, Y.S.; Sung, J.Y.; Lim, H.K.; Choi, H.; Lee, J. Efficacy and safety of ethanol ablation for thyroglossal duct cysts. Am. J. Neuroradiol. 2011, 32, 306–309.
- Chung, M.S.; Baek, J.H.; Lee, J.H.; Choi, Y.J.; Yoon, J.H.; Nam, S.Y.; Kim, S.C.; Sung, J.Y.; Baek, S.M.; Na, D.G. Treatment Efficacy and Safety of Ethanol Ablation for Thyroglossal Duct Cysts: A Comparison with Surgery. Eur. Radiol. 2017, 27, 2708–2716.
- Chow, T.L.; Choi, C.Y.; Yee-Hing Hui, J. Thyroglossal duct cysts in adults treated by ethanol sclerotherapy: A pilot study of a nonsurgical technique. Laryngoscope 2012, 122, 1262–1264.
- Park, S.I.; Baek, J.H.; Suh, C.H.; Chung, S.R.; Choi, Y.J.; Kim, T.Y.; Lee, Y.-M.; Lee, J.H. Chemical ablation using ethanol or OK-432 for the treatment of thyroglossal duct cysts: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 9048–9056.
- Lee, E.; Park, I.; Elzomor, A.; Li, L.; Lloyd, A.; Benito, D.A.; Goodman, J.F.; Thakkar, P.G.; Joshi, A. Efficacy of ethanol ablation as a treatment of benign head and neck cystic lesions. Am. J. Otolaryngol. 2021, 42, 103082.
- Yang, C.C.; Hsu, Y.; Liou, J.Y. Efficacy of Ethanol Ablation for Benign Thyroid Cysts and Predominantly Cystic Nodules: A Systematic Review and Meta-Analysis. Endocrinol. Metab. 2021, 36, 81–95.
- Cesareo, R.; Tabacco, G.; Naciu, A.M.; Crescenzi, A.; Bernardi, S.; Romanelli, F.; Deandrea, M.; Trimboli, P.; Palermo, A.; Castellana, M. Long-term efficacy and safety of percutaneous ethanol injection (PEI) in cystic thyroid nodules: A systematic review and meta-analysis. Clin. Endocrinol. 2022, 96, 97–106.
- Mulita, F.; Tchabashvili, L.; Verras, G.I.; Liolis, E.; Siouti, S.; Panagopoulos, K.; Vailas, M. Thyroid abscess as a complication of percutaneous ethanol ablation of cystic thyroid nodules. Endokrynol. Pol. 2021, 72, 284–285.
- Hahn, S.Y.; Shin, J.H.; Na, D.G.; Ha, E.J.; Ahn, H.S.; Lim, H.K.; Lee, J.H.; Park, J.S.; Kim, J.H.; Sung, J.Y.; et al. Ethanol Ablation of the Thyroid Nodules: 2018 Consensus Statement by the Korean Society of Thyroid Radiology. Korean J. Radiol. 2019, 20, 609–620.
- Orloff, L.A.; Noel, J.E.; Stack, B.C., Jr.; Russell, M.D.; Angelos, P.; Baek, J.H.; Brumund, K.T.; Chiang, F.Y.; Cunnane, M.B.; Davies, L.; et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck 2022, 44, 633–660.
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedus, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur. Thyroid J. 2020, 9, 172–185.
- Feldkamp, J.; Grunwald, F.; Luster, M.; Lorenz, K.; Vorlander, C.; Fuhrer, D. Non-Surgical and Non-Radioiodine Techniques for Ablation of Benign Thyroid Nodules: Consensus Statement and Recommendation. Exp. Clin. Endocrinol. Diabetes 2020, 128, 687–692.
- Park, H.S.; Yim, Y.; Baek, J.H.; Choi, Y.J.; Shong, Y.K.; Lee, J.H. Ethanol ablation as a treatment strategy for benign cystic thyroid nodules: A comparison of the ethanol retention and aspiration techniques. Ultrasonography 2019, 38, 166.
- Kim, D.W.; Rho, M.H.; Kim, H.J.; Kwon, J.S.; Sung, Y.S.; Lee, S.W. Percutaneous ethanol injection for benign cystic thyroid nodules: Is aspiration of ethanol-mixed fluid advantageous? Am. J. Neuroradiol. 2005, 26, 2122–2127.
- Hirshoren, N.; Neuman, T.; Udassin, R.; Elidan, J.; Weinberger, J.M. The imperative of the Sistrunk operation: Review of 160 thyroglossal tract remnant operations. Otolaryngol. Head Neck Surg. 2009, 140, 338–342.
- Maddalozzo, J.; Venkatesan, T.K.; Gupta, P. Complications associated with the Sistrunk procedure. Laryngoscope 2001, 111, 119–123.
- Lekkerkerker, I.; van Heurn, E.L.; van der Steeg, A.F.; Derikx, J.P. Pediatric thyroglossal duct cysts: Post-operative complications. Int. J. Pediatr. Otorhinolaryngol. 2019, 124, 14–17.




