Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anne-Kathrin Pfrieme | -- | 4388 | 2023-10-25 09:28:55 | | | |
| 2 | Jason Zhu | Meta information modification | 4388 | 2023-10-26 03:44:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pfrieme, A.; Will, T.; Pillen, K.; Stahl, A. Wheat Dwarf Virus and Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/50775 (accessed on 08 February 2026).
Pfrieme A, Will T, Pillen K, Stahl A. Wheat Dwarf Virus and Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/50775. Accessed February 08, 2026.
Pfrieme, Anne-Kathrin, Torsten Will, Klaus Pillen, Andreas Stahl. "Wheat Dwarf Virus and Disease" Encyclopedia, https://encyclopedia.pub/entry/50775 (accessed February 08, 2026).
Pfrieme, A., Will, T., Pillen, K., & Stahl, A. (2023, October 25). Wheat Dwarf Virus and Disease. In Encyclopedia. https://encyclopedia.pub/entry/50775
Pfrieme, Anne-Kathrin, et al. "Wheat Dwarf Virus and Disease." Encyclopedia. Web. 25 October, 2023.
Copy Citation
Wheat dwarf disease (WDD) is an important disease of monocotyledonous species, including economically important cereals. The causative pathogen, wheat dwarf virus (WDV), is persistently transmitted mainly by the leafhopper Psammotettix alienus and can lead to high yield losses. Due to climate change, the periods of vector activity increased, and the vectors have spread to new habitats, leading to an increased importance of WDV in large parts of Europe. In the light of integrated pest management, cultivation practices and the use of resistant/tolerant host plants are currently the only effective methods to control WDV.
wheat dwarf virus
WDV
resistance
mastrevirus
1. Introduction
As early as the 8th century AD, the Japanese Anthology described the first observations of viroses on Eupatorium chinense L., which, according to current knowledge, were caused by geminiviruses [1]. As a consequence of climate change, insect-transmitted viruses are gaining increased importance because vectors may benefit from a temperature increase in different ways [2][3][4][5]. Damage caused by viruses in agriculture includes not only yield and biomass losses but also the weakening of infected plants, making them more susceptible to abiotic and biotic stressors, so that quality losses may also occur [6]. Currently, there are no approved options for direct chemical control of viruses. So, appropriate measures in accordance with integrated pest management include farm hygiene, quarantine programs for the import and export of plant products, production of virus-free seeds and planting materials, breeding of resistant varieties, and, as a last measure, the control of vector insects by the use of chemical insecticides [7][8].
In Europe, more than 30 different viruses are known to occur in cereals [9]. These include wheat dwarf virus (WDV, family Geminiviridae, genus Mastrevirusas the causal agent of wheat dwarf disease (WDD). The virus is transmitted from plant to plant exclusively by leafhoppers [10][11][12]. The first occurrence was described in the former Czechoslovakia [10], followed by subsequent outbreaks in the 1990s [13][14][15][16]. Outbreaks vary from year to year and differ in the damage they cause, with early infections in the fall leading to drastic yield losses [17][18]. Lindblad and Waern [17] put the average yield losses in winter wheat fields at 35–90% for sites studied in Sweden, while a study in southern Finland found losses of 20–100% [18].
2. Wheat Dwarf Virus (WDV)
2.1. Classification and Genomic Organization of WDV
WDV belongs to a group of viruses originally described as wheat dwarfing viruses within the family Geminiviridae, genus Mastrevirus [19][20][21][22].
Geminiviruses themselves are defined as plant pathogenic circular single-stranded DNA (ssDNA) viruses [23]. Their virion consists of twinned (geminate) icosahedra with a bipartite capsid [24][25] and a genome packaged in 11 subunits [1][25][26]. In addition to nanoviruses (family Nanoviridae), they are the only phytopathogenic representatives with a genome consisting of a circular ssDNA [27]. Actual research on the family Geminiviridae began in the 1980s, although they have been known since the beginning of the 20th century, mainly as causal agents of yield loss in tomato, sugar beet, cassava, maize, and cotton in tropical and subtropical countries [28][29][30][31]. Based on their genome structure, vector, host range, and phylogeny, geminiviruses are classified into 14 genera with 520 species (Figure 1) [20][21][22][32][33][34].
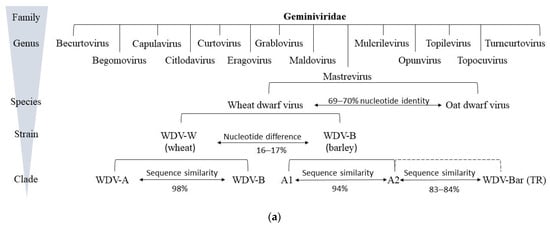
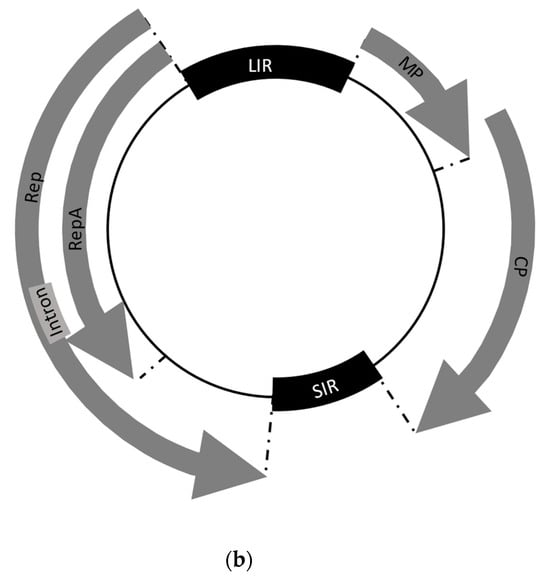
Figure 1. Classification and genomic organization of wheat dwarf virus (WDV): (a) classification of the family Geminiviridae is based on their molecular and biological characteristics. WDV species belong to the mastreviruses and consist of the main strains of wheat and barley, to which the various isolates are subordinated in clades. The percentage of nucleotide similarity is given for the species, strains, and clades. WDV Bar [TR] refers to the recombinant isolate between a barley isolate and a yet unknown member of the mastreviruses. (b) Genomic organization of mastreviruses, which include wheat dwarf virus (WDV). These have a circular ssDNA genome (black circle) and four ORFs. Code of viral proteins: MP—movement protein, CP—capsid protein, RepA—replication-associated protein, Rep—replication initiation protein. Also shown are the non-coding regions of the large intergenic region (LIR) and small intergenic region (SIR).
Currently, 45 different mastreviruses are known, which type species is Maize streak virus (MSV) [33][35], and share a common phylogenetic tree [36][37][38]. They predominantly infect monocotyledonous plants, with a few exceptions, such as Tobacco yellow dwarf virus [39], Bean yellow dwarf virus [40], and Chickpea redleaf virus [41][42], which can infect susceptible dicotyledonous host plants. Transmission of these viruses to host plants is mainly persistent and non-propagative through leafhoppers as vectors [41][42]. The Mastrevirus genus has a monopartite circular ssDNA genome with a length of 2.6–2.8 kb [43][44]. The genome of WDV [19], which belongs to this group, is 2.73–2.75 kb in size [14][24][45][46].
The circular genome contains two open reading frames (ORFs) on the sense side and two ORFs on the antisense side, separated by two noncoding regions that encode four viral proteins. On the virion sense strand, ORFs V1 and V2 are responsible for encoding the viral movement protein (MP) and the coat protein (CP). On the complementary sense strand are C1 and C2, which encode the replication-associated proteins (Rep, RepA) and are expressed through script splicing [47][48][49][50][51][52]. The two strands are separated by a large (LIR) and a small (SIR) non-coding intergenic unit, whose sequences are substantially involved in viral replication and regulation of gene expression [53] and control bidirectional transcription based on promoter (transcription initiation step) and terminator (transcription termination step) sequences [54][55]. Between the 5′ ends of the Rep/RepA and MP genes is the LIR sequence [56].
