Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prathap Somu | -- | 1736 | 2023-10-25 10:40:01 | | | |
| 2 | Alfred Zheng | Meta information modification | 1736 | 2023-10-26 03:16:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Somu, P.; Basavegowda, N.; Gomez, L.A.; Jayaprakash, H.V.; Puneetha, G.K.; Yadav, A.K.; Paul, S.; Baek, K. Heat Shock Proteins Immunostimulatory Properties-based Cancer Immunotherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/50779 (accessed on 08 February 2026).
Somu P, Basavegowda N, Gomez LA, Jayaprakash HV, Puneetha GK, Yadav AK, et al. Heat Shock Proteins Immunostimulatory Properties-based Cancer Immunotherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/50779. Accessed February 08, 2026.
Somu, Prathap, Nagaraj Basavegowda, Levin Anbu Gomez, Hulikunte Veeranna Jayaprakash, Gangadahosahalli Krishnegowda Puneetha, Akhilesh Kumar Yadav, Subhankar Paul, Kwang-Hyun Baek. "Heat Shock Proteins Immunostimulatory Properties-based Cancer Immunotherapy" Encyclopedia, https://encyclopedia.pub/entry/50779 (accessed February 08, 2026).
Somu, P., Basavegowda, N., Gomez, L.A., Jayaprakash, H.V., Puneetha, G.K., Yadav, A.K., Paul, S., & Baek, K. (2023, October 25). Heat Shock Proteins Immunostimulatory Properties-based Cancer Immunotherapy. In Encyclopedia. https://encyclopedia.pub/entry/50779
Somu, Prathap, et al. "Heat Shock Proteins Immunostimulatory Properties-based Cancer Immunotherapy." Encyclopedia. Web. 25 October, 2023.
Copy Citation
The development of multidrug resistance (MDR) against chemotherapeutic agents has become a major impediment in cancer therapy. Understanding the underlying mechanism behind MDR can guide future treatment for cancer with better therapeutic outcomes. Recent studies evidenced that crossroads interaction between the heat shock proteins (HSP) and inflammatory responses under the tumor microenvironment plays a pivotal role in modulating drug responsiveness and drug resistance through a complex cytological process.
cancer
tumor microenvironment
1. Introduction
Cancer includes a group of diseases that occur due to the uncontrolled division of the cells that can spread and invade throughout body parts. Cancer is the second most significant factor in morbidity worldwide, behind cardiovascular disease, and kills approximately 10 million people each year, according to the WHO (World Health Organization). Further, it is projected that 1,958,310 new cancer cases and 609,820 fatalities due to cancer emerge worldwide in 2023. The most common types of cancer are breast, lung, colorectal, and prostate cancers [1]. It has further been estimated that around 2030, the number of new cancer cases will rise by nearly 70% [2].
Currently practiced therapeutic intervention for cancer treatment includes radiation therapy, immunotherapy, surgery, chemotherapy, and targeted therapy. Radiation therapy and surgery have frequently been practiced to treat localized and non-metastatic cancers [3][4]. Chemotherapy is the most clinically practiced therapeutic intervention for treating cancer cells that have spread into distant organs in the whole body, which are no longer treated with localized therapeutic methods such as surgery and radiotherapy clinically [5][6]. The cytotoxic potential of these chemotherapeutic agents is due to their ability to interfere with DNA synthesis and mitosis of rapidly proliferating cells, thereby causing their death (rapid proliferation is one of the essential characteristics of neoplastic cells such as cancer cells). For instance, Taxol is a commonly used chemotherapy agent that hinders microtubule depolarization and hyper-stabilizes the microtubule. Another widely used and the most important chemotherapy agent is doxorubicin, which inhibits topoisomerase II, an essential enzyme for relaxing supercoils in DNA during cell division.
Despite the achievements made in cancer treatment, chemoresistance, or drug resistance to chemotherapeutic agents, has become a significant problem in cancer therapies and is responsible for most tumor relapses and poor prognosis [7]. The cytotoxic potential of these chemotherapeutic agents is due to their ability to interfere with the synthesis of DNA of rapidly dividing cells, which is non-specific in nature and thereby causes side-effects of the rapidly dividing healthy cells [8][9].