The replication-associated proteins (Rep, RepA) are encoded by a gene and are expressed by a complementary sense transcript. Both forms differ due to an intron in the Rep gene [16][50][57][58][59][60] and are involved in the early stages of infection [26]. Rep is involved in viral replication, while RepA affects the control of the host cell cycle to support viral replication [26]. Translation of RepA occurs directly from the native RNA transcript, whereas production of the Rep protein requires a splice cut of the RNA molecule. Therefore, the proteins have identical N-terminal sequences [61].
MP, as a product of V2, is a 10.9-kDa protein involved in systemic infection of the host by increasing the exclusion limit of plasmodesmata, allowing intercellular spread of viral DNA [62][63]. The functions of the coat protein (CP) have been studied most extensively for mastreviruses [64]. In addition to encapsulating viral DNA with a capsid, it is involved in various functions in the infection cycle, i.e., virus–vector interaction during transmission [61]. Thus, it plays an important role in vector specificity [65], viral nuclear import [66], insect transmission, systemic viral movement, and symptom development [47][64]. For the establishment of systemic infection, both MP and CP (V1 and V2) have been found to be essential, although they do not contribute to virus replication. CP binds ssDNA and dsDNA in vitro in this process, so its presence is essential for the accumulation of viral ssDNA in infected host cells and protoplasts [67].
The geminiviral transcriptional activator protein (TrAP) plays a role in pathogenicity by inhibiting a plant’s transcriptional and post-transcriptional gene silencing [68][69][70][71][72][73][74][75][76]. Enhanced viral replication is initiated by the replication enhancer protein (Ren), which interacts with host factors and Rep [65].
In several wheat isolates, a putative fifth ORF was discovered on the complementary (−) strand, coding for a protein (14.6 kDa) whose function is still unknown [14][45][46][77]. An additional ORF has not yet been detected in barley-adapted WDV isolates [78][79].
2.2. Life Cycle of the Virus
The life cycle of geminiviruses require both host proteins and viral proteins. Infection of the host plant begins as soon as the virus-bearing insect vector secretes saliva into the host plantit. Deposition and unpacking of the viral genome occurs in the phloem companion cells [80][81][82]. Replication of geminiviruses takes place in the nucleus of the companion cells because the sieve elements do not have a nucleus as a consequence of ontogenesis [83]. The entry of viral DNA into the nucleus is supported by the coat protein (CP). This is thought to interact with host-specific transport receptors. Within the intergenic regions, there are signal motifs controlling the two phases of replication. The onset of DNA synthesis is initiated specifically for representatives of the genus by a primer (approximately 80 bp long) located in the SIR, which is complementary to the intergenic region [48][49]. In the first phase, ssDNA is converted into a double-stranded (ds) DNA intermediate [84], which serves as a template for the production of complementary and virus-sense transcripts [54][55]. Replication of the genomic (+) DNA strand is initiated (ori) by cleavage of the virion-sense strand at a specific, highly conserved nona-nucleotide motif (5′ TAATATT ↓ AC 3′) by Rep (replication initiator protein) within the LIR sequence [56][81]. The motif is partially enclosed within the head of a stem-loop structure and contains the initiation point (↓) of the second replication phase to produce the (+) DNA strand using a rolling circle replication process [40][60][84][85][86][87].
For the amplification of viral dsDNA and the production of ssDNA genomes, the dsDNA intermediate is used as a template. Starting from the LIR, passing through the (−) and (+) strands, and continuing to the SIR, bidirectional transcription of the DNA occurs using host DNA polymerase [88]. Geminiviruses do not code for a DNA polymerase in this process, so the production of dsDNA using complementary DNA synthesis depends exclusively on host factors recruited during the early stages of replication [81]. Synthesis of the complementary minus (−) DNA strand begins at the 3′ end of a short complementary primer. This is packaged into viral particles and can hybridize with a sequence in the SIR region [84]. Transcription is bidirectional, with coding regions diverging from the LIR in both strands. For gene expression, geminiviruses use multiple overlapping transcripts [81].
The movement of the virus depends on the outcome of interaction with different parts of the cell (cytoskeleton), the type of plasmodesmata, and the ability of the virus to replicate in different cells [89]. In infected plants, electron microscopy has revealed altered nuclei in the phloem companion and in the parenchyma cells of roots and leaves [90]. In these cells, there is an accumulation of virus particles arranged in groups and rows, filling almost the entire nucleoplasm. High particle concentrations have been detected, especially in plants with wilted leaves in the stem region [91].
To spread the infection, the virus must overcome barriers such as the nuclear envelope and spread between adjacent cells [92]. Viral DNA is transported from the nucleus to the cell membrane as a V2-DNA complex with the help of the transport protein (MP), which binds to host receptors [43]. To spread the infection from one cell to another, the virus must pass through plasmodesmata. This is possible exclusively between the companion cells (CC) and the sieve element (SE) of the CC/SE complex because they are isolated from the surrounding phloem parenchyma cells, as indicated by a very low number of plasmodesmata in barley [93] and their absence in wheat [94]. Depending upon the developmental stage, the size of the protein that can pass through the plasmodesmata varies, as shown forwheat [95]. The authors furthermore demonstrated that a viral movement protein is able to increase the open width of plasmodesmata so that proteins with higher molecular weight can pass through, independent of the leaves’ developmental stage. This would facilitate the systemic movement of a virus such as WDV. WDV is distributed together with photoassimilates and other nutrients along the sieve tube with transport based on turgor-driven mass flow from source to sink [92]. For maize streak virus in maize, it has been shown that younger leaves formed after inoculation are more likely to be infected with the virus than older leaves because the viral antigen is distributed according to the age of the tissue. The virus can, therefore, be detected in the basal meristem of young leaves as it reaches them through the phloem with the metabolites of older leaves. For long-distance transport, probably only the thin-walled SEs that form the above-mentioned CC/SE complexes are relevant, while the thick-walled SEs lack CCs and, thus, the basis for virus replication [96].
Regarding the molecular mechanisms of spread and the associated interaction with host components, many questions remain open in the relationship between geminiviruses and hosts. Cell-to-cell spread is ensured by phosphorilization of the transport protein (MP) by host kinases [97][98][99]. A study of begomoviruses (Geminiviridae) in tomato (Solanum lycopersicum) and soybean (Glycine max [L.] Merr) identified the cellular interaction partners that support the transport of the viral genome from the nucleus to the cytoplasm. For both plant species, a membrane-associated plant species–specific kinase belonging to the LRR-RLK family of proteins (leucine-rich-repeat receptor-like kinase) was discovered. Within the highly specific interaction, short-term formation of a complex of nuclear shuttle protein (NSP) and NSP-interacting kinase (NIK) occurs, which provides targeted and active recognition of nuclear pores, plasma membrane, and plasmodesmata modes. The complex presumably serves to regulate the biochemical activity of the viral protein in phosphorylating the transport protein. In this case, NSP would regulate the movement of viral DNA through the kinase activity of transmembrane receptors for this purpose. Host kinase as enzyme and viral NSP as substrate are related here [97]. Therefore, the non-host relationship between the wheat and barley strains of WDV could be due to the non-recognition of the viral protein by the plant receptor. In this case, the low incidence of winter barley infected with the wheat strain and winter wheat infected with the barley strain could be attributed to a sequence swap resulting from a mutation [100].
2.3. Phylogenetics
Based on phylogenetic analyses of WDV sequences from isolates of different host species, WDV has been shown to form a clade that is distinctly different from other mastreviruses and consists of multiple strains [101][102]. WDV sequence identity is below the delimitation criterion of <75% for the Mastrevirus species [35][103].
A further Mastrevirus species was later identified in Avena fatua in Germany, based on sequences of isolates collected from plant samples from cereal fields. Oat dwarf virus (ODV) is closely related to the WDV species but is distinct from wheat and barley strains and appears to be one of the causal agents of WDD in oats [103], with symptoms comparable to those of WDD (Figure 1a). Although some relationships exist between WDV and ODV based upon a sequence analysis, the whole genome of ODV has only a nucleotide sequence similarity of approx. 70% compared to the wheat and barley strains of WDV. Based on a phylogenetic analysis, a revision of the classification of the Mastrevirus species into five phylogenetic groups (A–E) was proposed in 2013. In this context, WDV strains that preferentially infect wheat (WDV-W) or barley (WDV-B) should be assigned to groups A and C, respectively [36]. Phylogenetic analysis of 230 isolates identified six strains (A–F) based on sequence similarity. Strains A- and F- were assigned to WDV-B (Figure 1, Clade A1, A1, WDV-Bar), and strains B–E were mainly assigned to WDV-W (Figure 1, Clade WDV-A, WDV-B) [104].
Macdowell et al. [14] and Matzeit [24] sequenced a 2749 bp Swedish isolate (WDV-S), which was isolated from wheat in 1969 [77]. Two other wheat-adapted isolates from the Czech Republic (WDV-C) [45] and France (WDV-F) [46] showed a genome size of 2750 bp. Sequence analyses showed that barley WDV isolates had at least 94% similarity, whereas wheat isolates had at least 98.3 to 98.8% sequence similarity with the respective strains [45][46][77]. LIR and SIR represent the most variable parts of the WDV genome [103]. Within the genomes, nucleotide exchanges in coding regions were observed but did not result in amino acid sequence substitutions, so this had no effect on the gene products [77].
Depending on the WDV isolate, differences in WDV virulence can be observed. Significantly increased symptoms of a WDV infection can be attributed to amino acid substitutions in the CP gene. This was reported in a Ukrainian study in which the Ukrainian isolate Khm-K-Ukr caused a significantly greater reduction in seeds per ear and thousand-grain weight compared to the isolate MIP-12-Ukr, which had fewer mutations in the CP gene than Khm-K-Ukr. The authors of the study suggested that the isolate MIP-K-Ukr has a higher divergence potential so that the CP sequence contains more non-synonymous changes that are subject to selection [105]. This has already been observed for the maize streak virus, where even a few changes in nucleotide sequence have large effects on virus functionality [106].
Within a host, different WDV populations can occur [107], and a lack of antagonism between isolates may favor recombination between viral sequences during host infection. Such a case has already been described for the isolate WDV Bar [TR]. The isolate is a variant of the barley WDV strain described in infected barley in Turkey [108]. Whole genome sequence analysis showed that the barley WDV isolate partially corresponds to a novel WDV-like Mastrevirus species [109]. In addition to the WDV Bar [TR] isolate, sequence alignment analysis of field isolates revealed regions of the viral genome with short, few-nucleotide recombination patterns between wheat and barley strains. This suggests that sequences from barley strains were replaced by functionally homologous sequences from wheat strains [107]. Moreover, intra-specific recombinant genomes were detected with two WDV wheat strains in China [110]. In this context, it should be noted that defective forms of wheat and barley strains containing at least part of the SIR and LIR sequences have also been detected in WDV-infected plants [15][107]. Putative recombinant isolates have also been identified for other members of the Mastrevirus genus, such as the maize streak virus [111].
3. Wheat Dwarf Disease (WDD)
3.1. History
The first dwarfing of wheat in Europe was observed in the early 20th century, with characteristic heavy tillering, dwarfing, and deformation of the plants and subsequent death, while the first similar symptoms were described as early as 1863 in a region that is now part of Poland [112]. In Sweden, the leafhopper species Psammotettix alienus was made responsible for this by Tullgren in 1918 [113]. At that time, it was assumed that other insects besides P. alienus were involved in the transmission of the so-called slidsjuka, or sheath disease, due to the partially stuck ears in the leaf sheaths. Overall, there were differing opinions on the cause, but it was consistently observed that the damage occurred particularly in dry and hot years [114]. Field prevalence was relatively low in the 20th century, and thus, there are few descriptions of dwarfing symptoms in the scientific literature, but sometimes in the context of severe outbreaks in wheat [115][116][117][118][119]. Slidsjuka, or WDD, declined in Sweden around 1950 and occurred only sporadically in the following 30–40 years until the 1980s/1990s [120][121][122]. This decline was attributed to changes in agricultural practices. The abandonment of undersowing in winter wheat, which was common in the first half of the century, or even the increased use of combine harvesters, was considered to have had a positive effect on disease control [123].
The direct relationship between virus, vector, and symptoms was first reported in 1961 using samples from wheat fields in western parts of the Czechoslovakia [10][124]. However, there was still confusion about the cause, as no clear virus particles or possible pathogens could be detected [13]. The identification and current taxonomic classification of the virus did not occur until 1980, when, after three decades, there was again an increased incidence of the disease in a number of European countries [19]. In the late 1980s, a new disease (pieds chétifs) occurred in central France, causing severe damage in wheat, with yield losses of more than 50%, and was associated with a high incidence of the leafhopper P. alienus [125]. Initially, only Mycoplasma-like organisms were diagnosed in this context [116]. In collaboration with a Swedish research group, the disease-causing pathogen was identified as WDV [126].
From this time on, the occurrence of vectors and viruses was studied, with WDV occurring mainly in central France and adjacent areas but not in the coastal regions and south of the country [127][128]. The level of knowledge at that time was very low and was mainly based on studies from the Czech Republic [10], Sweden [19], and France [129]. In Germany, the first record probably occurred in 1990 near Dresden by Vacke [91] (Figure 2).
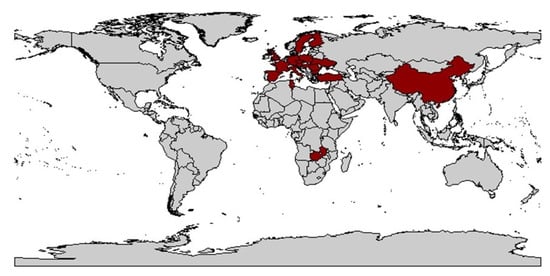
Figure 2. World map with countries where WDV could be detected (marked in red). WDV was reported in Ukraine [130], Romania [13], Bulgaria [130], Hungary [130], Italy [117], France [46], Sweden [19], Poland [131], Finland [18], Spain [132], the United Kingdom [107], Austria [107] and Slovenia [133], as well as regions in Iran [134], the Middle East (Turkey [108], Africa (Tunisia [119] and Zambia [135]), West Asia (Syria [136], and China [137][138]). [139].
A concrete dispersal route cannot be deduced from the data. However, based on the biology of the animals and their activity, a natural spread over land seems most likely. The virus has been detected in the main Eurasian cereal-growing areas and in its region of origin in the Middle East. This can possibly be attributed to the fact that the climatic requirements for wheat cultivation, for example, match with those of P. alienus. Exceptions like India, as well as Canada and Australia, underline these theories.
The reason for the increasing spread of WDV and the increased occurrence in areas where WDV has been previously reported is not clearly understood but is probably caused by changes in agricultural practices. One of the main causes is assumed to be the increased use of ploughless tillage. Also, the EU regulation on the use of a large part of stubble fields after winter wheat cultivation as set-aside areas was thought to be favorable for P. alienus reproduction and overwintering. Avoiding set-aside areas after the occurrence of WDV-infected wheat and avoiding undersowing crops were therefore considered as possible control measures in Sweden [120]. Furthermore, harvesting with short stubble, early tillage in autumn, and avoiding early sowing had a positive effect on reducing the population of P. alienus [120]. Global climate change may also play a role in promoting the spread of vector-borne diseases. In this context, higher temperatures may favor the colonization of new habitats and hosts. Field monitoring is therefore essential, especially in cereal-growing regions, to identify additional regions where P. alienus may spread together with WDV [115][116][117][118][119] since the spread of WDV results from the migration of virulent vectors from wild or cultivated reservoirs into cereal fields [120][140].