2. Heat Shock Proteins and their Immunomodulatory Effect in the Tumor Microenvironment, Drug Resistance, and Treatment
HSP overexpression contributes to tumor development and progression and in determining their response to various treatments, including chemotherapy. For example, as discussed above, HSP27 overexpression has contributed to the poor prognosis of osteosarcomas and gastric carcinomas [10].
Zheng et al. (2018) recently showed that HSP27 played an important role in SCCT cells for chemoresistance via synergistic extracellular and intracellular signaling [11]. In the intrinsic pathway, the reduction of TLR5 or the restored IκBα protein level of IB proteins interrupts the transactivation of extracellular HSP27-induced NF-κB transactivation resulting in drug resistance. In the extrinsic pathway, intracellular HSP27 interacts with BAX and BIM to suppress their translocation to the mitochondrion, thus blocking the successive release of cytochrome C and preventing cells from apoptosis by communicating with Cyto C [11][12][13]. HSP27 exhibited biological effects on monocytes, producing IL10 and TNFα and blocking monocyte differentiation to normal dendritic cells and tumor-associated macrophages to stimulate cancer progression and chemoresistance as shown in Figure 1 [11][14].
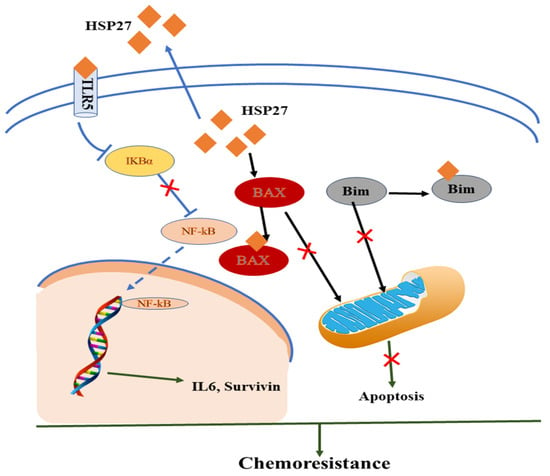
Figure 1. Mechanistic representation of HSP and immunomodulatory effect in the tumor microenvironment in acquiring chemoresistance.
However, there are also reports stating the increase in HSP expression in improving chemotherapy outcome, such as in the case of HSP70 in osteosarcomas, where enhanced expression of HSP70 was found to significantly influence the prognosis of breast cancer [15]. Furthermore, the circulating levels of HSPs along with their antibodies in blood provide attractive biomarkers for analyzing stage and aggressiveness in various types of cancer and monitoring the treatment process during treatment due to the extraordinary relationship between HSPs and drug resistance. More importantly, one of the immune system’s essential functions in tumor prevention is tumor immune surveillance, which particularly recognizes and eradicates cancerous and/or precancerous tumors by analyzing the expression level of tumor-specific antigens or molecules that are induced as a result of cellular stress. In the tumor microenvironment, some HSP plays a critical role in managing the delicate balance of protective and destructive immunological responses; thus, it is quite evident that HSP possesses a central understanding role in oncoimmunology. Thus, the expression or activity of HSP modulation provides a new therapeutic strategy in cancer immunotherapy [16].
The application of HSP-based immunotherapeutic approaches may contribute enormously to the field of oncoimmunology to obtain effective anticancer regimens. For example, antitumor T-cell immunity might be enhanced by the application of tumor-based HSP90 inhibition. HSP90 has essential functional roles that complement the role in innate and adaptive immune responses, including activation/maturation of dendritic cell antigen presentation, lymphocyte activation, and cross-priming [17][18]. Thus, targeted inhibition of HSP90 can stimulate a dual regulatory role in both immunosuppressive and immunostimulatory effects. Accumulating evidence suggests that T-cell-mediated antitumor immunity promotes increased tumor immune surveillance and recognition through therapeutic exploitation of client proteins’ dependent and independent mechanisms.