3.2. Host Range
The host range of WDV includes mainly monocotyledonous plants [36][141]. In addition to a variety of members of the Poaceae family, including important cereals such as wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), rye (Secale cereale L.), oats (Avena sativa), and triticale [11][13][142], WDV also infects various wild and cultivated grasses, including Bromus secalinus L., Lolium multiflorum Lam. [13], Avena fatua L., B. inermis Leyss., B. tectorum L., H. murinum L., L. perenne L., L. temulentum L. [143], A. sterilis L., A. strigosa Schreb., Poa annua L. [102], L. remotum Schrk., Lagurus ovatus L. [144], and Apera spica-venti (L.) P. Beauv. [143], which are considered virus reservoirs [13].
3.3. Symptoms of WDD
The name of the virus is derived from its main characteristics, the disruption of the shoot growth and the formation of numerous shoots in wheat, resulting in the typical dwarf and bushy growth (Figure 3).

Figure 3. Eight-week-old wheat plants with different degrees (symptom scoring 1, 2, 5, 6, 8) of dwarfing in the greenhouse depending on their genotype (a) and at BBCH stage 30–39 in May 2021 under field conditions (b) after artificial inoculation with symptom-bearing in the middle of the image. (c) Leaves of WDV-infected plants (left) show a stripe-like lightening compared to healthy leaves (right), which later develops into yellowing.
Furthermore, symptoms of WDV infection in wheat also include chlorosis, reduced root size, intense yellow or red discoloration of leaves with or without a mosaic pattern, deformation of leaves, reduced growth hardiness, delayed ear emergence, reduced number of ears as well as sterile flowers, significant yield losses and even complete plant death during early developmental stages of winter wheat and winter barley in winter and spring [13][120][145][146][147][148]. These are partly due to the side effects of infection, such as the effects of expression of viral suppressors of RNA silencing. Symptoms may also affect plant defense responses, leading to plant overreaction in the form of necrosis [149], chlorotic spots, and demarcated streaks on the leaves. The symptoms themselves first appear on the youngest and later on older leaves in association with small cracks and deformations on the youngest leaf, which are characteristic of the infection. This is followed by yellowing of the leaves at the leaf tips and margins with possible partial red coloration [13]. Symptomatic plants usually appear in patches in the field [11][13][147].
In addition to the described symptoms in wheat, the intensity of symptom expression varies among the other infested species. Symptoms in winter barley are similar to those of winter wheat, with no red coloration. Spring barley responds with a lower degree of dwarfing and yellowing of the leaf tips. Similar symptoms occur in winter rye, often associated with anthocyanin formation in leaves and culms. Spring rye shows only minor developmental depression, few leaf spots, and no disruption of generative plants. Oats show minor developmental depression, yellowing, and light red coloration [13]. Triticale shows no increased tillering after WDV infection compared to control plants, but spike-bearing culms shorten by half [150]. In A. spica, growth reductions of 20%, severe tillering, yellowing, and chlorotic spots were observed [13][102]. The wild grass Poa annua shows no symptoms after infection, while Lolium perenne and Lolium multiflorum showed tolerance to WDV in studies with longer plant viability after infection [11].
The extent of damage and the development of symptoms depends on the time of infection. Early infections of winter cereals at the 2–3 leaf stage during fall result in reduced winter hardiness, as well as severe developmental disorders, with pronounced symptoms and negative effects on yield as a result of ear formation that is often partially stuck in the leaf sheaths. The quality of the grains is reduced as they are dried out, shriveled, and partially unable to germinate [13][90]. The root system is also affected by WDV infection. As a result of the infection, there is a reduced formation of secondary roots. The roots appear shorter and thinner overall [90].
Infections in spring result in shortening of internodes and, in some cases, ears. In spring wheat, no severe developmental disorders but shortening of shoots could be observed when infestation occurred from the beginning of shooting to ear swelling (BBCH 31–45). Usually, the first signs of disease in winter wheat appear 18–25 days after infection. In general, symptoms in early-sown wheat are considered to usually appear four to six weeks after infection, while in late-sown wheat, the corresponding symptoms do not become visible until spring, provided the plants are able to overwinter. If infection occurs in spring or early summer, the incubation period lasts three to four weeks. In spring wheat, under greenhouse conditions, the first symptoms are expected 10–15 days after infection, while infections in the field have an incubation period of three weeks [13].
Symptoms caused by infection with Barley yellow dwarf virus (BYDV), which belongs to the Luteoviridae family and is transmitted by aphids, are visually similar to those caused by WDV. When infected in early fall, it causes WDV-like growth depression. The two viruses can only be distinguished from each other by double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) or polymerase chain reaction (PCR), so prior to the discovery of WDV, plants were probably often assigned to BYDV on the basis of dwarfism [150][151].
References
- Buck, K.W. Geminiviruses (Geminiviridae). In Encyclopedia of Virology; Granoff, A., Webster, R.G., Eds.; Academic Press: Cambridge, MA, USA, 1999; pp. 597–606.
- Canto, T.; Aranda, M.A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Chang. Biol. 2009, 15, 1884–1894.
- Habekuß, A.; Riedel, C.; Schliephake, E.; Ordon, F. Breeding for resistance to insect-transmitted viruses in barley—An emerging challenge due to global warming. J. Für Kult. 2009, 61, 53–61.
- Roos, J.; Hopkins, R.; Kvarnheden, A.; Dixelius, C. The impact of global warming on plant diseases and insect vectors in Sweden. Eur. J. Plant Pathol. 2011, 129, 9–19.
- Ziesche, T.M.; Bell, J.; Ordon, F.; Schliephake, E.; Will, T. Long-term monitoring of insects in agricultural landscapes. Mitteilungen Der DGaaE 2020, 22, 101–106.
- Barnett, O.W.; Main, C.E. Plant Virus Disease—Economic Aspects. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1318–1326.
- Waterworth, H.E.; Hadidi, A. Economic Losses due to Plant Viruses. In Plant Virus Disease control; APS Press: St. Paul, MN, USA, 1998.
- Fraser, R.S.S. Plant Resistance to Viruses|Natural Resistance. In Encyclopedia of Virology, 2nd ed.; Granoff, A., Webster, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1300–1307.
- van Regenmortel, M.H.; Fauquet, C.M.; Bishop, D.H.; Carstens, E.B.; Estes, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Virus Taxonomy: Classification and Nomenclature of Viruses. In Seventh Report of the International Committee on Taxonomy of Viruses; Academic Press: New York, NY, USA, 2000.
- Vacke, J. Wheat dwarf virus disease. Biol. Plant 1961, 3, 228–233.
- Mehner, S.; Manurung, B.; Gruntzig, M.; Habekuss, A.; Witsack, W.; Fuchs, E. Investigations into the ecology of the Wheat dwarf virus (WDV) in Saxony-Anhalt, Germany. J. Plant Dis. Prot. 2003, 110, 313–323.
- Manurung, B.; Witsack, W.; Mehner, S.; Gruntzig, M.; Fuchs, E. Studies on biology and population dynamics of the leafhopper Psammotettix alienus Dahlb. (Homoptera: Auchenorrhyncha) as vector of Wheat dwarf virus (WDV) in Saxony-Anhalt, Germany. J. Plant Dis. Prot. 2005, 112, 497–507.
- Vacke, J. Host plants range and symptoms of wheat dwarf virus. Věd Pr Výz Ust. Rostl Výroby Praha-Ruzyně 1972, 17, 151–162.
- MacDowell, S.W.; Macdonald, H.; Hamilton, W.D.O.; Coutts, R.H.A.; Buck, K.W. The nucleotide sequence of cloned wheat dwarf virus DNA. EMBO J. 1985, 4, 2173–2180.
- Macdonald, H.; Coutts, R.H.A.; Buck, K.W. Characterization of a Subgenomic DNA Isolated from Triticum Aestivum Plants Infected with Wheat Dwarf. J. Gen. Virol. 1988, 69, 1339–1344.
- Schalk, H.J.; Matzeit, V.; Schiller, B.; Schell, J.; Gronenborn, B. Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. EMBO J. 1989, 8, 359–364.
- Lindblad, M.; Waern, P. Correlation of wheat dwarf incidence to winter wheat cultivation practices. Agric. Ecosyst. Environ. 2002, 92, 115–122.
- Lemmetty, A.; Huusela-Veistola, E. First Report of Wheat dwarf virus in Winter Wheat in Finland. Plant Dis. 2005, 89, 912.
- Lindsten, K.; Lindsten, B.; Abdelmoeti, M.; Junti, N. Purification and some properties of wheat dwarf virus. In Proceedings of the 3rd Conference on Virus Diseases of Gramineae in Europe, Rothamsted, UK, 28–30 May 1980; pp. 27–31.
- Fauquet, C.M.; Briddon, R.W.; Brown, J.K.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X. Geminivirus strain demarcation and nomenclature. Arch. Virol. 2008, 153, 783–821.
- Bernardo, P.; Golden, M.; Akram, M.; Naimuddin, N.N.; Fernandez, E.; Granier, M.; Rebelo, A.G.; Peterschmitt, M.; Martin, D.P.; Roumagnac, P. Identification and characterisation of a highly divergent geminivirus: Evolutionary and taxonomic implications. Virus Res. 2013, 177, 35–45.
- Varsani, A.; Navas-Castillo, J.; Moriones, E.; Hernández-Zepeda, C.; Idris, A.; Brown, J.K.; Murilo Zerbini, F.; Martin, D.P. Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 2014, 159, 2193–2203.
- Agrios, G.N. Plant Pathology, 3rd ed.; Academic Press: New York, NY, USA, 1988; pp. 3–39.
- Matzeit, V. Wheat Dwarf Virus—Ein Geminivirus Monokotyledoner Pflanzen-DNA-Sequenz, Replikation und Einsatz Seines Genoms zur Amplifikation und Expression Fremder Gene. Ph.D. Thesis, Universität zu Köln, Köln, Germany, 1988.
- Zhang, W.; Olson, N.H.; Baker, T.S.; Faulkner, L.; Agbandje-McKenna, M.; Boulton, M.I.; Davies, J.W.; McKenna, R. Structure of the Maize Streak Virus Geminate Particle. Virology 2001, 279, 471–477.
- Boulton, M.I. Functions and interactions of mastrevirus gene products. Physiol. Mol. Plant Pathol. 2002, 60, 243–255.
- Drews, G.; Adam, G.; Heinze, C. Molekulare Pflanzenvirologie; Springer: Berlin/Heidelberg, Germany, 2004.
- Adejare, G.O.; Coutts, R.H.A. The Isolation and Characterisation of a Virus from Nigerian Cassava Plants Affected by the Cassava Mosaic Disease, and Attempted Transmission of the Disease. J. Phytopathol. 1982, 103, 198–210.
- Harrison, B.D. Advances in Geminivirus Research. Annu. Rev. Phytopathol. 1985, 23, 55–82.
- Damsteegt, V.D.; Igwegbe, E.C.K. Epidemiology and Control of Maize streak disease. In Plant Virus Disease Control; APS Press: St. Paul, MN, USA, 1998; pp. 484–494.
- Moffat, A.S. Geminiviruses Emerge as Serious Crop Threat. Science 1999, 286446, 1835.
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717.
- Fiallo-Olivé, E.; Lett, J.-M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J. ICTV Virus Taxonomy Profile: Geminiviridae 2021. J. Gen. Virol. 2021, 1022, 001696.
- Family: Geminiviridae. Available online: https://ictv.global/report/chapter/geminiviridae/geminiviridae (accessed on 12 November 2022).
- Fauquet, C.M.; Bisaro, D.M.; Briddon, R.W.; Brown, J.K.; Harrison, B.D.; Rybicki, E.P.; Stenger, D.C.; Stanley, J. Virology division news: Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Arch. Virol. 2003, 148, 405–420.
- Muhire, B.; Martin, D.P.; Brown, J.K.; Navas-Castillo, J.; Moriones, E.; Zerbini, F.M.; Rivera-Bustamante, R.; Malathi, V.G.; Briddon, R.W.; Varsani, A. A Genome-Wide Pairwise-Identity-Based Proposal for the Classification of Viruses in the Genus Mastrevirus (Family Geminiviridae). Arch. Virol. 2013, 158, 1411–1424.
- Candresse, T.; Filloux, D.; Muhire, B.; Julian, C.; Galzi, S.; Fort, G.; Bernardo, P.; Daugrois, J.H.; Fernandez, E.; Martin, D.P.; et al. Appearances can be deceptive: Revealing a hidden viral infection with deep sequencing in a plant quarantine context. PLoS ONE 2014, 9, e102945.
- NCBI Virus. Available online: https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/virus?SeqType_s=Nucleotide&VirusLineage_ss=Mastrevirus,%20taxid:11212 (accessed on 22 September 2023).
- Morris, B.A.M.; Richardson, K.A.; Haley, A.; Zhan, X.; Thomas, J.E. The nucleotide sequence of the infectious cloned DNA component of tobacco yellow dwarf virus reveals features of geminiviruses infecting monocotyledonous plants. Virology 1992, 187, 633–642.
- Gutierrez, C. Geminivirus DNA replication. Mol. Life Sci. CMLS 1999, 56, 313–329.
- Thomas, J.E.; Parry, J.N.; Schwinghamer, M.W.; Dann, E.K. Two novel mastreviruses from chickpea (Cicer arietinum) in Australia. Arch. Virol. 2010, 155, 1777–1788.
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133.
- Gafni, Y.; Epel, B.L. The role of host and viral proteins in intra- and inter-cellular trafficking of geminiviruses. Physiol. Mol. Plant Pathol. 2002, 60, 231–241.
- Ramsell, J.N.E. Genetic Variability of Wheat Dwarf Virus. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2007.
- Woolston, C.J.; Barker, R.; Gunn, H.; Boulton, M.I.; Mullineaux, P.M. Agroinfection and nucleotide sequence of cloned wheat dwarf virus DNA. Plant Mol. Biol. 1988, 11, 35–43.
- Bendahmane, M.; Schalk, H.J.; Gronenborn, B. Identification and characterization of wheat dwarf virus from France using a rapid method for geminivirus DNA preparation. Phytopathology 1995, 851, 1449–1455.
- Dickinson, V.J.; Halder, J.; Woolston, C.J. The Product of Maize Streak Virus ORF V1 Is Associated with Secondary Plasmodesmata and Is First Detected with the Onset of Viral Lesions. Virology 1996, 220, 51–59.
- Gutierrez, C. Geminiviruses and the plant cell cycle. Plant Mol. Biol. 2000, 43, 763–772.
- Gutierrez, C. DNA replication and cell cycle in plants: Learning from geminiviruses. EMBO J. 2000, 19, 792–799.
- Gutierrez, C.; Ramirez-Parra, E.; Mar Castellano, M.; Sanz-Burgos, A.P.; Luque, A.; Missich, R. Geminivirus DNA replication and cell cycle interactions. Vet. Microbiol. 2004, 98, 111–119.