The enhancement in the anti-tumor T-cell immunity using tumor-based HSP90 inhibition has been reported. In addition, the invention that oncoprotein-directed dysregulation of PD-1/PD-L1 signaling can occur as an adaptive response for endogenous antitumor immunity elevates the capability of upstream interference via HSP90 blockade. However, the mechanism to integrate the process of HSP90 inhibition and blockade of immune checkpoints should be investigated to improve antitumor immune responses. Expect the above therapeutic approach, i.e., immune-checkpoint inhibitors for cancer immuno-therapy, and many other approaches such as cytokines, immune-checkpoint inhibitors, targeted antibody therapies, and adoptive cell transfer methodologies. Overall, compelling evidence suggests that HSPs are attractive targets for cancer immunotherapy.
3. Other HSP Immunostimulatory Properties-based Cancer Immunotherapy
The following unique immunostimulatory properties of HSP that can be used as physiological adjuvants for cancer immunotherapy are as follows [19]:
-
Assuring the desired specificity and sensitivity of antigen targeting possibly assured through receptor-mediated uptake of molecular chaperones via antigen-presenting cells (APCs).
-
Molecular chaperones are able to serve as danger signals and stimulate innate immune components for the progression of active immune responses.
These novel immunostimulatory properties of HSPs facilitate their utilization as adjuvants to develop various cancer immunotherapy approaches.
3.1. Tumor-based HSP Vaccine
The HSP possesses substantial properties that can be utilized for targeting dendritic cells (DCs) and can also used as a natural adjuvant for inducing adaptive immune responses based on the ability of HSP to deliver multiple antigens and serve as the secure constituent of current vaccines [20][21]. Under stress conditions or immunological danger signals, HSP-bound tumoral peptides could be released in the extracellular medium owing to the chaperone activity of HSP [19]. The interaction between HSP and APC cells involves multiple receptors, including CD40, CD91, and LOX-1. After endocytosis of HSP–peptide complexes, they were humiliated and subsequently led to cross-presentation of the tumor peptide to CD8+ T cells through the major histocompatibility complex 1 (MHC-1) molecules [22]. Vitespen is an autologous tumor-derived HSP Gp96, which has shown an excellent clinical impact for treatments of kidney cancer and melanoma in phase III clinical trials [23]. Generally, the immunogenicity of HSPs is primarily because of its two essential properties: first, their peptide-dependent capacity to stimulate and elicit adaptive CTL responses against antigenic peptides and, second, their peptide-independent immunomodulatory capacity.
Several reports have shown that particular HSPs, including HSP70 and Gp96, are effective carrier molecules for cross-presenting [24].
3.2. HSP as an Antigen for Immune Responses Stimulation
The immune system can be stimulated for cancer treatment using HSP as an antigen without chaperonin activity [19]. For example, intratumoral vaccination with a recombinant oncolytic adenovirus overexpressing HSP, in particular HSP70, thus reducing tumor growth [25]. Li et al. (2014) reported that HSP peptide-specific CTL (cytotoxic T lymphocytes) effectively reduced tumor burden in the mouse model of a myeloma xenograft. Treatment of CTLs engineered to target HSP27 and HSP90 peptides effectively decreased tumor growth in a mouse model with myeloma xenograft and stimulation of peripheral blood mononuclear cells (PBMC), resulting specifically in the generation of HSP peptide CTL [26].
3.3. HSP as an Adjuvant-Free Carrier to Stimulate Immune Responses
Adjuvants are substances that can enhance, stimulate, and modulate innate immune responses, as well as alter the quality and quantity of adaptive immune responses [21]. HSP was used as an adjuvant for stimulating the immune response of CTLs in various cancers and infectious diseases. Some reports revealed that immune activities reside within N- or C-terminal fragments. Thus, a small piece or part of HSP as an adjuvant in vaccines for cancer prevention can be used [27]. For instance, the N-terminal piece of Gp96 (NT-gp96) has been reported as a potential adjuvant that has been found to increase specific immune responses of CTLs against hepatitis B virus (HBV) and hepatocellular carcinoma infections [27][28].
The anti-tumor responses based on targeting the HSP70 family of fusion proteins and tumor-associated antigens (TAAs) were evaluated by Zhang and Huang, who reported that the C-terminal domain of HSP70 seemed to be the essential part of eliciting anti-tumor responses, including NK cell stimulation against B16 murine melanoma expressing tumor-associated antigens [29]. The investigation further proves the importance of the peptide-binding domain that mediates the binding of HSP70 and generated DNA encoding the E7/HSP70 vaccine. However, in conjunction with the HSP70 functional domain, the orientation of links between HSP70 and HPVE7 also has significant clinical importance in optimizing HSP70-based DNA vaccines [30].
In chronic non-progressive pneumonia, membrane-associated proteins elongation factor Tu and Mycoplasma ovipneumoniae elongation factor HSP70 induce significant levels of cytokines, such as TNF-α, IFN-γ, IgG, IL-4, IL-5, IL-6, and IL-12 [19]. In fact, recombinant HSP70 has the potential to act as a Th1 cytokine-like adjuvant in mice [31]. A similar study has been conducted using Small HSP, that is, HSP27 as an efficient adjuvant to improve HIV-1Nef antigen-specific immunity. A fusion protein, namely HSP27-Nef, secured from the fusion of HIV-1Nef and HSP27, significantly improved the Nef-specific T-cell response. In fact, these regimens induced high levels of IgG2a and IFN-γ, which are Th1-associated cytokines, as well as Granzyme B secretion. Thus, this research demonstrated the immunostimulatory properties of HSP27, which can be utilized for diverse immunization approaches other than Freund’s adjuvant, and these findings suggested that HSP27 can be used as an effective adjuvant in protein-based vaccines to boost HIV-1 Nef-specific B- and T-cell immune responses [32] and serve as a promising HIV-1 vaccine candidate also [33].
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193.
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584.
- Chabner, B.A.; Roberts, T.G., Jr. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72.
- Tekade, R.K.; Dutta, T.; Tyagi, A.; Bharti, A.C.; Das, B.C.; Jain, N.K. Surface-engineered dendrimers for dual drug delivery: A receptor up-regulation and enhanced cancer targeting strategy. J. Drug Target. 2008, 16, 758–772.
- Liu, C.; Zhao, G.; Liu, J.; Ma, N.; Chivukula, P.; Perelman, L.; Okada, K.; Chen, Z.; Gough, D.; Yu, L. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J. Control. Release 2009, 140, 277–283.
- Madden, E.C.; Gorman, A.M.; Logue, S.E.; Samali, A. Tumour cell secretome in chemoresistance and tumour recurrence. Trends Cancer 2020, 6, 489–505.
- Feng, S.-S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114.
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207.
- Uozaki, H.; Ishida, T.; Kakiuchi, C.; Horiuchi, H.; Gotoh, T.; Iijima, T.; Imamura, T.; Machinami, R. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol. Pract. 2000, 196, 665–673.
- Zheng, G.; Zhang, Z.; Liu, H.; Xiong, Y.; Luo, L.; Jia, X.; Peng, C.; Zhang, Q.; Li, N.; Gu, Y. HSP27-mediated extracellular and intracellular signaling pathways synergistically confer chemoresistance in squamous cell carcinoma of tongue. Clin. Cancer Res. 2018, 24, 1163–1175.
- Stetler, R.A.; Cao, G.; Gao, Y.; Zhang, F.; Wang, S.; Weng, Z.; Vosler, P.; Zhang, L.; Signore, A.; Graham, S.H. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J. Neurosci. 2008, 28, 13038–13055.
- Havasi, A.; Li, Z.; Wang, Z.; Martin, J.L.; Botla, V.; Ruchalski, K.; Schwartz, J.H.; Borkan, S.C. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J. Biol. Chem. 2008, 283, 12305–12313.
- Banerjee, S.; Lin, C.-F.L.; Skinner, K.A.; Schiffhauer, L.M.; Peacock, J.; Hicks, D.G.; Redmond, E.M.; Morrow, D.; Huston, A.; Shayne, M. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011, 71, 318–327.
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86.
- Das, J.K.; Xiong, X.; Ren, X.; Yang, J.-M.; Song, J. Heat shock proteins in cancer immunotherapy. J. Oncol. 2019, 2019, 3267207.
- Tsan, M.-F.; Gao, B. Heat shock proteins and immune system. J. Leucoc. Biol. 2009, 85, 905–910.
- Callahan, M.K.; Garg, M.; Srivastava, P.K. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc. Natl. Acad. Sci. USA 2008, 105, 1662–1667.