- Rojas, M.R.; Hagen, C.; Lucas, W.J.; Gilbertson, R.L. Exploiting chinks in the plant’s armor: Evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 2005, 43, 361–394.
- Briddon, R.W.; Martin, D.P.; Owor, B.E.; Donaldson, L.; Markham, P.G.; Greber, R.S.; Varsani, A. A novel species of mastrevirus (family Geminiviridae) isolated from Digitaria didactyla grass from Australia. Arch. Virol. 2010, 155, 1529–1534.
- Hofer, J.M.I.; Dekker, E.L.; Reynolds, H.V.; Woolston, C.J.; Cox, B.S.; Mullineaux, P.M. Coordinate Regulation of Replication and Virion Sense Gene Expression in Wheat Dwarf Virus. Plant Cell 1992, 4, 213–223.
- Morris-Krsinich, B.A.M.; Mullineaux, P.M.; Donson, J.; Boulton, M.I.; Markham, P.G.; Short, M.N.; Davies, J.W. Bidirectional transcription of maize streak virus DNA and identification of the coat protein gene. Nucleic Acids Res. 1985, 130, 7237–7256.
- Dekker, E.L.; Woolston, C.J.; Xue, Y.; Cox, B.; Mullineaux, P.M. Transcript mapping reveals different expression strategies for the bicistronic RNAs of the geminivirus wheat dwarf virus. Nucleic Acids Res. 1991, 195, 4075–4081.
- Fenoll, C.; Black, D.M.; Howell, S.H. The intergenic region of maize streak virus contains promoter elements involved in rightward transcription of the viral genome. EMBO J. 1988, 7, 1589–1596.
- Accotto, G.P.; Donson, J.; Mullineaux, P.M. Mapping of Digitaria streak virus transcripts reveals different RNA species from the same transcription unit. EMBO J. 1989, 8, 1033–1039.
- Mullineaux, P.M.; Guerineau, F.; Accotto, G.-P. Processing of complementary sense RNAs of Digitariastreak virus in its host and in transgenic tobacco. Nucleic Acids Res. 1990, 184, 7259–7265.
- Wright, E.A.; Heckel, T.; Groenendijk, J.; Davies, J.W.; Boulton, M.I. Splicing features in maize streak virus virion- and complementary-sense gene expression. Plant J. 1997, 12, 1285–1297.
- Palmer, K.E.; Rybicki, E.P. The Molecular Biology of Mastreviruses. Adv. Virus Res. 1998, 50, 183–234.
- Wang, Y.; Mao, Q.; Liu, W.; Mar, T.; Wei, T.; Liu, Y.; Wang, X. Localization and Distribution of Wheat dwarf virus in Its Vector Leafhopper, Psammotettix alienus. Phytopathology 2014, 104, 897–904.
- Noueiry, A.O.; Lucas, W.J.; Gilbertson, R.L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 1994, 76, 925–932.
- Liu, H.; Boulton, M.I.; Oparka, K.J.; Davies, J.W. Interaction of the movement and coat proteins of Maize streak virus: Implications for the transport of viral DNA. J. Gen. Virol. 2001, 82, 35–44.
- Liu, H.; Andrew, L.P.; Davies, J.W.; Boulton, M.I. A single amino acid change in the coat protein of Maize streak virus abolishes systemic infection, but not interaction with viral DNA or movement protein. Mol. Plant Pathol. 2001, 2, 223–228.
- Noris, E.; Vaira, A.M.; Caciagli, P.; Masenga, V.; Gronenborn, B.; Accotto, G.P. Amino Acids in the Capsid Protein of Tomato Yellow Leaf Curl Virus That Are Crucial for Systemic Infection, Particle Formation, and Insect Transmission. J. Virol. 1998, 722, 10050–10057.
- Liu, H.; Boulton, M.I.; Thomas, C.L.; Prior, D.A.M.; Oparka, K.J.; Davies, J.W. Maize Streak Virus Coat Protein Is Karyophyllic and Facilitates Nuclear Transport of Viral DNA. Mol. Plant Microb. Interact. 1999, 120, 894–900.
- Kotlizky, G.; Boulton, M.I.; Pitaksutheepong, C.; Davies, J.W.; Epel, B.L. Intracellular and Intercellular Movement of Maize Streak Geminivirus V1 and V2 Proteins Transiently Expressed as Green Fluorescent Protein Fusions. Virology 2000, 274, 32–38.
- Sunter, G.; Bisaro, D.M. Transactivation of Geminivirus AR1 and BR1 Gene Expression by the Viral AL2 Gene Product Occurs at the Level of Transcription. Plant Cell 1992, 40, 1321–1331.
- Hong, Y.; Saunders, K.; Hartley, M.R.; Stanley, J. Resistance to Geminivirus Infection by Virus-Induced Expression of Dianthin in Transgenic Plants. Virology 1996, 220, 119–127.
- Voinnet, O.; Pinto, Y.M.; Baulcombe, D.C. Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 1999, 964, 14147–14152.
- Shivaprasad, P.V.; Akbergenov, R.; Trinks, D.; Rajeswaran, R.; Veluthambi, K.; Hohn, T.; Pooggin, M.M. Promoters, Transcripts, and Regulatory Proteins of Mungbean Yellow Mosaic Geminivirus. J. Virol. 2005, 793, 8149–8163.
- Trinks, D.; Rajeswaran, R.; Shivaprasad, P.V.; Akbergenov, R.; Oakeley, E.J.; Veluthambi, K.; Hohn, T.; Pooggin, M.M. Suppression of RNA Silencing by a Geminivirus Nuclear Protein, AC2, Correlates with Transactivation of Host Genes. J. Virol. 2005, 79, 2517–2527.
- Wang, H.; Buckley, K.J.; Yang, X.; Buchmann, R.C.; Bisaro, D.M. Adenosine Kinase Inhibition and Suppression of RNA Silencing by Geminivirus AL2 and L2 Proteins. J. Virol. 2005, 792, 7410–7418.
- Chowda-Reddy, R.V.; Dong, W.; Felton, C.; Ryman, D.; Ballard, K.; Fondong, V.N. Characterization of the cassava geminivirus transcription activation protein putative nuclear localization signal. Virus Res. 2009, 145, 270–278.
- Castillo-González, C.; Liu, X.; Huang, C.; Zhao, C.; Ma, Z.; Hu, T.; Sun, F.; Zhou, X.; Wang, X.J.; Zhang, X. Geminivirus-Encoded TrAP Suppressor Inhibits the Histone Methyltransferase SUVH4/KYP to Counter Host Defense. eLife 2015, 4, e06671.
- Kumar, V.; Mishra, S.K.; Rahman, J.; Taneja, J.; Sundaresan, G.; Mishra, N.S.; Mukherjee, S.K. Mungbean yellow mosaic Indian virus encoded AC2 protein suppresses RNA silencing by inhibiting Arabidopsis RDR6 and AGO1 activities. Virology 2015, 486, 158–172.
- Kvarnheden, A.; Lindblad, M.; Lindsten, K.; Valkonen, J.P.T. Genetic diversity of Wheat dwarf virus. Arch. Virol. 2002, 147, 205–216.
- Koch, C. Die Bestimmung der DNA-Sequenz des Geminivirus WDV-ER Genoms und Versuche zur Übertragung des Virus auf Gerste mit Agrobacterium tumefaciens. Ph.D. Thesis, Universität Köln, Köln, Germany, 1990.
- Schubert, J.; Habekuß, A.; Rabenstein, F. Investigation of differences between wheat and barley forms of Wheat dwarf virus and their distribution in host plants. Plant Prot. Sci. Prague 2003, 38, 43–48.
- Jeske, H. Geminiviruses. In TT Viruses. Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 185–226.