- Somu, P.; Paul, S. Inter-Relationship Between the Inflammation and Heat Shock Protein in Cancer Development: A Possible Target for Diagnosis and Cancer Immunotherapy. In Heat Shock Proteins in Human Diseases; Springer: Cham, Switzerland, 2021; pp. 1–29.
- Meng, J.; Lv, Y.; Bao, W.; Meng, Z.; Wang, S.; Wu, Y.; Li, S.; Jiao, Z.; Tian, Z.; Ma, G.; et al. Generation of whole tumor cell vaccine for on-demand manipulation of immune responses against cancer under near-infrared laser irradiation. Nat. Commun. 2023, 14, 4505.
- Dai, W.; Yao, G.; Deng, X.; Zang, G.; Liu, J.; Zhang, G.; Chen, Y.; Lv, M.; Chen, T. Heat shock protein: A double-edged sword linking innate immunity and hepatitis B virus infection. J. Virus Erad. 2023, 9, 100322.
- Calderwood, S.K.; Mambula, S.S.; Gray, P.J., Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann. N. Y. Acad. Sci. 2007, 1113, 28–39.
- Wood, C.; Srivastava, P.; Bukowski, R.; Lacombe, L.; Gorelov, A.I.; Gorelov, S.; Mulders, P.; Zielinski, H.; Hoos, A.; Teofilovici, F. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: A multicentre, open-label, randomised phase III trial. Lancet 2008, 372, 145–154.
- Bolhassani, A.; Rafati, S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev. Vaccines 2008, 7, 1185–1199.
- Li, J.L.; Liu, H.L.; Zhang, X.R.; Xu, J.P.; Hu, W.K.; Liang, M.; Chen, S.Y.; Hu, F.; Chu, D.T. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009, 16, 376–382.
- Li, R.; Qian, J.; Zhang, W.; Fu, W.; Du, J.; Jiang, H.; Zhang, H.; Zhang, C.; Xi, H.; Yi, Q. Human heat shock protein-specific cytotoxic T lymphocytes display potent antitumour immunity in multiple myeloma. Br. J. Haematol. 2014, 166, 690–701.
- Bolhassani, A.; Talebi, S.; Anvar, A. Endogenous and exogenous natural adjuvants for vaccine development. Mini Rev. Med. Chem. 2017, 17, 1442–1456.
- Li, H.; Zhou, M.; Han, J.; Zhu, X.; Dong, T.; Gao, G.F.; Tien, P. Generation of murine CTL by a hepatitis B virus-specific peptide and evaluation of the adjuvant effect of heat shock protein glycoprotein 96 and its terminal fragments. J. Immunol. 2005, 174, 195–204.
- Zhang, H.; Huang, W. Fusion proteins of Hsp70 with tumor-associated antigen acting as a potent tumor vaccine and the C-terminal peptide-binding domain of Hsp70 being essential in inducing antigen-independent anti-tumor response in vivo. Cell Stress Chaperones 2006, 11, 216.
- Li, Y.; Subjeck, J.; Yang, G.; Repasky, E.; Wang, X.-Y. Generation of anti-tumor immunity using mammalian heat shock protein 70 DNA vaccines for cancer immunotherapy. Vaccine 2006, 24, 5360–5370.
- Jiang, F.; He, J.; Navarro-Alvarez, N.; Xu, J.; Li, X.; Li, P.; Wu, W. Correction: Elongation Factor Tu and Heat Shock Protein 70 Are Membrane-Associated Proteins from Mycoplasma ovipneumoniae Capable of Inducing Strong Immune Response in Mice. PLoS ONE 2017, 12, e0189562.
- Milani, A.; Bolhassani, A.; Shahbazi, S.; Motevalli, F.; Sadat, S.M.; Soleymani, S. Small heat shock protein 27: An effective adjuvant for enhancement of HIV-1 Nef antigen-specific immunity. Immunol. Lett. 2017, 191, 16–22.
- Milani, A.; Bolhassani, A.; Heshmati, M. Delivery of HIV-1 Nef linked to heat shock protein 27 using a cationic polymer is more effective than cationic lipid in mammalian cells. Bratisl. Lek. Listy 2017, 118, 334–338.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
26 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