- Hanley-Bowdoin, L.; Bejarano, E.R.; Robertson, D.; Mansoor, S. Geminiviruses: Masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 2013, 111, 777–788.
- Wu, B.; Shang, X.; Schubert, J.; Habekuß, A.; Elena, S.F.; Wang, X. Global-scale computational analysis of genomic sequences reveals the recombination pattern and coevolution dynamics of cereal-infecting geminiviruses. Sci. Rep. 2015, 5, 8153.
- Van Bel, A.J.E. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149.
- Kammann, M.; Schalk, H.-J.; Matzeit, V.; Schaefer, S.; Schell, J.; Gronenborn, B. DNA replication of wheat dwarf virus, a geminivirus, requires two cis-acting signals. Virology 1991, 184, 786–790.
- Heyraud, F.; Matzeit, V.; Schaefer, S.; Schell, J.; Gronenborn, B. The conserved nonanucleotide motif of the geminivirus stem-loop sequence promotes replicational release of virus molecules from redundant copies. Biochimie 1993, 75, 605–615.
- Laufs, J.; Jupin, I.; David, C.; Schumacher, S.; Heyraud-Nitschke, F.; Gronenborn, B. Geminivirus replication: Genetic and biochemical characterization of Rep protein function, a review. Biochimie 1995, 770, 765–773.
- Hanley-Bowdoin, L.; Settlage, S.B.; Orozco, B.M.; Nagar, S.; Robertson, D. Geminiviruses: Models for Plant DNA Replication, Transcription, and Cell Cycle Regulation. Crit. Rev. Plant Sci. 1999, 18, 71–106.
- Bosque-Pérez, N.A. Eight decades of maize streak virus research. Virus Res. 2000, 71, 107–121.
- Astier, S.; Albouy, J.; Maury, Y.; Robaglia, C.; Lecoq, H. Principles of Plant Virology: Genome, Pathogenicity, Virus Ecology; Institut National de la Recherche Agronomique: Paris, France, 2007.
- Tomenius, K.; Oxelfelt, P. Preliminary Observations of Viruslike Particles in Nuclei in Cells of Wheat Infected with the Wheat Dwarf Disease. J. Phytopathol. 1981, 101, 163–167.
- Huth, W.; Lesemann, D.-E. Nachweis des wheat dwarf virus in Deutschland. Nachrichtenblatt Dtsch. Pflanzenschutzdienstes 1994, 46, 105–106.
- Hehnle, S.; Wege, C.; Jeske, H. Interaction of DNA with the Movement Proteins of Geminiviruses Revisited. J. Virol. 2004, 784, 7698–7706.
- Evert, R.F.; Russin, W.A.; Botha, C.E.J. Distribution and frequency of plasmodesmata in relation to photoassimilate pathways and phloem loading in the barley leaf. Planta 1996, 198, 572–579.
- Aoki, N.; Scofield, G.N.; Wang, X.-D.; Patrick, J.W.; Offler, C.E.; Furbank, R.T. Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 2004, 219, 176–184.
- Crawford, K.M.; Zambryski, P.C. Non-Targeted and Targeted Protein Movement through Plasmodesmata in Leaves in Different Developmental and Physiological States. Plant Physiol. 2001, 125, 1802–1812.
- Peterschmitt, M.; Quiot, J.B.; Reynaud, B.; Baudin, P. Detection of maize streak virus antigens over time in different parts of maize plants of a sensitive and a so-called tolerant cultivar by ELISA. Ann. Appl. Biol. 1992, 121, 641–653.
- Mariano, A.C.; Andrade, M.O.; Santos, A.A.; Carolino, S.M.B.; Oliveira, M.L.; Baracat-Pereira, M.C.; Brommonshenkel, S.H.; Fontes, E.P.B. Identification of a novel receptor-like protein kinase that interacts with a geminivirus nuclear shuttle protein. Virology 2004, 318, 24–31.
- Maule, A.; Leh, V.; Lederer, C. The dialogue between viruses and hosts in compatible interactions. Curr. Opin. Plant Biol. 2002, 5, 279–284.
- Plant Resistance to Geminiviruses. Available online: https://biblio.iita.org/documents/S20InbkPatilPlantNothomDev.pdf-c1c85057a36d00c1bca8600d973d2cdc.pdf (accessed on 23 September 2023).
- Mehner, S. Zur Ökologie des Wheat Dwarf Virus (WDV) in Sachsen-Anhalt. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2005.
- Commandeur, U.; Huth, W. Differentiation of strains of Wheat dwarf virus in infected wheat and barley plants by means of polymerase chain reaction. J. Plant Dis. Prot. 1999, 106, 550–552.
- Lindsten, K.; Vacke, J. A possible barley adapted strain of wheat dwarf virus (WDV). Acta Phytopathol. Entomol. Hung. 1991, 26, 175–180.
- Schubert, J.; Habekuß, A.; Kazmaier, K.; Jeske, H. Surveying cereal-infecting geminiviruses in Germany—Diagnostics and direct sequencing using rolling circle amplification. Virus Res. 2007, 127, 61–70.
- Wu, B.; Melcher, U.; Guo, X.; Wang, X.; Fan, L.; Zhou, G. Assessment of codivergence of Mastreviruses with their plant hosts. BMC Evol. Biol. 2008, 8, 335.
- Mishchenko, L.T.; Dunich, A.A.; Mishchenko, I.A.; Dashchenko, A.V.; Kozub, N.O.; Kyslykh, T.M.; Molodchenkova, O.O. Wheat dwarf virus in Ukraine: Occurrence, molecular characterization and impact on the yield. J. Plant Dis. Prot. 2022, 129, 107–116.
- Shepherd, D.N.; Martin, D.P.; McGivern, D.R.; Boulton, M.I.; Thomson, J.A.; Rybicki, E.P. A three-nucleotide mutation altering the Maize streak virus Rep pRBR-interaction motif reduces symptom severity in maize and partially reverts at high frequency without restoring pRBR–Rep binding. J. Gen. Virol. 2005, 86, 803–813.
- Schubert, J.; Habekuß, A.; Wu, B.; Thieme, T.; Wang, X. Analysis of complete genomes of isolates of the Wheat dwarf virus from new geographical locations and descriptions of their defective forms. Virus Genes 2014, 48, 133–139.
- Köklü, G.; Ramsell, J.N.E.; Kvarnheden, A. The complete genome sequence for a Turkish isolate of Wheat dwarf virus (WDV) from barley confirms the presence of two distinct WDV strains. Virus Genes 2007, 34, 359–366.
- Ramsell, J.N.E.; Boulton, M.I.; Martin, D.P.; Valkonen, J.P.T.; Kvarnheden, A. Studies on the host range of the barley strain of Wheat dwarf virus using an agroinfectious viral clone. Plant Pathol. 2009, 58, 1161–1169.
- Wu, X.; Weigel, D.; Wigge, P.A. Signaling in plants by intercellular RNA and protein movement. Genes Dev. 2002, 16, 151–158.
- Owor, B.E.; Shepherd, D.N.; Taylor, N.J.; Edema, R.; Monjane, A.L.; Thomson, J.A.; Martin, D.P.; Varsani, A. Successful application of FTA® Classic Card technology and use of bacteriophage ϕ29 DNA polymerase for large-scale field sampling and cloning of complete maize streak virus genomes. J. Virol. Methods 2007, 140, 100–105.
- Jungner, J. Die Zwergzikade (Cicadula sexnotata Fall.) und ihre Bekämpfung; Deutsche landwirtschafts-gesellschaft: Berlin, Germany, 1906.
- Tullgren, A. Zur Morphologie und Systematik der Hemipteren I. Entomol. Tidskr. Entomol. Föreningen I Stockh. 1918, 1918, 113–133.
- Lindsten, K.; Vacke, J.; Gerhardson, B. A preliminary report on three cereal virus diseases new to Sweden spread by Macrosteles and Psammotettix leafhoppers. Medd. Fran Statens Vaxtskyddsanst. 1970, 1423, 285–297.
- Gaborjanyi, R.; Vacke, J.; Bisztray, G. Wheat Dwarf Virus: A New Cereal Pathogen in Hungary; Novenytermeles: Debrecen, Hungary, 1988.
- Lapierre, H.; Cousin, M.T.; Della Giustina, W.; Moreau, J.P.; Khogali, M.; Roux, J. Nanisme blé: Agent pathogéne et vecteur. Description, biologie, interaction. Phytoma 1991, 432, 26–28.
- Conti, M. Leafhopper-borne plant viruses in Italy. Mem. Della Soc. Entomol. Ital. 1993, 72, 541–547.
- Jilaveanu, A.; Vacke, J. Isolation and identification of wheat dwarf virus (WDV) in Romania. Probl. Prot. Plantelor. 1995, 23, 51–62.
- Najar, A.; Makkouk, K.M.; Boudhir, H.; Kumari, S.G.; Zarouk, R.; Bessai, R.; Othman, F.B. Viral Diseases of Cultivated Legume and Cereal Crops in Tunisia; Firenze University Press: Florence, France, 2000; pp. 1000–1010.
- Lindsten, K.; Lindsten, B. Wheat dwarf—An old disease with new outbreaks in Sweden. J. Plant Dis. Prot. 1999, 106, 325–332.
- Sandgren, M.; Lindblad, M. Field studies of Wheat dwarf virus. In Proceedings of the 7th International Congress of Plant Pathology, Edinburgh, UK, 9–16 August 1998.
- Lindblad, M. What happened to the wheat dwarf disease. Växtskyddsnotiser 2000, 64, 11–13.
- Lindsten, K. Wheat dwarf—An old disease caused by a unique and earlier unknown virus. Vaextskyddsnotiser 1980.
- Dlabola, J. Zur Schädlichkeit der Zikaden in Getreidefeldern. Nachrichtenblatt Dtsch. Pflanzenschutzd. 1961, 14, 120–122.
- Moreau, J.-P.; Lapierre, H.; Navarro, D.; Debray, P.; Fohrer, F.; Lebrun, I. Distinction des effets du nanisme et de la jaunisse sur le blé. Phytoma Défense Végétaux 1992, 443, 21–25.
- Lindsten, K.; Lindsten, B. Occurrence and transmission of Wheat dwarf virus (WDV) in France. In Proceedings of the Third International Conference on Pest in Agriculture, Montpellier, France, 7–9 December 1993; pp. 7–9.
- Giustina, W.D.; Lebrun, I.; Lapierre, H.; Lochon, S.; Groupe de Travail „Biologie et Écologie de, P. alienus “. Distribution géographique du vecteur et du virus. Phytoma Défense Végétaux 1991, 432, 30–34.
- Anonym. New Knowledges about wheat dwarf virus. Phytoma Défense Végétaux 1992, 443, 17–20.
- Vacher, C.; Felix, I.; Bonnand, E. Lutte contre Psammotettix alienus, Cicadelle vectrice de la maladie des pieds chétifs. Perspect. Agric. 1991, 162, 86–89.
- Bisztray, G.; Gaborjanyi, R.; Vacke, J. Isolation and characterization of wheat dwarf virus found for the first time in Hungary. J. Plant Dis. Prot. 1989, 96, 449–454.
- Jezewska, J. First report of Wheat dwarf virus occurring in Poland. Phytopathol. Pol. 2001, 21, 93–100.
- Achon, M.A.; Serrano, L.; Ratti, C.; Rubies-Autonell, C. First Detection of Wheat dwarf virus in Barley in Spain Associated with an Outbreak of Barley Yellow Dwarf. Plant Dis. 2006, 90, 970.
- Viršček Marn, M.; Mavrič Pleško, I. First Report of the Occurrence of Wheat dwarf virus Infecting Wheat in Slovenia. Plant Dis. 2017, 101, 1336.
- Behjatnia, S.A.A.; Afsharifar, A.R.; Tahan, V.; Motlagh, M.H.A.; Gandomani, O.E.; Niazi, A.; Izadpanah, K. Widespread occurrence and molecular characterization of Wheat dwarf virus in Iran. Australas. Plant Pathol. 2011, 40, 12–19.
- Kapooria, R.G.; Ndunguru, J. Occurrence of viruses in irrigated wheat in Zambia. EPPO Bull. 2004, 34, 413–419.
- Ekzayez, A.M.; Kumari, S.G.; Ismail, I. First Report of Wheat dwarf virus and Its Vector (Psammotettix provincialis) Affecting Wheat and Barley Crops in Syria. Plant Dis. 2011, 95, 76.
- Xie, J.; Wang, X.; Liu, Y.; Peng, Y.; Zhou, G. First Report of the Occurrence of Wheat dwarf virus in Wheat in China. Plant Dis. 2007, 91, 111.
- Wang, X.; Wu, B.; Wang, J.F. First report of Wheat dwarf virus infecting barley in Yunnan, China. J. Plant Pathol. 2008, 90, 400.
- Bivand, R.; Lewin-Koh, N.; Pebesma, E.; Archer, E.; Baddeley, A.; Bearman, N.; Golicher, D. Package ‘maptools’, Version 1.1–4. 2022.
- Felix, I.; Larcher, J.M.; Maraby, J.; Philippeau, G.; Vinatier, K. Risques d’attaques de cicadelles et conditions d’efficacité des insecticides. Perspect. Agric. 1992, 173, 98–106.
- ICTV. Report Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012.
- Ramsell, J.N.E.; Lemmetty, A.; Jonasson, J.; Andersson, A.; Sigvald, R.; Kvarnheden, A. Sequence analyses of Wheat dwarf virus isolates from different hosts reveal low genetic diversity within the wheat strain. Plant Pathol. 2008, 57, 834–841.
- Vacke, J.; Cibulka, R. Silky bent grass (Apera spica-venti Beauv.)—A new host and reservoir of wheat dwarf virus. Plant Prot. Sci. 1999, 35, 47–50.
- Brunt, A.; Crabtree, K.; Dallwitz, M.; Gibbs, A.; Watson, L. Viruses of Plants; CAB International: Oxfordshire, UK, 1996.
- Fohrer, F.; Lebrun, I.; Lapierre, E.H. Acquisitions recéntes sur le virus du nanisme du blé. Phytoma Défense Végétaux 1992, 443, 18–20.
- Vacke, J.; Cibulka, R. Response of selected winter wheat varieties to wheat dwarf virus infection at an early growth stage. Czech J. Genet. Plant Breed. 2000, 36, 1–4.
- Manurung, B.; Witsack, W.; Mehner, S.; Gruntzig, M.; Fuchs, E. The epidemiology of Wheat dwarf virus in relation to occurrence of the leafhopper Psammotettix alienus in Middle-Germany. Virus Res. 2004, 100, 109–113.
- Širlová, L.; Vacke, J.; Chaloupková, M. Reaction of selected winter wheat varieties to autumnal infection with Wheat dwarf virus. Plant Prot. Sci 2005, 41, 1–7.
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233.
- Huth, W. Weizenverzwergung—Bisher übersehen? Pflanzenschutz Prax. 1994, 4, 37–39.
- Áy, Z.; Kerényi, Z.; Takács, A.; Papp, M.; Petróczi, I.; Gáborjányi, R.; Silhavy, D.; Pauk, J.; Kertész, Z. Detection of cereal viruses in wheat (Triticum aestivum L.) by serological and molecular methods. Cereal Res. Commun. 2008, 36, 215–224.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
26 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




